Abstract
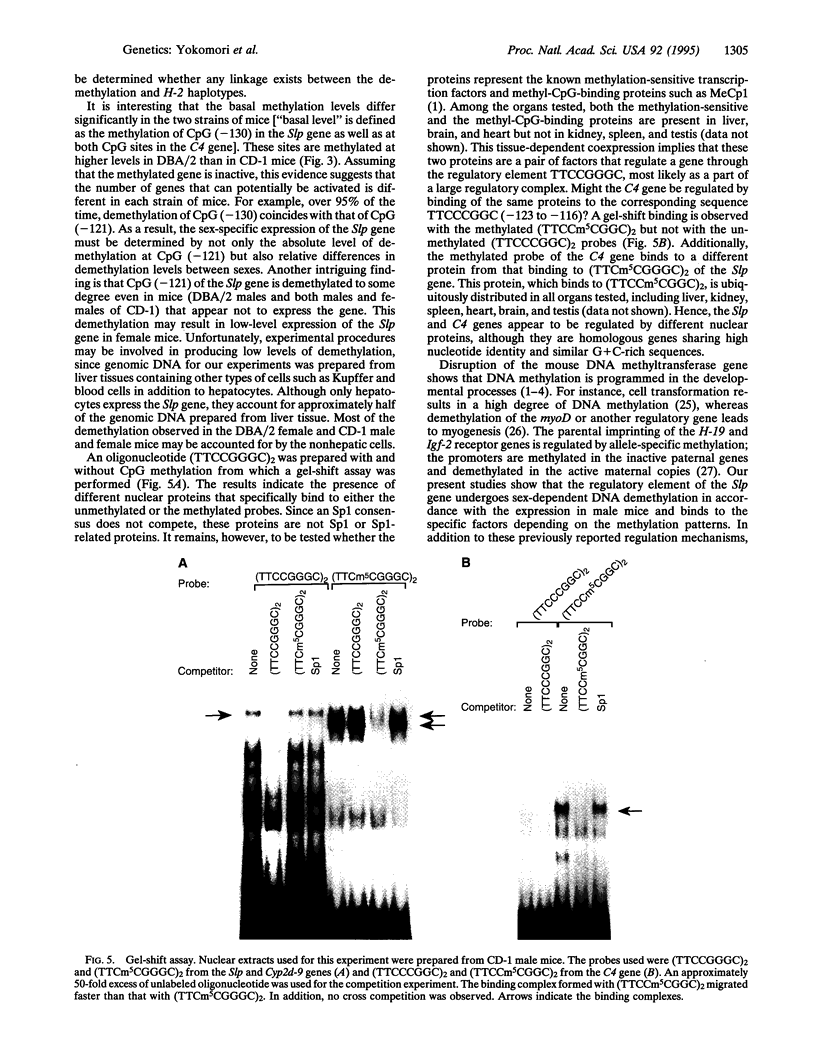
Mouse Slp, a duplicate of the fourth complement component (C4) gene, exhibits EDTA-independent complement activity with a hepatic expression that is male specific. To provide an underlying mechanism for the male-specific expression, we have analyzed the promoter activity of the various 5'-flanking sequences and CpG demethylation of the Slp gene. Transient transfections using HepG2 cells indicate that the element TTCCGGGC (nt -124 to -117) regulates the promoter activity. Moreover, CpG at position -121 of this regulatory element is demethylated to a much higher degree in males than in females. This sexually dimorphic DNA demethylation is consistent with the male-specific expression of the Slp gene in DBA/2 males. The regulatory element binds to the different TTCCGGGC-specific nuclear proteins depending on the methylation of the CpG site. In contrast, the corresponding CpG at position -119 of the C4 gene, which is expressed in both males and females, is demethylated at equal and high levels in both sexes. We therefore propose that the DNA demethylation and methylation-sensitive transcription factors may be a part of the regulatory mechanism for the male-specific expression of the Slp gene.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler A. J., Scheller A., Hoffman Y., Robins D. M. Multiple components of a complex androgen-dependent enhancer. Mol Endocrinol. 1991 Nov;5(11):1587–1596. doi: 10.1210/mend-5-11-1587. [DOI] [PubMed] [Google Scholar]
- Aguado F., Rodrigo J., Cacicedo L., Mellström B. Distribution of insulin-like growth factor-I receptor mRNA in rat brain. Regulation in the hypothalamo-neurohypophysial system. J Mol Endocrinol. 1993 Oct;11(2):231–239. doi: 10.1677/jme.0.0110231. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Frommer M., McDonald L. E., Millar D. S., Collis C. M., Watt F., Grigg G. W., Molloy P. L., Paul C. L. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatsou E., Bourgarel P., Meo T. Male-specific expression of mouse sex-limited protein requires growth hormone, not testosterone. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3626–3630. doi: 10.1073/pnas.90.8.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Li E., Beard C., Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993 Nov 25;366(6453):362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- Miyagoe Y., Georgatsou E., Varin-Blank N., Meo T. The androgen-dependent C4-Slp gene is driven by a constitutively competent promoter. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5786–5790. doi: 10.1073/pnas.90.12.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka M., Kimura H., Yeul Y. D., Yokoyama S., Nakayama K., Takahashi M. Identification of the 5'-flanking regulatory region responsible for the difference in transcriptional control between mouse complement C4 and Slp genes. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7883–7887. doi: 10.1073/pnas.83.20.7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noshiro M., Negishi M. Pretranslational regulation of sex-dependent testosterone hydroxylases by growth hormone in mouse liver. J Biol Chem. 1986 Dec 5;261(34):15923–15927. [PubMed] [Google Scholar]
