Abstract
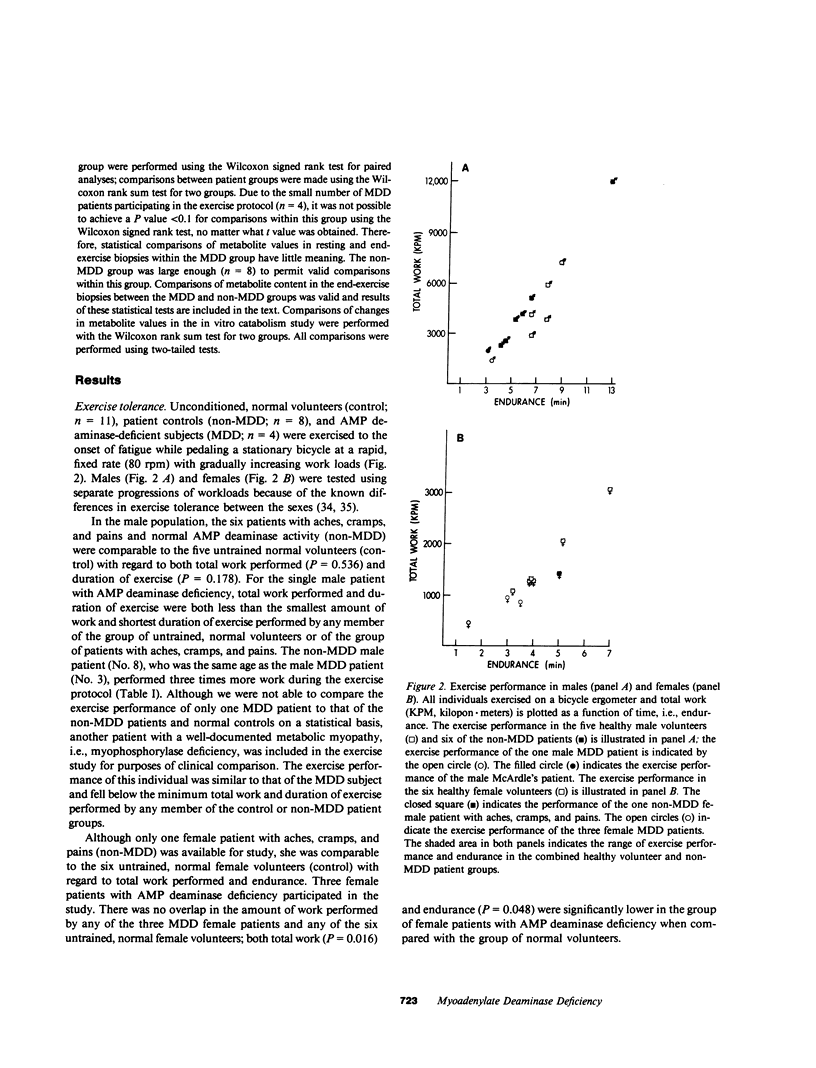
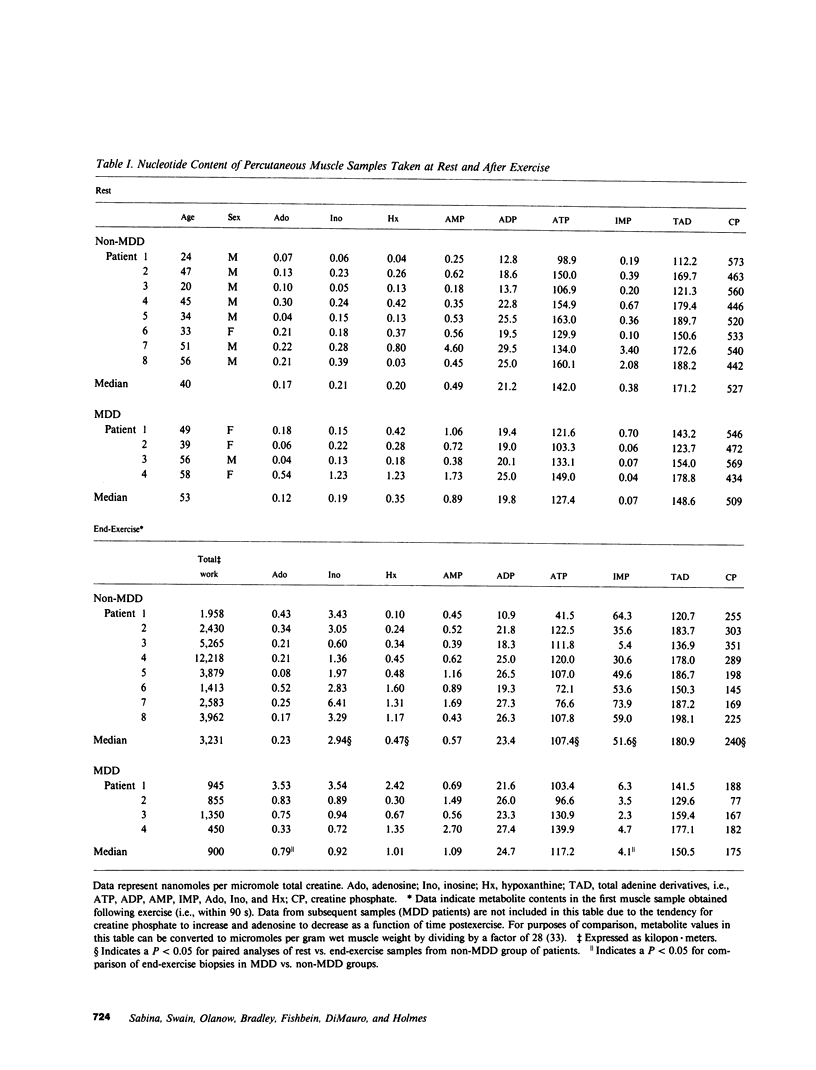
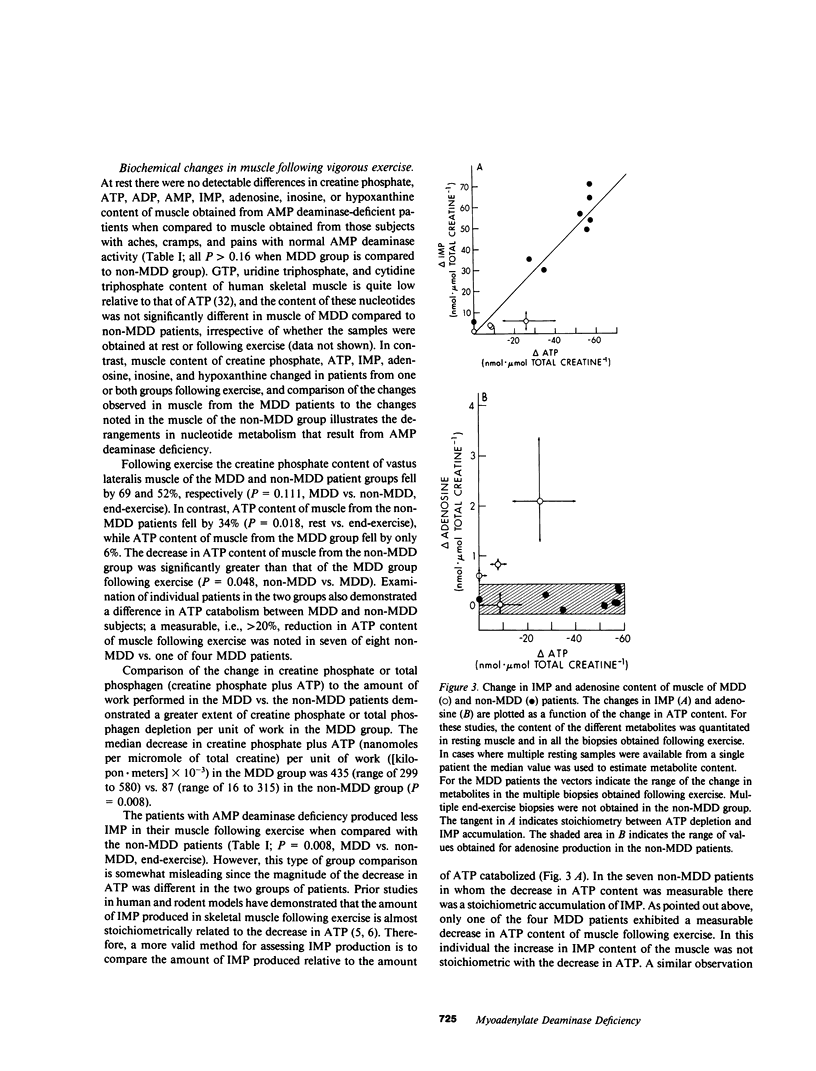
To assess the role of the purine nucleotide cycle in human skeletal muscle function, we evaluated 10 patients with AMP deaminase deficiency (myoadenylate deaminase deficiency; MDD). 4 MDD and 19 non-MDD controls participated in an exercise protocol. The latter group was composed of a patient cohort (n = 8) exhibiting a constellation of symptoms similar to those of the MDD patients, i.e., postexertional aches, cramps, and pains; as well as a cohort of normal, unconditioned volunteers (n = 11). The individuals with MDD fatigued after performing only 28% as much work as their non-MDD counterparts. Muscle biopsies were obtained from the four MDD patients and the eight non-MDD patients at rest and following exercise to the point of fatigue. Creatine phosphate content fell to a comparable extent in the MDD (69%) and non-MDD (52%) patients at the onset of fatigue. Following exercise the 34% decrease in ATP content of muscle from the non-MDD subjects was significantly greater than the 6% decrease in ATP noted in muscle from the MDD patients (P = 0.048). Only one of four MDD patients had a measurable drop in ATP compared with seven of eight non-MDD patients. At end-exercise the muscle content of inosine 5'-monophosphate (IMP), a product of AMP deaminase, was 13-fold greater in the non-MDD patients than that observed in the MDD group (P = 0.008). Adenosine content of muscle from the MDD patients increased 16-fold following exercise, while there was only a twofold increase in adenosine content of muscle from the non-MDD patients (P = 0.028). Those non-MDD patients in whom the decrease in ATP content following exercise was measurable exhibited a stoichiometric increase in IMP, and total purine content of the muscle did not change significantly. The one MDD patient in whom the decrease in ATP was measurable, did not exhibit a stoichiometric increase in IMP. Although the adenosine content increased 13-fold in this patient, only 48% of the ATP catabolized could be accounted for by the combined increases of adenosine, inosine, hypoxanthine, and IMP. Studies performed in vitro with muscle samples from seven MDD and seven non-MDD subjects demonstrated that ATP catabolism was associated