Abstract
AIM: To evaluate the significance of transforming growth factor beta (TGF β) expression, in correlation with histopathological parameters, at the front of invasion in T1 colorectal cancer (CRC) and presence of metastases.
METHODS: TGF β immunohistochemical expression was studied in 34 specimens of colorectal adenocarcinomas (pT1). A three-step avidin-biotinylated immuno-peroxidase (ABCu-NCL) staining technique was performed on 4-µm paraffin-embedded tissue sections with a monoclonal antibody to TGF β (Novocastra, NCL-TGFB, clone TGFB 17, dilution 1:40).
RESULTS: Seventeen (50%) out of 34 lesions were positive for TGF β expression. The TGF β-positive rate in patients with vascular invasion was significantly higher than in those without vascular invasion (11/14 cases, P<0.01, P = 0.005). The TGF β-positive rate was observed in 91.7% of patients with presence of tumor budding at the front of invasion (11/12 cases, P<0.01, P = 0.0003). A statistically significant correlation was found between the presence of lymph node metastases and positive expression of TGF β (14/16 cases, P<0.01, P = 0.0001). We also observed that the TGF β-positive rates in groups with distant and non-distant metastases were 92.8% and 20% respectively, and a significant correlation between TGF β expression and distant metastasis was shown (P<0.01, P = 0.00003).
CONCLUSION: The evaluation of TGF β expression of protein in association with histological parameters can be used as a parameter of the aggressiveness of pT1 CRC.
Keywords: TGF β, Tumor budding, Colorectal cancer
INTRODUCTION
Colorectal cancer (CRC) is one of the most common types of cancer in Poland and other western countries. According to statistics, the mortality of CRC is ranking second in western countries and third in Poland amongst all cancers. That is why its biology is still being investigated by researchers[1-3]. Transforming growth factor β (TGF β) is a multifunctional cytokine that can induce growth inhibition, apoptosis, and differentiation of intestinal epithelial cells[4,5].
Generally, TGF β inhibits the growth of normal intestinal epithelial cells but can switch to a growth stimulator with tumor progression and enhance malignant transformation[6,7].
Until now, the number of potential prognostic factors for CRC is continually increasing, which is associated with its biologically diverse malignancy, given the same stage of advancement. Some histological parameters such as vascular invasion and tumor budding at the front of invasion seem to give very important information about invasive potential and metastasis of CRC. A few published studies have associated tumor- budding with metastatic changes[8-12]. These results seem to confirm that tumor- budding can play a crucial role as a prognostic factor in CRC. Also, the observed presence of neoplastic cells and nets of cells in lymphatic and venous vessels (vascular invasion) is an important feature of invasiveness in CRC. On the other hand, the presence of lymph node and distant metastasis is a recognized parameter of metastatic potential. This is why the aims of the study were, to evaluate the vascular invasion and tumor-budding in CRC growth zone, to analyze its relationship with the expression of TGF β protein in tumors, and to find any correlation between the expression of TGF β and the presence of metastases.
MATERIALS AND METHODS
Collection of samples
Thirty-four T1 colorectal carcinoma specimens from 34 patients, treated by radical surgery at the Department of Surgery, J. Śniadecki Hospital, Białystok, Poland, between 1999 and 2003, were examined retrospectively. Patients’ clinical records and pathological reports were reviewed with special attention paid to the presence or absence of lymph node metastasis, local recurrence, and distant metastasis. Tissue specimens were collected immediately after tumor removal, fixed in 100 g/L formaldehyde, embedded in paraffin, and then histopathologically examined using standard hematoxylin-eosin staining according to the TNM classification.
Vascular invasion, lymphatic and venous invasion were examined and grouped. Tumor budding was examined according to the criteria of Morodomi et al[1].
Immunohistochemistry
Slides of 4-μm-thick serial sections of the primary tumor were prepared. A standard avidin-biotin immunoperoxidase (Novostain Super ABC Kit (universal), No. NCL-ABCu, Biokom, Poland) method was used for the detection of TGF β expression. Briefly, the slides were dewaxed using xylene, transferred to alcohol, placed in citric acid buffer (10 mmol/L) and heated in a microwave oven (700 W) for 15 min to expose antigens. Endogenous peroxidase activity was inhibited by incubating the section with 3% hydrogen peroxide in methanol for 5 min. The slides were then washed three times with phosphate-buffered saline (PBS) and incubated in 10 g/L normal horse serum for 20 min to reduce nonspecific antibody binding. After washing with PBS, the slides were incubated overnight at 4 °C with monoclonal antibodies. Anti-human TGF β protein monoclonal antibody (Novocastra, NCL-TGFB, clone TGFB 17, dilution 1:40, Biokom, Poland) was used. Nonspecific mouse IgG was used as a negative control. The reaction products were visualized with diaminobenzidine DAB (DAKO S3000, Dako, Poland). The sections were counterstained with hematoxylin, dehydrated, and mounted. The tissue sections known to be positive were used as positive controls.
