Abstract
The NADPH-dependent O2-.-generating oxidase in subcellular fractions from the neutrophils of three male patients with chronic granulomatous disease was compared with the corresponding preparations from normal neutrophils. The oxidase from normal neutrophils contained flavin adenine dinucleotide in an approximately 0.9:1 molar ratio with cytochrome b559. Each of the three chronic granulomatous disease patients had decreased amounts of the flavoprotein component of the oxidase fraction. The oxidase from two chronic granulomatous disease patients had undetectable amounts of cytochrome b559 whereas the third patient had a normal content of cytochrome b559, which was spectrally indistinguishable from the normal. The intrinsic cytochrome b559 in the oxidase fraction from stimulated neutrophils of the latter chronic granulomatous disease patient was not reduced by NADPH under anaerobic conditions, in distinction with the previously reported reduction of the normal cytochrome b559 under identical conditions. We conclude that the flavoprotein component of the oxidase may mediate transfer of electrons from NADPH to the cytochrome b559 in normal neutrophils, and that deficiency of this flavoprotein is associated with the chronic granulomatous disease phenotype in the three patients studied.
Full text
PDF

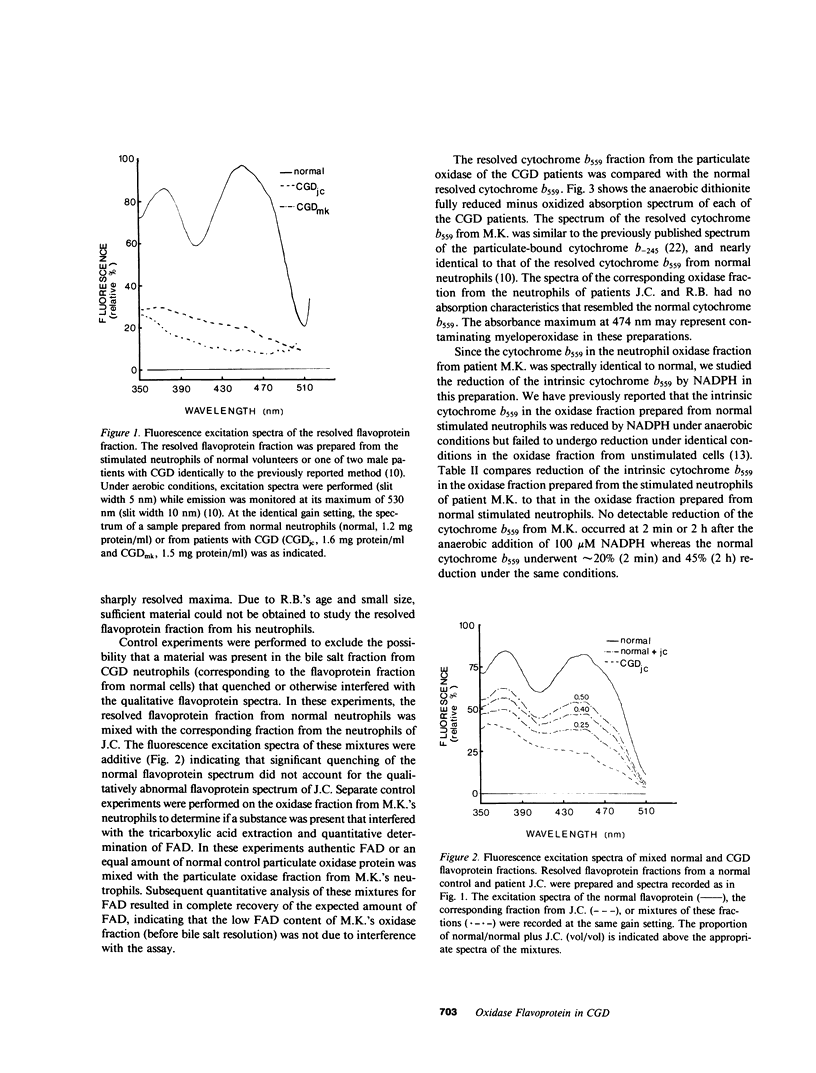
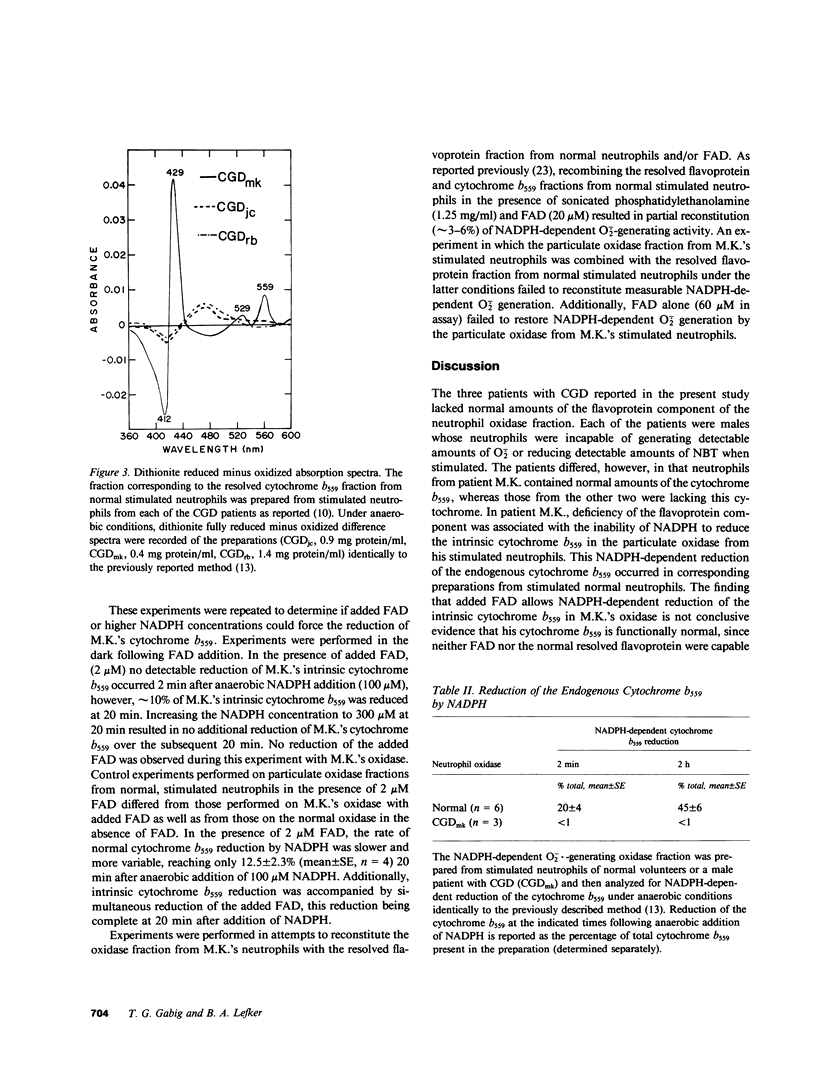

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Peters W. A. The O2--producing enzyme of human neutrophils. Further properties. J Biol Chem. 1981 Mar 10;256(5):2321–2323. [PubMed] [Google Scholar]
- Beinert H., Orme-Johnson W. H., Palmer G. Special techniques for the preparation of samples for low-temperature EPR spectroscopy. Methods Enzymol. 1978;54:111–132. doi: 10.1016/s0076-6879(78)54013-5. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Higson F. K., Jones O. T., Harper A. M., Segal A. W. The enzymic reduction and kinetics of oxidation of cytochrome b-245 of neutrophils. Biochem J. 1982 May 15;204(2):479–485. doi: 10.1042/bj2040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T., Garcia R., Segal A. W. The association of FAD with the cytochrome b-245 of human neutrophils. Biochem J. 1982 Dec 15;208(3):759–763. doi: 10.1042/bj2080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T., Harper A. M., Segal A. W. Oxidation-reduction properties of the cytochrome b found in the plasma-membrane fraction of human neutrophils. A possible oxidase in the respiratory burst. Biochem J. 1981 Feb 15;194(2):599–606. doi: 10.1042/bj1940599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabig T. G., Babior B. M. The killing of pathogens by phagocytes. Annu Rev Med. 1981;32:313–326. doi: 10.1146/annurev.me.32.020181.001525. [DOI] [PubMed] [Google Scholar]
