Abstract
AIM: To investigate the effects of autologous tumor vaccine on recurrence of hepatocellular carcinoma (HCC).
METHODS: Sixty patients with HCC who had undergone curative resection, were randomly divided into HCC vaccine group and control group. Three vaccinations at 2-wk intervals were performed after curative hepatic resection. Delayed-type- hypersensitivity (DTH) test was performed before and after vaccination. Primary endpoints were the time of recurrence.
RESULTS: Four patients in control group and 6 patients in HCC vaccine group were withdrawn from the study. The vaccine containing human autologous HCC fragments showed no essential adverse effect in a phase II clinical trial and 17 of 24 patients developed a DTH response against the fragments. Three of 17 DTH-positive response patients and 5 of 7 DTH- negative response patients had recurrences after curative resection. After the operation, 1-, 2- and 3-year recurrence rates of HCC vaccine group were 16.7%, 29.2% and 33.3%, respectively. But, 1-, 2- and 3-year recurrence rates of the control group were 30.8%, 53.8% and 61.5%, respectively. The time before the first recurrence in the vaccinated patients was significantly longer than that in the control patients (P<0.05).
CONCLUSION: Autologous tumor vaccine is of promise in decreasing recurrence of human HCC.
Keywords: Hepatocellular carcinoma, HCC vaccine, Recurrence
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, and its incidence is inceasing[1,2]. Although curative treatments such as hepatic resection, orthotopic liver transplantation or percutaneous regional treatments offer the only chances of cure, the long-term results are still disappointed because of the high frequency of postoperative recurrence[3-9]. Recurrence control is the primary goal of novel approaches for HCC treatment. Active specific immunotherapy using the patient’s own tumor to elicit a long-term cell-mediated immune response has been successfully applied to melanoma, renal carcinoma and colon cancer[10].
Tumor vaccines have been used to enhance T-cell responses in many forms[11-16], such as whole tumor cells, tumor cell lysates, genetically modified tumor cells and peptide-pulsed autologous dendritic cells. Tumor cells themselves might be used as immunogens because it is believed that tumor cells could express a set of tumor-specific peptide-MHC complexes recognized by cytotoxic T lymohocytes (CTL). It has been reported that highly specific autologous CTL are induced from peripheral blood on formalin-fixed paraffin-embedded tumor sections[17,18]. Using a formalin-fixed autologous tumor cell line as target cells, CTL could continuously amplify. When T lymphocytes are stimulated with formalin-fixed primary target cells derived from glioblastoma multiformis as tumor antigens, human tumor-specific CTL are expanded in vitro. HLA-A-2402-restricted and carcinoembryonic-antigen (CEA)-specific CTL could be induced by culturing human peripheral blood mononuclear cells (PBMC) with autologous formalin-fixed adhesive PBMC pre-loaded with CEA-bound latex beads[19]. These observations have led us to propose that solid antigens in the size able to be phagocytosed by antigen presenting cells (APC) is one of the efficient tumor vaccines[20]. We here reported a HCC vaccine against HCC recurrence.
MATERIALS AND METHODS
Patients
The following protocol was approved by the ethical authorities of the First Affiliated Hospital of Sun Yat-Sen University. All patients were histologically confirmed as HCC and underwent hepatectomy. Eligibility criteria included International Union Against Cancer (UICC) (1997, 5th edition) clinical tumor-node-metastasis (TNM) groupings of stages I, II, or IIIA. No patient received any immunosuppressive drugs including steroids within one month prior to receiving the vaccine. Furthermore, patients who had adequate hematologic, hepatic, and renal functions (hepatic function Child-Pugh class A or B, white cell count >3×109/L, platelets >5×1010/L, and creatinine <88.4 µmol/L) were enrolled, but those with any severe cardiac or psychiatric disease or distant metastases were excluded, after written informed consent was obtained. Exclusion criteria included evidence of extra-hepatic metastases (stage III-B or IV), hepatic function Child-Pugh C, other malignancies or history of other malignancies in the past five years, postoperative dysfunction of any organ for more than 2 wk, systemic active infections, autoimmune diseases at any period of study, systematic steroid therapy within one month prior to the vaccination and chemotherapy, radiation therapy or biological therapy within one month prior to the vaccination. Sixty patients with HCC underwent curative resection between January 2000 and June 2003, 7 of them received left lateral lobectomy, 15 left hepatectomy, 6 right hepatectomy and 32 irregular hepatectomy. All patients were randomly assigned into two groups. Thirty patients served as control group with no adjuvant treatment but with regular follow-up observation in our hospital, and thirty patients treated with HCC vaccination served as treatment group.
