Abstract
AIM: To investigate the changes of platelet endothelial cell adhesion molecule-1 (PECAM-1) expression on polymorphonuclear leukocytes (PMNs) in peripheral circulation and pancreatic microcirculation in cerulein-induced acute edematous pancreatitis (AEP).
METHODS: Fifty Wistar rats were randomly divided into control group (n = 10) and AEP group (n = 40). A model of AEP was established by subcutaneous injection of cerulein 5.5 and 7.5 μg/kg at 0 and 1 h after the beginning of experiment respectively. PECAM-1 expression on PMNs from splenic vein and inferior vena cava was determined by RT-PCR at mRNA level and determined by flow cytometry at protein level.
RESULTS: In experimental rats, an increased PECAM-1 mRNA expression was seen from 4 to 8 h of AEP in peripheral circulation (0.77±0.25%, 0.76±0.28%, 0.89±0.30%, 1.00±0.21%), while in pancreatic microcirculation, expression decreased from 2 h and reached the lowest level at 6 h of AEP (0.78±0.29%, 0.75±0.26%, 0.62±0.28%, 0.66±0.20%). There were significant differences at 8-h time point of AEP between peripheral circulation and pancreatic microcirculation (1.00±0.21% vs 0.66±0.20%, P<0.05). Meanwhile, the difference at protein level was also found.
CONCLUSION: A reverse expression of PECAM-1 on PMNs was found between peripheral circulation and pancreatic microcirculation, suggesting that inhibition of PECAM-1 expression may improve the pathological change of AEP.
Keywords: Pancreatitis, Platelet endothelial cell adhesion molecule-1, Microcirculation, Cerulein
INTRODUCTION
Acute pancreatitis (AP) is a potentially lethal disease and characterized by acinar cell necrosis, extensive interstitial edema, and migration of neutrophils to the damaged gland. However, the development and etiology of AP remain poorly understood.
Cerulein-induced acute edematous pancreatitis (AEP) is a widely used model in investigating the pathophysiological events of the disease. In this regard, rapid induction of mild disease with a highly reproducible course and easily detected changes of acute interstitial pancreatitis have made this secretagogue-induced model favorable for the investigation of the pathogenesis of the disease.
Platelet endothelial cell adhesion molecule -1(PECAM-1, CD31) is a cell adhesion molecule that belongs to the Ig superfamily expressed on endothelial cells as well as circulating leukocytes including neutrophils [1,2], monocytes and NK cells[3,4]. The homophilic interaction of neutrophil or monocyte PECAM-1 with endothelial PECAM-1 is very important to neutrophil and monocyte transendothelial migration as demonstrated in studies in several different laboratories[5-9]. The interaction may be mediated by interdigitating PECAM-1 molecules from neutrophils or monocytes and endothelial cells, forming a zipper which promotes their adhesion[10-12].
Recently, several studies have demonstrated that AP is frequently associated with sequestration of inflammatory cells, particularly leukocytes[13-15]. However, the expression of PECAM-1 on leukocytes and the role of PECAM-1 in pancreatic injury in AP are not very clear. Therefore, we conducted this study to investigate the expression of PECAM-1 on PMNs and the role of PECAM-1 in microcirculatory injury in AEP.
MATERIALS AND METHODS
Animals
Male Wistar rats (250-350 g) were provided by the Center of Experimental Animals, Sichuan University (Chengdu, China). All animals were fasted for 12 h before the experiments but had free access to water. They were treated in accordance with the protocols approved by the local Animal Use and Care Committee and executed according to the National Animal Welfare Law.
Induction of acute pancreatitis
All experimental rats were given subcutaneous injection of cerulein (Sigma Co, USA) 5.5 and 7.5 μg/kg at 0 and 1 h after the beginning of experiment respectively, while control rats were given subcutaneous injection of 0.9% saline solution.
Experimental protocols
The rats were randomly assigned into control group (n = 10) and AEP group (n = 40). Rats in AEP group were sacrificed at 2 (n = 10), 4 (n = 10), 6 (n = 10) and 8 h (n = 10) after induction of AEP.