Evaluation of samples
The immunostaining of cytoplasm was observed under a light microscope for TGF β. TGF β expression was semi-quantitatively assessed in neoplastic cells of primary tumors. Cases were considered positive when >20% of cancerous cells was stained with TGF β and negative when <20% of cancerous cells was stained with TGF β. The percentage of TGF β-positive cells was calculated in at least 500 neoplastic cells per sample, under light microscope (×400). The overall results of immunohistochemical examinations are presented in Tables 1, 2, 3, 4.
Table 1.
TGF β expression in main mass of CRC with and without vascular invasion.
| Parameter |
Expression of TGF β, n (%) |
Positive | Total (%) | |
| n | Negative | |||
| With vascular invasion | 14 | 3 (21.4) | 11 (78.6) | 41.2 |
| Without vascular invasion | 20 | 14 (70) | 6 (30) | 58.8 |
P = 0.005, P<0.01.
Table 2.
TGF β expression in main mass of CRC with and without lymph node metastasis.
| Parameter |
Expression of TGF β, n (%) |
Positive | Total (%) | |
| n | Negative | |||
| With lymph node metastasis | 16 | 2 (12.5) | 14 (87.5) | 47.1 |
| Without lymph node metastasis | 18 | 15 (83.3) | 3 (16.7) | 52.9 |
P = 0.0001, P<0.01.
Table 3.
TGF β expression in main mass of CRC with and without distant metastasis.
| Parameter |
Expression of TGF β, n (%) |
Positive | Total (%) | |
| n | Negative | |||
| With distant metastasis | 14 | 1 (7.2) | 13 (92.8) | 41.2 |
| Without distant metastasis | 20 | 16 (80.0) | 4 (20.0) | 58.8 |
P = 0.00003, P<0.01.
Table 4.
TGF β expression in main mass of CRC with and without tumor budding.
| Parameter |
Expression of TGF β, n (%) |
Positive | Total (%) | |
| n | Negative | |||
| With tumor budding | 12 | 1 (8.3) | 11 (91.7) | 35.3 |
| Without tumor budding | 22 | 16 (72.7) | 6 (27.3) | 64.7 |
P = 0.00003, P<0.01.
Statistical analysis
The association between TGF β expression and clinico-pathological parameters was examined using χ2 test. Fisher’s exact test was used for statistical analysis. P<0.05 was considered statistically significant.
RESULTS
In our study, 17 (50%) out of 34 colorectal carcinomas displayed positive cytoplasmatic TGF β protein reactivity (Figure 1). There was a significant difference between the expression of TGF β in patients with and without vascular invasion (78.6% and 30% respectively, P<0.01). A significant difference also existed between the expression of TGF β in primary tumor and lymph node metastases (87.5%, P<0.01) and distant metastases (92.8%, P<0.01). The presence of tumor- budding at the front of invasion showed a statistically significant correlation with the expression of TGF β in the primary tumor (P<0.01).
Figure 1.
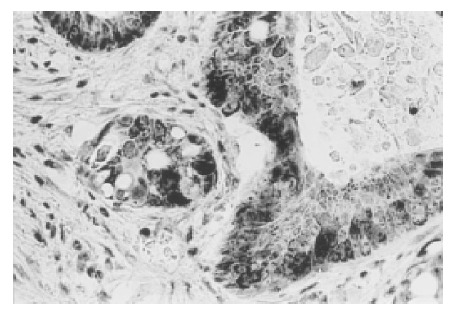
Strong cytoplasmic expression of TGF β in neoplastic cells ×200.