Preparation of tumor vaccine and method for injection
Lecithin solution was prepared by adding 0.5 mL of 99.5% ethanol into 2.0 grams of lecithin, then dissolved at 45 °C in a water bath for approximately 1 h and filtered with 0.22 μm pore-size Milex-GV filters. α-tocopherol was dissolved at 45 °C in a water bath for less than 30 min and filtered with Steradisc 13 γ-ray sterile 0.45 μm filters. The two solutions were mixed. Cholesterol solution was prepared by dissolving 25 mg of cholesterol in 2 mL of 99.5% ethanol. Twenty-four microliters of cholesterol solution was added to 0.6 mL mixture solution of the α-tocopherol and lecithin, and then vortexed thoroughly to mix the solution. Then 0.1 mL of 107 IU/mL human IL-2 and human GM-CSF solution was added to 0.6 mL of the solution, and mixed.
Resected and neutral formalin-fixed HCC tissues (2 g or more) were homogenized. The fragments were filtered through 40 μm nylon meshes, washed and sterilized with 70% alcohol, then incubated in RPMI- 1640 medium at 37 °C for 2 d. The tumor fragments were washed three times with saline, aliquoted into 50 mL per Eppendorf tube and stored at -80 °C until use.
HCC vaccine consisted of Vac-1 and Vac-2. Vac-1 contained (doses per injected site) 10 μL of fixed HCC fragments, 1000 IU of hGM-CSF, 1000 IU of hIL-2 and 0.05 μg of tuberculin. Vac-2 cytokine-grease contained 6000 IU of hGM-CSF and 6000 IU of hIL-2. Vac-1 (0.1 mL) was immunized by intradermal injection into the upper arm per injected site. After thirty minutes, 0.1 mL of Vac-2 was injected exactly into the Vac-1-injected site. One month after hepatic operation, eligible patients had the first vaccination. Autologous HCC vaccine was injected intra dermally into the upper arm at a dose of 0.1 mL/site to 5 separate sites. The patients received three vaccinations at two-week intervals.
Delayed-type hypersensitivity (DTH) test
Patients who underwent curative hepatic resection were submitted to DTH test-1 before the first vaccination and to DTH test-2 two weeks after the final vaccine injection. Peripheral blood for PBMC preparation (10 mL) was taken for flow cytometry, and then, a suspension (0.1 mL in saline) of the autologous tumor fragments was injected intra-dermally into the left forearm. Fourty-eight hours later, if erythema and induration shared a skin-area of more than 10 mm in diameter, it was defined positive. Slight responses (5-10 mm in diameter) though definitely observable, were recorded as weakly positive.
Follow up
Hepatic ultrasonography and serum alpha-fetoprotein (AFP) two weeks after DTH test-2 injection and then every two months, computed tomography (CT) scan and chest radiography at every six months after operations were performed. The protective effects of postoperative vaccine therapy on recurrence of HCC included the time to the first recurrence after operation, recurrence-free survival and overall survival. Once the recurrence was confirmed, follow-up was stopped, and routine treatments such as second hepatic resection, ultrasound-guided intratumoral ablation, transcatheter chemoembolisation (TACE) or systemic chemotherapy were performed.
Evaluation of adverse events
Although safety was confirmed in the preceding phase I study[20], when any of the following events happened during the vaccination period, severe toxicity was considered. Using WHO grading of acute and sub-acute toxicity for reference[21], the evaluation standards for toxicity of vaccine therapy were as follows. The toxicity to some main organs, such as heart, liver or kidney, reached grade 3 or grade 4 of WHO standards, and the toxicity to skin reached grade 3 or grade 4 of WHO standards in two of three injections, acute fulminant hepatitis or any kind of autoimmune diseases was established.
Flow cytometry
Suspended cells (1×106 cells/mL) were washed three times with phosphate-buffered solution (PBS) (-), stained with monoclonal antibodies for 30 min, and FITC-labeled goat anti-mouse IgG polyclonal antibody for 5 min. The cells were again washed with PBS(-) containing 4% fibrinogens in fetal bovine serum (FBS), re-suspended in the same buffer at a concentration of 1×106 cells/mL and immediately analyzed by fluorescence-activated cell sorter (FACS) (Becton Dickinson, Co.) as previously described[22]. The proportion of CD3+, CD4+, CD8+, CD16+, and CD56+ cells was determined using mouse anti- human monoclonal antibodies.