Collection of samples
At different time points (2, 4, 6, and 8 h) after induction of AEP, the experimental animals underwent laparotomy under anesthesia by intraperitoneal injection of 50 mg/kg sodium pentobarbital (Sanofi, Libourne, France). Blood samples were immediately obtained from splenic vein by retrograde catheterization from portal vein and inferior vena cava and samples of pancreatic head were taken immediately after the animals were killed. Control animals underwent laparotomy and were sampled in the same fashion as animals with AEP.
Amylase measurement
Serum amylase levels were measured at 37 °C by an enzymatic assay with a spectrophotometer according to the manufacturer’s instructions. All serum samples were assayed in duplicate, and the results were averaged at the end of the experiment.
Edema assessment
Pancreatic edema was evaluated by measuring the wet-to-dry weight ratio. A segment of the pancreas was trimmed of fat and weighed. The water content was determined by calculating the wet-to-dry weight ratio from the initial weight (wet weight) and its weight after incubation at 160 °C for 24 h (dry weight).
Reverse transcriptase-polymerase chain reaction
Total RNA was isolated from blood using TRIzol® reagent kits (Gibco BRL, USA). RT-PCR was performed with the Access RT-PCR kit (Takara, Japan) according to the manufacturer’s protocol. The primer for rat PECAM-1 was constructed based on published human and mouse PECAM-1 nucleotide sequences and synthesized by Life Technology (Hong Kong, China). We obtained two different bands from RT-PCR corresponding to the splice variants. The band densities of the two splice variants behaved in the same manner. The primers are indicated in Table 1. RT-PCR reactions were performed in 50 μL reaction volume and run in Gene Amp 9600 machine (Perkin-Elmer, USA). The conditions of RT-PCR were as follows: 1 cycle for 30 min at 50 °C; 1 cycle for 2 min at 94 °C; 30 cycles for 30 s at 94 °C, for 30 s at 55 °C, for 30 s at 72 °C; 1 cycle for 10 min at 72 °C. The PCR products were separated by electrophoresis in 2% agarose gels and stained with ethidium bromide. The densities of cDNA bands were analyzed by scanning densitometry using GelDoc 2000 (Bio-Rad, USA). The band densities were normalized to the β-actin band densities and the results were expressed as ratio.
Table 1.
Primer list of RT-PCR.
| mRNA | Upper primer | Lower primer | Fragment size |
| b-actin | 5’-GATGGTGGGTATGGGTXAGAA-3’ | 5’-CTAGGAGCCAGGGCAGTAATC-3’ | 346 bp |
| PECAM-1 | 5’-AGGGCTCATTGCGGTGGTTGTCAT-3 | 5’-TAAGGGAGCCTTCCGTTCTAGAGT-3 | 348, 404 bp |
PECAM-1: platelet endothelial cell adhesion molecule-1.
Neutrophils preparation
Blood was immediately mixed with heparin (50 U/mL) and centrifuged in a discontinuous Percoll gradient to yield a fraction of approximately 97% purity. Rat PMNs were isolated by a modification of the technique. Cell viabilities, as assessed by trypan blue exclusion, were above 96% under all experimental conditions.
Flow cytometry
To determine PECAM-1 protein expression, rat PMNs were incubated with phycoerythrin (PE)-anti-PECAM-1 monoclonal antibody (BD PharMingen, USA) at 4 °C in the dark for 20 min. After washing with PBS and fixed in 0.5% paraformaldehyde in phosphate-buffered saline, cells were resuspended and mean fluorescence intensity was measured by flow cytometry (ELITE ESP, Coulter, USA).
Statistical analysis
The results were expressed as mean±SE, individual comparisons of group means were performed with one-way ANOVA, P<0.05 was considered statistically significant.
RESULTS
Serum amylase
After induction of acute pancreatitis by subcutaneous injection of cerulein as shown in Table 2, serum amylase increased sharply and reached the peak at 4-h time point, then dropped slightly at 8-h time point. Compared to control rats, all AEP rats demonstrated hyperamylasemia (P<0.05).
Table 2.