with a fivefold greater increase in IMP in non-MDD muscle. There were significant increases in AMP and ADP content of the muscle from MDD patients following ATP catabolism in vitro, while there was no detectable increase in AMP or ADP in non-MDD muscle. Adenosine content of MDD muscle increased following ATP catabolism, but there was no detectable increase in adenosine content of non-MDD muscle following ATP catabolism in vitro. These studies demonstrate that AMP deaminase deficiency leads to reduced entry of adenine nucleotides into the purine nucleotide cycle during exercise. We postulate that the resultant disruption of the purine nucleotide cycle accounts for the muscle dysfunction observed in these patients.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASMUSSEN E., MATHIASEN P. Some physiologic functions in physical education students re-investigated after twenty-five years. J Am Geriatr Soc. 1962 May;10:379–387. doi: 10.1111/j.1532-5415.1962.tb00309.x. [DOI] [PubMed] [Google Scholar]
- ASTRAND I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl. 1960;49(169):1–92. [PubMed] [Google Scholar]
- Aragón J. J., Tornheim K., Goodman M. N., Lowenstein J. M. Replenishment of citric acid cycle intermediates by the purine nucleotide cycle in rat skeletal muscle. Curr Top Cell Regul. 1981;18:131–149. doi: 10.1016/b978-0-12-152818-8.50014-1. [DOI] [PubMed] [Google Scholar]
- Aragón J. J., Tornheim K., Lowenstein J. M. On a possible role of IMP in the regulation of phosphorylase activity in skeletal muscle. FEBS Lett. 1980 Aug 25;117 (Suppl):K56–K64. doi: 10.1016/0014-5793(80)80570-9. [DOI] [PubMed] [Google Scholar]
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Bessman S. P., Geiger P. J. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981 Jan 30;211(4481):448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- Conway E. J., Cooke R. The deaminases of adenosine and adenylic acid in blood and tissues. Biochem J. 1939 Apr;33(4):479–492. doi: 10.1042/bj0330479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMauro S., Miranda A. F., Hays A. P., Franck W. A., Hoffman G. S., Schoenfeldt R. S., Singh N. Myoadenylate deaminase deficiency--muscle biopsy and muscle culture in a patient with gout. J Neurol Sci. 1980 Aug;47(2):191–202. doi: 10.1016/0022-510x(80)90003-9. [DOI] [PubMed] [Google Scholar]