Statistical analysis
The F-test was used to analyze the data using Excel software. The prognostic relevance data of HCC tumor vaccine therapy were analyzed using log-rank test. P value less than 0.05 was considered statistically significant.
RESULTS
Base-line data of HCC patients
Four patients in the control group and 6 patients in the HCC vaccine group were withdrawn from the study, because they gave up tumor vaccine therapy or lost follow-up. Base-line data of 26 control patients and 24 vaccinated patients are shown in Table 1. No essential difference in the base-line data was observed between the vaccinated and control patients. Age, cause of liver injury, Child-Pugh classes, serum alanine-aminotransferase level, percentage of patients with cirrhosis, operation, American Joint Commission for Cancer (AJCC) stages, blood loss and transfusion in the operation were all in proximity. The major axis of resected tumor was 63±22 mm and 56±36 mm in the vaccinated and control patients, respectively.
Table 1.
Base-line data of vaccinated and control patients (mean±SD).
| Vaccinated (n = 24, %) | Control (n = 26, %) | |
| Age (yr) | 53±9 | 49±15 |
| Male | 22 (92) | 21 (81) |
| Female | 2 (8) | 5 (19) |
| Cause of liver injury | ||
| Hepatitis B | 19 (79.1) | 18 (69.2) |
| Hepatitis C | 1 (4.2) | 2 (7.7) |
| Unknown | 4 (16.7) | 6 (23.1) |
| Child-Pugh class A | 23 (96) | 24 (92) |
| Child-Pugh class B | 1 (4) | 2 (8) |
| Alanine aminotransferase (U/L) | 75±35 | 50±95 |
| Cirrhosis | 16 (67) | 16 (62) |
| a-fetoprotein | ||
| ≥400 mg/L | 17 (71.3) | 16 (61.5) |
| <400 mg/L | 7 (28.7) | 10 (38.5) |
| Tumor size (major axis, mm) | 63±22 | 56±36 |
| AJCC stage I | 4 (17) | 2 (8) |
| AJCC stage II | 20 (83) | 24 (92) |
| Style of operation | ||
| Left lateral lobectomy | 3 | 4 |
| Left hepatectomy | 8 | 5 |
| Right hepatectomy | 2 | 3 |
| Irregular hepatectomy | 11 | 14 |
| Blood loss during operation (mL) | 812±536 | 769±620 |
| Blood transfusion during operation (mL) | 542±521 | 638±612 |
Adverse events in patients
The ultimate aim of this study was to detect toxicity. The vaccination was well tolerated and no severe adverse effect was observed in all patients. Erythema, dry desquamation and pruritus at the vaccinated sites were observed after each vaccination. These adverse effects disappeared 2 wk later and required no medical intervention. No exacerbation of cutaneous toxicities such as moist desquamation, ulceration or necrosis were observed. Lymphadenopathy and systemic reactions such as fever or chills, or vaccination-related impairments of the function of vital organs such as liver, kidney and bone marrow were not found. Occurrence of autoimmune diseases and skin toxicity was not observed. No patient withdrew from the study because of the adverse effects.
DTH responses induced by HCC vaccine
Delayed-type hypersensitivity (DTH) test was performed before vaccination and two weeks after the third vaccination. Negative responses were observed in all patients before vaccination. All patients demonstrated DTH responses two weeks after the third vaccination. Seventeen of 24 patients had delayed-type hypersensitivity (DTH) response to the HCC fragments, three weak-positive responses and fourteen positive responses were observed in the vaccinated patients. Seven patients showed a negative DTH response.
Tumor vaccine against recurrence of HCC
After three vaccinations at 2 wk intervals, seventeen of 24 patients had delayed-type hypersensitivity (DTH) response against the fragments. Three of 17-DTH positive response patients had HCC recurrence 13, 15 and 16 mo after curative resection. Five of 7-DTH negative response patients had HCC recurrence 8, 9, 10, 10, and 16 mo after operation. After the operation, the 1-, 2- and 3-year recurrence rates in HCC vaccine group were 16.7%, 29.2% and 33.3%, respectively. But the 1-, 2- and 3-year recurrence rates in the control group were 30.8%, 53.8% and 61.5%, respectively. The time before the first recurrence in the vaccinated patients was significantly longer than that in the control patients (P<0.05). The recurrence-free HCC patients with or without tumor vaccine therapy is shown in Figure 1. The difference of the two curves was statistically significant (log-rank test, P<0.05).These data suggested that vaccination of HCC patients with the autologous vaccine was a well-tolerated treatment and could induce tumor fragment-specific immunity.