Serum amylase level in AEP (mean±SE).
| Group | Serum amylase (IU/L) |
| Control | 319.5±78.7 |
| AEP 2 h | 1493.3±308.9a |
| AEP 4 h | 2095±956.7b |
| AEP 6 h | 1662.7±544.6a |
| AEP 8 h | 1514.2±634.2a |
P<0.05 vs control group;
P<0.001 vs control group.
Wet-to-dry weight ratio
As shown in Table 3, subcutaneous injection of cerulein resulted in an increase in the wet/dry weight ratio in AEP rats. Compared to control rats, all AEP rats had a significant increase in the wet/dry weight ratio (P<0.05).
Table 3.
Wet/dry pancreatic weight ratio in AEP (mean±SE).
| Group | Wet/dry weight ratio (%) |
| Control | 3.4±0.2 |
| AEP 2 h | 3.9±0.3a |
| AEP 4 h | 4.0±0.3a |
| AEP 6 h | 4.3±0.4a |
| AEP 8 h | 4.1±0.5a |
aP<0.05 vs control group.
RT-PCR demonstration of PECAM-1 expression on PMNs
To determine the role of PECAM-1 in AEP, we evaluated the PECAM-1 mRNA expression on PMNs by RT-PCR. As shown in Figure 1, in experimental rats, the PECAM-1 mRNA expression slightly increased from 4 to 8-h time points of AEP in peripheral circulation, while in pancreatic microcirculation, the expression decreased from 2 h and reached the lowest level at 6-h time point of AEP and the PECAM-1 mRNA expression difference became significant between peripheral circulation and pancreatic microcirculation at 8-h time point of AEP (P<0.05).
Figure 1.
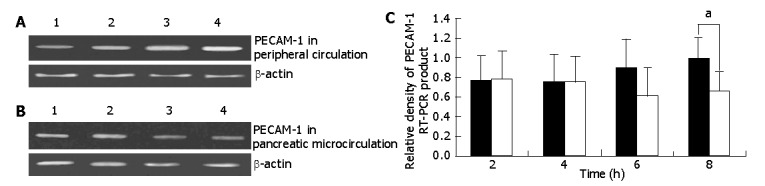
RT-PCR analysis of PMNs PECAM-1. A: Expression of on PMNs PECAM-1 mRNA in peripheral circulation. Lanes 1, 2, 3 and 4: PECAM-1 cDNA after amplification from RNA of PMNs from inferior vena cava at 2, 4, 6 and 8-h time points of AEP respectively; B: Expression of on PMNs PECAM-1 mRNA on in pancreatic microcirculation. Lanes 1, 2, 3 and 4: PECAM-1 cDNA after amplification from RNA of PMNs from splenic vein at 2, 4, 6 and 8-h time points of AEP respectively; C: Values of the density of PECAM-1 RT-PCR product normalized to that of β-actin in the same RNA sample. Symbols are: (ν) peripheral circulation; (ο) pancreatic microcirculation. aP<0.05, by one-way ANOVA.
Flow cytometry demonstration of PECAM-1 expression on PMNs
At protein level, we analyzed the PECAM-1 expression on the surface of PMNs by flow cytometry. As shown in Figure 2, in experimental rats, the expression of PECAM-1 on PMNs had no significant difference between peripheral circulation and pancreatic microcirculation at 2 and 4-h time points of AEP. Then from 4 to 8-h time points, the expression of PECAM-1 was up-regulated in peripheral circulation, while it was down-regulated in pancreatic microcirculation. As a result, the expression had a significant difference between peripheral circulation and pancreatic microcirculation at 8-h time point of AEP (P<0.05).
Figure 2.
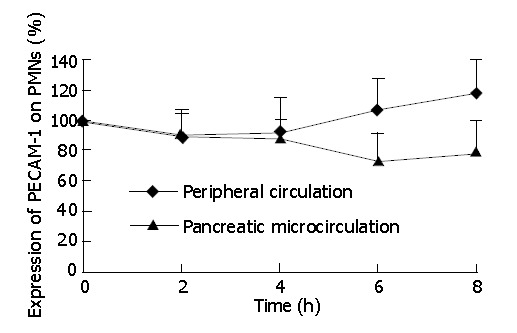
Flow cytometry analysis of PECAM-1 on PMNs. aP<0.05 vs peripheral circulation values.