DISCUSSION
Recent studies reported that many tumor cells, including colon cancer cells, can secrete TGF β[13,14]. It has been shown that elevated levels of human TGF β protein in colorectal cancer correlate with an increased metastatic potential[15]. There is a significant correlation between tumor expression of TGF β 1 and a shorter post-operative survival[16]. It has been reported that plasma concentrations of active total TGF β1 are significantly higher in patients with CRC than in healthy volunteers[17]. Patients with CRC in stages C-D have significantly higher expressions of TGF β1 in tumors[16]. Some authors observed that the expression of TGF β1 is closely related to a higher rate of lymph node metastases in gastric cancer[18]. Our results also showed that the expression of TGF β in CRC pT1 with lymph node and distant metastasis was higher. All these findings are consistent with the results from Robson et al[19] who reported a positive expression in 58% of tumors. Similarly, Bellone et al[20] reported that colon carcinoma progression is associated with gradual and significant increases in the expression of TGF-β1, TGF-β2 mRNA and proteins. Tsushima et al[21] showed that preoperative TGF β1 level is a predictive factor for liver metastasis after curative resection.
The novel histopathological parameter in CRC associated with invasion is known as ‘tumor- budding’. Recent studies of the prognostic factors for CRC have paid attention to tumor-budding as a potential prognostic factor[1,8-12]. We found a statistically significant correlation between the intensity of tumor- budding and lymph node involvement[2]. Hase et al[8] examined 663 patients with CRC for tumor- budding, and suggested that tumor- budding is an important prognostic factor in patients with CRC. Morodomi et al[1] reported that tumor- budding represents the neoplastic cells that are directly involved in host tissue invasion. According to these authors, if the degree of differentiation in colorectal adenocarcinoma is moderate (G2), lymph node involvement is highly probable. However, even in these moderately differentiated tumors, lymphatic involvement is probable but less likely. In well-differentiated adenocarcinomas (G1), tumor-budding and lymphatic invasion are usually not observed. If tumor-budding occurs in such cases, then lymph node involvement is highly probable.
We found that the expression of TGF β in investigated tumors (T1) was strongly correlated with the presence of tumor -budding, vascular invasion at the front of invasion and presence of lymph node and distant metastases. These results suggest that TGF seems to be closely related to the aggressiveness of CRC.
References
- 1.Morodomi T, Isomoto H, Shirouzu K, Kakegawa K, Irie K, Morimatsu M. An index for estimating the probability of lymph node metastasis in rectal cancers. Lymph node metastasis and the histopathology of actively invasive regions of cancer. Cancer. 1989;63:539–543. doi: 10.1002/1097-0142(19890201)63:3<539::aid-cncr2820630323>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Guzińska-Ustymowicz K, Sulkowska M, Famulski W, Sulkowski S. Tumour 'budding' and its relationship to p53 and Bcl-2 expression in colorectal cancer. Anticancer Res. 2003;23:649–653. [PubMed] [Google Scholar]
- 3.Famulski W, Guzińska-Ustymowicz K, Sulkowska M, Chabowski A, Zalewski B, Piotrowski Z, Stasiak-Barmuta A, Sulkowski S. Tumour budding intensity in relation to cathepsin D expression and some clinicopathological features of colorectal cancer. Folia Histochem Cytobiol. 2001;39 Suppl 2:171–172. [PubMed] [Google Scholar]
- 4.Fujiwara T, Stolker JM, Watanabe T, Rashid A, Longo P, Eshleman JR, Booker S, Lynch HT, Jass JR, Green JS, et al. Accumulated clonal genetic alterations in familial and sporadic colorectal carcinomas with widespread instability in microsatellite sequences. Am J Pathol. 1998;153:1063–1078. doi: 10.1016/S0002-9440(10)65651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markowitz SD, Roberts AB. Tumor suppressor activity of the TGF-beta pathway in human cancers. Cytokine Growth Factor Rev. 1996;7:93–102. doi: 10.1016/1359-6101(96)00001-9. [DOI] [PubMed] [Google Scholar]