- ENGEL A. G., POTTER C. S., ROSEVEAR J. W. NUCLEOTIDES AND ADENOSINE MONOPHOSPHATE DEAMINASE ACTIVITY OF MUSCLE IN PRIMARY HYPOKALAEMIC PERIODIC PARALYSIS. Nature. 1964 May 16;202:670–672. doi: 10.1038/202670a0. [DOI] [PubMed] [Google Scholar]
- Fishbein W. N., Armbrustmacher V. W., Griffin J. L. Myoadenylate deaminase deficiency: a new disease of muscle. Science. 1978 May 5;200(4341):545–548. doi: 10.1126/science.644316. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Edwards R. H., Hultman E., Nordesjö L. O., Nylind B., Sahlin K. The time course of phosphorylcreatine resynthesis during recovery of the quadriceps muscle in man. Pflugers Arch. 1976 Dec 28;367(2):137–142. doi: 10.1007/BF00585149. [DOI] [PubMed] [Google Scholar]
- Hayes D. J., Summers B. A., Morgan-Hughes J. A. Myoadenylate deaminase deficiency or not? Observations on two brothers with exercise-induced muscle pain. J Neurol Sci. 1982 Jan;53(1):125–136. doi: 10.1016/0022-510x(82)90086-7. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W., Mommsen T. P. Protons and anaerobiosis. Science. 1983 Mar 25;219(4591):1391–1397. doi: 10.1126/science.6298937. [DOI] [PubMed] [Google Scholar]
- Holloszy J. O., Booth F. W. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- Jansson E. Diet and muscle metabolism in man with reference to fat and carbohydrate utilization and its regulation. Acta Physiol Scand Suppl. 1980;487:1–24. [PubMed] [Google Scholar]
- Juengling E., Kammermeier H. Rapid assay of adenine nucleotides or creatine compounds in extracts of cardiac tissue by paired-ion reverse-phase high-performance liquid chromatography. Anal Biochem. 1980 Mar 1;102(2):358–361. doi: 10.1016/0003-2697(80)90167-0. [DOI] [PubMed] [Google Scholar]
- Julius S., Amery A., Whitlock L. S., Conway J. Influence of age on the hemodynamic response to exercise. Circulation. 1967 Aug;36(2):222–230. doi: 10.1161/01.cir.36.2.222. [DOI] [PubMed] [Google Scholar]
- Kar N. C., Pearson C. M. Muscle adenylate deaminase deficiency. Report of six new cases. Arch Neurol. 1981 May;38(5):279–281. doi: 10.1001/archneur.1981.00510050045005. [DOI] [PubMed] [Google Scholar]
- Karlsson J., Diamant B., Saltin B. Muscle metabolites during submaximal and maximal exercise in man. Scand J Clin Lab Invest. 1970 Dec;26(4):385–394. doi: 10.3109/00365517009046250. [DOI] [PubMed] [Google Scholar]