DISCUSSION
This study demonstrates that PECAM-1 mRNA expression on PMNs between peripheral circulation and pancreatic microcirculation is in a reverse pattern in cerulein-induced AEP. In peripheral circulation, the PECAM-1 mRNA expression is up-regulated, while it is down-regulated in pancreatic microcirculation, especially in the late stage of AEP. Moreover, PECAM-1 gene activation is in good correlation with its protein expression.
Since the concept of autodigestion is accepted generally, considerable progress has been made in the understanding of pathogenesis of AEP. In recent years, basic researches on the morphology of pancreatic microcirculation have revealed that intralobular arterioles could be considered as ‘end-arteries’. Furthermore, they have no anastomosis with adjacent intralobular arterioles and their branches. This anatomic feature makes pancreas susceptible to the pancreatic microcirculatory impairment. However, the key factors for local microcirculatory disturbance remain obscure.
Several lines of evidence have shown that PECAM-1 is required for leukocyte transmigration through endothelial cell monolayer[16-18] and PMNs play an important role in microcirculatory injury during inflammation[19,20]. But these researches mainly focused on the expression of PECAM-1 on leukocytes in peripheral circulation and rarely investigated its expression in microcirculation. Moreover, the expression of PECAM-1 and its role in pancreatic injury in vivo have been ambiguous. Therefore, we examined the PECAM-1 expression on PMNs in pancreatic microcirculation and peripheral circulation during AEP.
PECAM-1, expressed on the surface of most leukocytes and endothelial cells, can up-regulate leukocyte integrin affinity by homophillic engagement of PECAM-1 molecules between circulating leukocytes and the underlying endothelial cell monolayer, facilitating leukocyte transmigration subsequently[20-22]. Neutralized antibodies to PECAM-1 could inhibit neutrophil and monocyte transendothelial migration by 80% in vitro and in vivo. The anti-PECAM-1 antibodies could inhibit tissue recruitment of neutrophils and monocytes in vivo by inhibiting the transmigration of neutrophils and monocytes between endothelial cells[23]. Studies using intravital videomicroscopy have demonstrated that anti-PECAM-1 antibodies do not inhibit other steps in neutrophil or monocyte recruitment such as rolling on endothelium or activation-dependent adhesion to endothelium[24]. In our experiments, we found that PECAM-1 expression on PMNs in pancreatic microcirculation was significantly down-regulated, in comparison with its expression in peripheral circulation in AEP. In addition, the more the serum amylase and wet/dry weight ratio increased, the more the PECAM-1 expression decreased on PMNs in pancreatic microcirculation, suggesting that PECAM-1 expression on PMNs in pancreatic microcirculation is correlated with the development of AEP. It is possible that the up-regulation of PECAM-1 may prepare PMNs for transmigrating through the monolayer of endothelial cells in microvessels, and down-regulation of PECAM-1 may reflect the activation of PMNs. While the activated PMNs go through pancreas, they can swim from the microvessel lumen, across the endothelium, to the inflammatory tissue more easily, causing the deterioration of pancreatic injury. Thus we hypothesize that inhibition of PECAM-1 expression on PMNs may block the interaction between endothelial cells and PMNs, thereby preventing PMNs’ transmigration.
In conclusion, our study is the first to investigate the expression of PECAM-1 mRNA and protein on PMNs in pancreatic microcirculation and peripheral circulation. PECAM-1 expression on PMNs between peripheral circulation and pancreatic microcirculation is in a reverse direction in AEP. Furthermore, down-regulation of PECAM-1 expression may be one of the most important parameters of pancreatic microcirculatory injury and inhibition of PECAM-1 expression may alleviate the pathological changes of AEP.