- Gabig T. G., Schervish E. W., Santinga J. T. Functional relationship of the cytochrome b to the superoxide-generating oxidase of human neutrophils. J Biol Chem. 1982 Apr 25;257(8):4114–4119. [PubMed] [Google Scholar]
- Gabig T. G. The NADPH-dependent O-.2-generating oxidase from human neutrophils. J Biol Chem. 1983 May 25;258(10):6352–6356. [PubMed] [Google Scholar]
- Ghisla S., Massey V., Lhoste J. M., Mayhew S. G. Fluorescence and optical characteristics of reduced flavines and flavoproteins. Biochemistry. 1974 Jan 29;13(3):589–597. doi: 10.1021/bi00700a029. [DOI] [PubMed] [Google Scholar]
- Gifford R. H., Malawista S. E. A simple rapid micromethod for detecting chronic granulomatous disease of childhood. J Lab Clin Med. 1970 Mar;75(3):511–519. [PubMed] [Google Scholar]
- Hatefi Y. Introduction--preparation and properties of the enzymes and enzymes complexes of the mitochondrial oxidative phosphorylation system. Methods Enzymol. 1978;53:3–4. doi: 10.1016/s0076-6879(78)53004-8. [DOI] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light D. R., Walsh C., O'Callaghan A. M., Goetzl E. J., Tauber A. I. Characteristics of the cofactor requirements for the superoxide-generating NADPH oxidase of human polymorphonuclear leukocytes. Biochemistry. 1981 Mar 17;20(6):1468–1476. doi: 10.1021/bi00509a010. [DOI] [PubMed] [Google Scholar]
- Newburger P. E., Chovaniec M. E., Cohen H. J. Activity and activation of the granulocyte superoxide-generating system. Blood. 1980 Jan;55(1):85–92. [PubMed] [Google Scholar]
- Newburger P. E., Cohen H. J., Rothchild S. B., Hobbins J. C., Malawista S. E., Mahoney M. J. Prenatal diagnosis of chronic granulomatous disease. N Engl J Med. 1979 Jan 25;300(4):178–181. doi: 10.1056/NEJM197901253000406. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Cramer R., Moncalvo S., Rossi F., Romeo D. Enzymatic basis of metabolic stimulation in leucocytes during phagocytosis: the role of activated NADPH oxidase. Arch Biochem Biophys. 1971 Jul;145(1):255–262. doi: 10.1016/0003-9861(71)90034-8. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Cross A. R., Garcia R. C., Borregaard N., Valerius N. H., Soothill J. F., Jones O. T. Absence of cytochrome b-245 in chronic granulomatous disease. A multicenter European evaluation of its incidence and relevance. N Engl J Med. 1983 Feb 3;308(5):245–251. doi: 10.1056/NEJM198302033080503. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. Absence of cytochrome b reduction in stimulated neutrophils from both female and male patients with chronic granulomatous disease. FEBS Lett. 1980 Jan 28;110(1):111–114. doi: 10.1016/0014-5793(80)80035-4. [DOI] [PubMed] [Google Scholar]