- 6.Polyak K. Negative regulation of cell growth by TGF beta. Biochim Biophys Acta. 1996;1242:185–199. doi: 10.1016/0304-419x(95)00009-5. [DOI] [PubMed] [Google Scholar]
- 7.Hsu S, Huang F, Hafez M, Winawer S, Friedman E. Colon carcinoma cells switch their response to transforming growth factor beta 1 with tumor progression. Cell Growth Differ. 1994;5:267–275. [PubMed] [Google Scholar]
- 8.Hase K, Shatney C, Johnson D, Trollope M, Vierra M. Prognostic value of tumor "budding" in patients with colorectal cancer. Dis Colon Rectum. 1993;36:627–635. doi: 10.1007/BF02238588. [DOI] [PubMed] [Google Scholar]
- 9.Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour 'budding' as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology. 2002;40:127–132. doi: 10.1046/j.1365-2559.2002.01324.x. [DOI] [PubMed] [Google Scholar]
- 10.Okuyama T, Oya M, Ishikawa H. Budding as a risk factor for lymph node metastasis in pT1 or pT2 well-differentiated colorectal adenocarcinoma. Dis Colon Rectum. 2002;45:628–634. doi: 10.1007/s10350-004-6259-0. [DOI] [PubMed] [Google Scholar]
- 11.Okuyama T, Oya M, Yamaguchi M. Budding (sprouting) as a useful prognostic marker in colorectal mucinous carcinoma. Jpn J Clin Oncol. 2002;32:412–416. doi: 10.1093/jjco/hyf089. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Hashiguchi Y, Ueno H, Hase K, Mochizuki H. Tumor budding at the invasive margin can predict patients at high risk of recurrence after curative surgery for stage II, T3 colon cancer. Dis Colon Rectum. 2003;46:1054–1059. doi: 10.1007/s10350-004-7280-z. [DOI] [PubMed] [Google Scholar]
- 13.Grégoire M, Garrigue L, Blottière HM, Denis MG, Meflah K. Possible involvement of TGF beta 1 in the distinct tumorigenic properties of two rat colon carcinoma clones. Invasion Metastasis. 1992;12:185–196. [PubMed] [Google Scholar]
- 14.Fischer JR, Darjes H, Lahm H, Schindel M, Drings P, Krammer PH. Constitutive secretion of bioactive transforming growth factor beta 1 by small cell lung cancer cell lines. Eur J Cancer. 1994;30A:2125–2129. doi: 10.1016/0959-8049(94)00364-b. [DOI] [PubMed] [Google Scholar]
- 15.Narai S, Watanabe M, Hasegawa H, Nishibori H, Endo T, Kubota T, Kitajima M. Significance of transforming growth factor beta1 as a new tumor marker for colorectal cancer. Int J Cancer. 2002;97:508–511. doi: 10.1002/ijc.1631. [DOI] [PubMed] [Google Scholar]
- 16.Shim KS, Kim KH, Han WS, Park EB. Elevated serum levels of transforming growth factor-beta1 in patients with colorectal carcinoma: its association with tumor progression and its significant decrease after curative surgical resection. Cancer. 1999;85:554–561. doi: 10.1002/(sici)1097-0142(19990201)85:3<554::aid-cncr6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 17.Xiong B, Yuan HY, Hu MB, Zhang F, Wei ZZ, Gong LL, Yang GL. Transforming growth factor-beta1 in invasion and metastasis in colorectal cancer. World J Gastroenterol. 2002;8:674–678. doi: 10.3748/wjg.v8.i4.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maehara Y, Kakeji Y, Kabashima A, Emi Y, Watanabe A, Akazawa K, Baba H, Kohnoe S, Sugimachi K. Role of transforming growth factor-beta 1 in invasion and metastasis in gastric carcinoma. J Clin Oncol. 1999;17:607–614. doi: 10.1200/JCO.1999.17.2.607. [DOI] [PubMed] [Google Scholar]
- 19.Robson H, Anderson E, James RD, Schofield PF. Transforming growth factor beta 1 expression in human colorectal tumours: an independent prognostic marker in a subgroup of poor prognosis patients. Br J Cancer. 1996;74:753–758. doi: 10.1038/bjc.1996.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellone G, Carbone A, Tibaudi D, Mauri F, Ferrero I, Smirne C, Suman F, Rivetti C, Migliaretti G, Camandona M, et al. Differential expression of transforming growth factors-beta1, -beta2 and -beta3 in human colon carcinoma. Eur J Cancer. 2001;37:224–233. doi: 10.1016/s0959-8049(00)00391-9. [DOI] [PubMed] [Google Scholar]
- 21.Tsushima H, Ito N, Tamura S, Matsuda Y, Inada M, Yabuuchi I, Imai Y, Nagashima R, Misawa H, Takeda H, et al. Circulating transforming growth factor beta 1 as a predictor of liver metastasis after resection in colorectal cancer. Clin Cancer Res. 2001;7:1258–1262. [PubMed] [Google Scholar]