Figure 1.
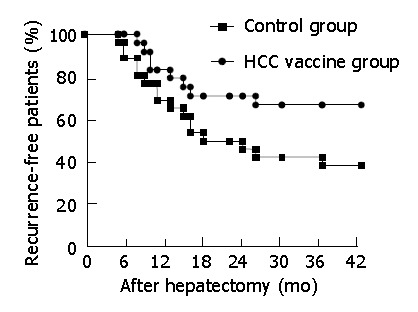
Recurrence-free HCC patients with or without tumor vaccine therapy (Kaplan-Meier curves).
Flow cytometric evaluation of surface phenotypes of peripheral blood lymphocytes
Twenty-four HCC patients who received the tumor vaccine were analyzed for surface phenotypes of peripheral blood lymphocytes before and after vaccination. After tumor vaccination, the patients had a significantly higher number of CD3+ CD8+- positive lymphocytes and number of CD4+/CD8+ significantly lower (P<0.05 Table 2).
Table 2.
Surface phenotypes of peripheral blood lymphocytes (mean±SD).
| Control (n=26) | Before vaccination (n=24) | After vaccination (n=24) | |
| CD3+ | 73.5±5.8 | 70.4±7.2 | 71.9±6.5 |
| CD3+CD4+ | 45.2±9.7 | 48.3±7.6 | 49.0±9.2 |
| CD3+CD8+ | 29.3±9.8a | 27.9±7.5a | 39.9±10.5a |
| CD4+/CD8+ | 2.02±0.39c | 2.15±0.98c | 1.03±1.13c |
| CD3-CD16+56+ | 18.5±3.9 | 16.9±8.3 | 19.7±9.3 |
P<0.05,
P<0.05 after tumor vaccination vs control or before tumor vaccination.
DISCUSSION
The present results suggest that a vaccine comprised of fixed HCC tissue fragments, cytokine controlled-release formulation, and adjuvant could elicit anti-tumor immune responses in human phase II clinical trials. The vaccine containing autologously fixed HCC fragments has no essential adverse effect; the time to the first recurrence in the vaccinated patients, especially in the DTH-positive response patients, is significantly longer than that in the control group (Figure 1).
In the clinical trial, we used DTH reaction, a monitoring method, for detecting antigen-specific immunity. Vaccination of patients with HCC fragments combined with hGM-CSF/hIL-2 controlled-release formulation and tuberculin was capable of inducing antitumor cellular immune response. We investigated DTH response to tumor fragments, which were processed by APC for effective presentation to effector T cells. After three vaccinations, 17 of 24 patients demonstrated postive DTH response. Furthermore, anti-hepatocellular carcinoma immune reaction was found to inhibit the recurrence. The results suggest that fixed HCC vaccination is well tolerated and is able to induce antitumor immunity, and should be considered for further clinical evaluation to define its potential therapeutic efficacy.
The major advantage of the present human HCC vaccine is that it contains personalized and very stable tumor antigens. HCC vaccine consisted unidentified autologous tumour antigens in fixed tumor tissues that can be preferably used in many medical institutions where vaccine is prepared with MHC-matched peptides and complicated recombinant techniques. Compared to the tumor vaccine containing live dendritic cells, which are considered the essential component, non-live cell-containing stable vaccines are easy to handle at bedside, thus increasing its use.