- Kelemen J., Rice D. R., Bradley W. G., Munsat T. L., DiMauro S., Hogan E. L. Familial myoadenylate deaminase deficiency and exertional myalgia. Neurology. 1982 Aug;32(8):857–863. doi: 10.1212/wnl.32.8.857. [DOI] [PubMed] [Google Scholar]
- Lowenstein J. M. Ammonia production in muscle and other tissues: the purine nucleotide cycle. Physiol Rev. 1972 Apr;52(2):382–414. doi: 10.1152/physrev.1972.52.2.382. [DOI] [PubMed] [Google Scholar]
- Lowenstein J. M., Goodman M. N. The purine nucleotide cycle in skeletal muscle. Fed Proc. 1978 Jul;37(9):2308–2312. [PubMed] [Google Scholar]
- Matsuda Y., Ogawa H., Fukutome S., Shiraki H., Nakagawa H. Adenylosuccinate synthetase in rat liver: the existence of two types and their regulatory roles. Biochem Biophys Res Commun. 1977 Sep 23;78(2):766–771. doi: 10.1016/0006-291x(77)90245-5. [DOI] [PubMed] [Google Scholar]
- Mercelis R., Martin J. J., Dehaene I., de Barsy T., Van den Berghe G. Myoadenylate deaminase deficiency in a patient with facial and limb girdle myopathy. J Neurol. 1981;225(3):157–166. doi: 10.1007/BF00313744. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Terjung R. L. AMP deamination and IMP reamination in working skeletal muscle. Am J Physiol. 1980 Jul;239(1):C32–C38. doi: 10.1152/ajpcell.1980.239.1.C32. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Goto H., Yamada Y., Watanabe T. Distribution of AMP-deaminase isozymes in rat tissues. Eur J Biochem. 1978 Jun 15;87(2):297–304. doi: 10.1111/j.1432-1033.1978.tb12378.x. [DOI] [PubMed] [Google Scholar]
- PURZYCKA J. AMP and adenosine aminohydrolases in rat tissues. Acta Biochim Pol. 1962;9:83–93. [PubMed] [Google Scholar]
- Rahim Z. H., Perrett D., Lutaya G., Griffiths J. R. Metabolic adaptation in phosphorylase kinase deficiency. Changes in metabolite concentrations during tetanic stimulation of mouse leg muscles. Biochem J. 1980 Jan 15;186(1):331–341. doi: 10.1042/bj1860331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpf K. W., Wagner H., Kaiser H., Meinck H. M., Goebel H. H., Scheler F. Increased ammonia production during forearm ischemic work test in McArdle's disease. Klin Wochenschr. 1981 Dec 1;59(23):1319–1320. doi: 10.1007/BF01711182. [DOI] [PubMed] [Google Scholar]