- Ogata R. T., Zepf N. E. The murine Slp gene. Additional evidence that sex-limited protein has no biologic function. J Immunol. 1991 Oct 15;147(8):2756–2763. [PubMed] [Google Scholar]
- Shreffler D C, Owen R D. A Serologically Detected Variant in Mouse Serum: Inheritance and Association with the Histocompatibility-2 Locus. Genetics. 1963 Jan;48(1):9–25. doi: 10.1093/genetics/48.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreffler D. C., Atkinson J. P., Chan A. C., Karp D. R., Killion C. C., Ogata R. T., Rosa P. A. The C4 and Slp genes of the complement region of the murine H-2 major histocompatibility complex. Philos Trans R Soc Lond B Biol Sci. 1984 Sep 6;306(1129):395–403. doi: 10.1098/rstb.1984.0100. [DOI] [PubMed] [Google Scholar]
- Stavenhagen J. B., Robins D. M. An ancient provirus has imposed androgen regulation on the adjacent mouse sex-limited protein gene. Cell. 1988 Oct 21;55(2):247–254. doi: 10.1016/0092-8674(88)90047-5. [DOI] [PubMed] [Google Scholar]
- Tate P. H., Bird A. P. Effects of DNA methylation on DNA-binding proteins and gene expression. Curr Opin Genet Dev. 1993 Apr;3(2):226–231. doi: 10.1016/0959-437x(93)90027-m. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M. DNA methylation: a phoenix rises. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8761–8762. doi: 10.1073/pnas.90.19.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G., Itakura T., Kawajiri K., Skow L., Negishi M. Gene family of male-specific testosterone 16 alpha-hydroxylase (C-P-450(16 alpha)) in mice. Organization, differential regulation, and chromosome localization. J Biol Chem. 1989 Feb 15;264(5):2920–2927. [PubMed] [Google Scholar]
- Wong G., Kawajiri K., Negishi M. Gene family of male-specific testosterone 16 alpha-hydroxylase (C-P-450(16) alpha) in mouse liver: cDNA sequences, neonatal imprinting, and reversible regulation by androgen. Biochemistry. 1987 Dec 29;26(26):8683–8690. doi: 10.1021/bi00400a029. [DOI] [PubMed] [Google Scholar]
- Wu J., Issa J. P., Herman J., Bassett D. E., Jr, Nelkin B. D., Baylin S. B. Expression of an exogenous eukaryotic DNA methyltransferase gene induces transformation of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8891–8895. doi: 10.1073/pnas.90.19.8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H., Lang M., Wong G., Negishi M. A specific cis-acting element regulates in vitro transcription of sex-dependent mouse steroid 16 alpha-hydroxylase (C-P450(16 alpha)) gene. J Biol Chem. 1990 Aug 25;265(24):14612–14617. [PubMed] [Google Scholar]
- van den Berg C. W., Démant P., Aerts P. C., Van Dijk H. Slp is an essential component of an EDTA-resistant activation pathway of mouse complement. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10711–10715. doi: 10.1073/pnas.89.22.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]