Footnotes
Supported by National Natural Science Foundation of China, No. 39925032
Edited by Wang XL and Zhu LH
References
- 1.Wakayama T, Hamada K, Yamamoto M, Suda T, Iseki S. The expression of platelet endothelial cell adhesion molecule-1 in mouse primordial germ cells during their migration and early gonadal formation. Histochem Cell Biol. 2003;119:355–362. doi: 10.1007/s00418-003-0528-1. [DOI] [PubMed] [Google Scholar]
- 2.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421:748–753. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 3.Pu FR, Williams RL, Markkula TK, Hunt JA. Expression of leukocyte-endothelial cell adhesion molecules on monocyte adhesion to human endothelial cells on plasma treated PET and PTFE in vitro. Biomaterials. 2002;23:4705–4718. doi: 10.1016/s0142-9612(02)00219-3. [DOI] [PubMed] [Google Scholar]
- 4.Kawabata K, Nakai S, Miwa M, Sugiura T, Otsuka Y, Shinzato T, Hiki N, Tomimatsu I, Ushida Y, Hosono F, et al. CD31 expression on leukocytes is downregulated in vivo during hemodialysis. Nephron. 2001;89:153–160. doi: 10.1159/000046062. [DOI] [PubMed] [Google Scholar]
- 5.Zaremba J, Losy J. sPECAM-1 in serum and CSF of acute ischaemic stroke patients. Acta Neurol Scand. 2002;106:292–298. doi: 10.1034/j.1600-0404.2002.01339.x. [DOI] [PubMed] [Google Scholar]
- 6.Luscinskas FW, Ma S, Nusrat A, Parkos CA, Shaw SK. Leukocyte transendothelial migration: a junctional affair. Semin Immunol. 2002;14:105–113. doi: 10.1006/smim.2001.0347. [DOI] [PubMed] [Google Scholar]
- 7.Radi ZA, Kehrli ME, Ackermann MR. Cell adhesion molecules, leukocyte trafficking, and strategies to reduce leukocyte infiltration. J Vet Intern Med. 2001;15:516–529. doi: 10.1892/0891-6640(2001)015<0516:camlta>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Neubauer K, Ritzel A, Saile B, Ramadori G. Decrease of platelet-endothelial cell adhesion molecule 1-gene-expression in inflammatory cells and in endothelial cells in the rat liver following CCl(4)-administration and in vitro after treatment with TNFalpha. Immunol Lett. 2000;74:153–164. doi: 10.1016/s0165-2478(00)00203-0. [DOI] [PubMed] [Google Scholar]
- 9.Neubauer K, Wilfling T, Ritzel A, Ramadori G. Platelet-endothelial cell adhesion molecule-1 gene expression in liver sinusoidal endothelial cells during liver injury and repair. J Hepatol. 2000;32:921–932. doi: 10.1016/s0168-8278(00)80096-3. [DOI] [PubMed] [Google Scholar]
- 10.Lefer AM. Role of the beta2-integrins and immunoglobulin superfamily members in myocardial ischemia-reperfusion. Ann Thorac Surg. 1999;68:1920–1923. doi: 10.1016/s0003-4975(99)01017-6. [DOI] [PubMed] [Google Scholar]
- 11.Nakada MT, Amin K, Christofidou-Solomidou M, O'Brien CD, Sun J, Gurubhagavatula I, Heavner GA, Taylor AH, Paddock C, Sun QH, et al. Antibodies against the first Ig-like domain of human platelet endothelial cell adhesion molecule-1 (PECAM-1) that inhibit PECAM-1-dependent homophilic adhesion block in vivo neutrophil recruitment. J Immunol. 2000;164:452–462. doi: 10.4049/jimmunol.164.1.452. [DOI] [PubMed] [Google Scholar]
- 12.Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol. 1999;66:698–704. doi: 10.1002/jlb.66.5.698. [DOI] [PubMed] [Google Scholar]
- 13.de Dios I, Perez M, de La Mano A, Sevillano S, Orfao A, Ramudo L, Manso MA. Contribution of circulating leukocytes to cytokine production in pancreatic duct obstruction-induced acute pancreatitis in rats. Cytokine. 2002;20:295–303. doi: 10.1006/cyto.2002.2011. [DOI] [PubMed] [Google Scholar]