- Sabina R. L., Kernstine K. H., Boyd R. L., Holmes E. W., Swain J. L. Metabolism of 5-amino-4-imidazolecarboxamide riboside in cardiac and skeletal muscle. Effects on purine nucleotide synthesis. J Biol Chem. 1982 Sep 10;257(17):10178–10183. [PubMed] [Google Scholar]
- Sabina R. L., Swain J. L., Bradley W. G., Holmes E. W. Quantitation of metabolites in human skeletal muscle during rest and exercise: a comparison of methods. Muscle Nerve. 1984 Jan;7(1):77–82. doi: 10.1002/mus.880070113. [DOI] [PubMed] [Google Scholar]
- Sabina R. L., Swain J. L., Hines J. J., Holmes E. W. A comparison of methods for quantitation of metabolites in skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):624–627. doi: 10.1152/jappl.1983.55.2.624. [DOI] [PubMed] [Google Scholar]
- Sabina R. L., Swain J. L., Patten B. M., Ashizawa T., O'Brien W. E., Holmes E. W. Disruption of the purine nucleotide cycle. A potential explanation for muscle dysfunction in myoadenylate deaminase deficiency. J Clin Invest. 1980 Dec;66(6):1419–1423. doi: 10.1172/JCI109995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K., Palmskog G., Hultman E. Adenine nucleotide and IMP contents of the quadriceps muscle in man after exercise. Pflugers Arch. 1978 May 18;374(2):193–198. doi: 10.1007/BF00581301. [DOI] [PubMed] [Google Scholar]
- Scisłowski P. W., Aleksandrowicz Z., Swierczyński J. Purine nucleotide cycle as a possible anaplerotic process in rat skeletal muscle. Experientia. 1982 Sep 15;38(9):1035–1037. doi: 10.1007/BF01955351. [DOI] [PubMed] [Google Scholar]
- Shumate J. B., Kaiser K. K., Carroll J. E., Brooke M. H. Adenylate deaminase deficiency in a hypotonic infant. J Pediatr. 1980 May;96(5):885–887. doi: 10.1016/s0022-3476(80)80569-5. [DOI] [PubMed] [Google Scholar]
- Shumate J. B., Katnik R., Ruiz M., Kaiser K., Frieden C., Brooke M. H., Carroll J. E. Myoadenylate deaminase deficiency. Muscle Nerve. 1979 May-Jun;2(3):213–216. doi: 10.1002/mus.880020309. [DOI] [PubMed] [Google Scholar]
- Tornheim K., Lowenstein J. M. The purine nucleotide cycle. 3. Oscillations in metabolite concentrations during the operation of the cycle in muscle extracts. J Biol Chem. 1973 Apr 25;248(8):2670–2677. [PubMed] [Google Scholar]
- Tornheim K. Oscillations of the glycolytic pathway and the purine nucleotide cycle. J Theor Biol. 1979 Aug 21;79(4):491–541. doi: 10.1016/0022-5193(79)90240-6. [DOI] [PubMed] [Google Scholar]