- 14.Bhatnagar A, Wig JD, Majumdar S. Immunological findings in acute and chronic pancreatitis. ANZ J Surg. 2003;73:59–64. doi: 10.1046/j.1445-2197.2003.02621.x. [DOI] [PubMed] [Google Scholar]
- 15.Mann DV, Kalu P, Foulds S, Edwards R, Glazer G. Neutrophil activation and hyperamylasaemia after endoscopic retrograde cholangiopancreatography: potential role for the leukocyte in the pathogenesis of acute pancreatitis. Endoscopy. 2001;33:448–453. doi: 10.1055/s-2001-14260. [DOI] [PubMed] [Google Scholar]
- 16.Solowiej A, Biswas P, Graesser D, Madri JA. Lack of platelet endothelial cell adhesion molecule-1 attenuates foreign body inflammation because of decreased angiogenesis. Am J Pathol. 2003;162:953–962. doi: 10.1016/S0002-9440(10)63890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Brien CD, Lim P, Sun J, Albelda SM. PECAM-1-dependent neutrophil transmigration is independent of monolayer PECAM-1 signaling or localization. Blood. 2003;101:2816–2825. doi: 10.1182/blood-2002-08-2396. [DOI] [PubMed] [Google Scholar]
- 18.Thompson RD, Noble KE, Larbi KY, Dewar A, Duncan GS, Mak TW, Nourshargh S. Platelet-endothelial cell adhesion molecule-1 (PECAM-1)-deficient mice demonstrate a transient and cytokine-specific role for PECAM-1 in leukocyte migration through the perivascular basement membrane. Blood. 2001;97:1854–1860. doi: 10.1182/blood.v97.6.1854. [DOI] [PubMed] [Google Scholar]
- 19.Dangerfield J, Larbi KY, Huang MT, Dewar A, Nourshargh S. PECAM-1 (CD31) homophilic interaction up-regulates alpha6beta1 on transmigrated neutrophils in vivo and plays a functional role in the ability of alpha6 integrins to mediate leukocyte migration through the perivascular basement membrane. J Exp Med. 2002;196:1201–1211. doi: 10.1084/jem.20020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okouchi M, Okayama N, Shimizu M, Omi H, Fukutomi T, Itoh M. High insulin exacerbates neutrophil-endothelial cell adhesion through endothelial surface expression of intercellular adhesion molecule-1 via activation of protein kinase C and mitogen-activated protein kinase. Diabetologia. 2002;45:556–559. doi: 10.1007/s00125-001-0773-6. [DOI] [PubMed] [Google Scholar]
- 21.Thompson RD, Wakelin MW, Larbi KY, Dewar A, Asimakopoulos G, Horton MA, Nakada MT, Nourshargh S. Divergent effects of platelet-endothelial cell adhesion molecule-1 and beta 3 integrin blockade on leukocyte transmigration in vivo. J Immunol. 2000;165:426–434. doi: 10.4049/jimmunol.165.1.426. [DOI] [PubMed] [Google Scholar]
- 22.Cid MC, Cebrián M, Font C, Coll-Vinent B, Hernández-Rodríguez J, Esparza J, Urbano-Márquez A, Grau JM. Cell adhesion molecules in the development of inflammatory infiltrates in giant cell arteritis: inflammation-induced angiogenesis as the preferential site of leukocyte-endothelial cell interactions. Arthritis Rheum. 2000;43:184–194. doi: 10.1002/1529-0131(200001)43:1<184::AID-ANR23>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 23.Su WH, Chen HI, Jen CJ. Differential movements of VE-cadherin and PECAM-1 during transmigration of polymorphonuclear leukocytes through human umbilical vein endothelium. Blood. 2002;100:3597–3603. doi: 10.1182/blood-2002-01-0303. [DOI] [PubMed] [Google Scholar]
- 24.Sun Z, Wang X, Lasson A, Böjesson A, Annborn M, Andersson R. Effects of inhibition of PAF, ICAM-1 and PECAM-1 on gut barrier failure caused by intestinal ischemia and reperfusion. Scand J Gastroenterol. 2001;36:55–65. doi: 10.1080/00365520150218066. [DOI] [PubMed] [Google Scholar]