Active immunotherapy can induce tumor-specific cytotoxic T lymphocytes (CTL) and achieve a long-term antitumor immune response[10,11,22]. AFP, a HCC-associated antigen, could serve as a target for T-cell immunotherapy in animals[23,24], but patients carrying matched major histocompatibility complex (MHC) alleles could benefit from tumor-associated antigen-based vaccine[12]. Antigen pre-loaded dendritic cells (DCs) could elicit strong antitumor immune response[25,26], but DC-based approaches are cumbersome and expensive, and unsuitable for large-scale clinical trials. Until HCC-specific antigens are identified, the tumor cell itself is still the best source of tumor antigen. With autologous formalin-fixed paraffin-embedded tumor sections, we successfully induced tumor-specific cytotoxic T lymphocytes (CTL) from the peripheral blood of tumor-bearing patients. From the present results, precise mechanisms of the anti-tumor immune response are obscure. However, we prepared fixed HCC as a fragment suspension in the vaccine. Phagocytosis of the particular antigen might be an important pathway to present the antigenic peptide on MHC-class I molecules[27-30]. The present results suggest that antigens in a particular form could elicit CD8+ MHC class I-restricted CTL response (Table 2). Soluble antigens could elicit responses of CD4+ MHC class II-restricted lymphocytes[31]. CD4+ T cells could then transfer their immune information to stimulate antibody production. Therefore, direct induction of CTL against soluble antigens is difficult, except when the antigenic peptide is loaded on the antigen presenting dendritic cells[32]. However, studies have also reported that macrophages could efficiently induce CTL response in vitro when particular carriers are used to deliver antigenic short peptides[33]. In our hypothesis, fixed tumor tissue can provide many tumor antigens that are recognized by the immune system of patients and elicit a specific antitumor response. Local use of hGM-CSF could activate dermal antigen-presenting cells (APC), hIL-2 could expand the proliferation of induced CTL, and the elicited antitumor immune response is then amplified. Slow release of these cytokines from cytokine controlled release formulation could maintain long-term stimulation of the immune system and induce persistent antitumor immunity.
Considering the simplicity in preparation, bed-side handling of the tumor vaccine is one of the most important factors. In this respect, vaccines including live cells in the formulation are disadvantageous because of the complicated preparation techniques. Tumor-associated antigenic peptides, DNA vaccines, and tumor cell lysate, are therefore the attractive alternatives. Unfortunately, peptides are only effective on patients carrying matched major histocompatibility complex (MHC) alleles[12]. Peptides and tumor cell lysate are also rather weak immunogens. Antigen pre-loaded dendritic cells might be a promising vaccine[34],which also require live cell handling. DNA vaccines could provide a definite amount of antigens[14].
In conclusion, HCC vaccine can significantly control early post-operative recurrence of HCC and improve overall survival. This cell-free immunization mode should be recommended for a large-scale use in patients with HCC.
Footnotes
Supported by the Natural Science Foundation of Guangdong Province, No. 021889
Edited by Kumar M and Wang XL
References
- 1.Peto J. Cancer epidemiology in the last century and the next decade. Nature. 2001;411:390–395. doi: 10.1038/35077256. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999;353:1253–1257. doi: 10.1016/S0140-6736(98)09148-X. [DOI] [PubMed] [Google Scholar]
- 4.Zhou XD, Tang ZY, Yang BH, Lin ZY, Ma ZC, Ye SL, Wu ZQ, Fan J, Qin LX, Zheng BH. Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer. 2001;91:1479–1486. doi: 10.1002/1097-0142(20010415)91:8<1479::aid-cncr1155>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 7.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–799; discussion 799-800. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 9.Lee WC, Jeng LB, Chen MF. Estimation of prognosis after hepatectomy for hepatocellular carcinoma. Br J Surg. 2002;89:311–316. doi: 10.1046/j.0007-1323.2001.02034.x. [DOI] [PubMed] [Google Scholar]
- 10.Vermorken JB, Claessen AM, van Tinteren H, Gall HE, Ezinga R, Meijer S, Scheper RJ, Meijer CJ, Bloemena E, Ransom JH, et al. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet. 1999;353:345–350. doi: 10.1016/S0140-6736(98)07186-4. [DOI] [PubMed] [Google Scholar]
- 11.Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger JC, Hodi FS, Liebster L, Lam P, Mentzer S, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci USA. 1998;95:13141–13146. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Protti MP, Bellone M. Immunotherapy: natural versus synthetic peptides. Immunol Today. 1998;19:98. [PubMed] [Google Scholar]
- 13.Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997;3:558–561. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 14.Pardoll DM. Cancer vaccines. Nat Med. 1998;4:525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Zhang JK, Zhuo SH, Chen HB. Effect of a cancer vaccine prepared by fusions of hepatocarcinoma cells with dendritic cells. World J Gastroenterol. 2001;7:690–694. doi: 10.3748/wjg.v7.i5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Y, Wu M, Chen H, Wang X, Liu G, Li G, Ma J, Sy MS. Effective tumor vaccine generated by fusion of hepatoma cells with activated B cells. Science. 1994;263:518–520. doi: 10.1126/science.7507262. [DOI] [PubMed] [Google Scholar]
- 17.Liu SQ, Saijo K, Todoroki T, Ohno T. Induction of human autologous cytotoxic T lymphocytes on formalin-fixed and paraffin-embedded tumour sections. Nat Med. 1995;1:267–271. doi: 10.1038/nm0395-267. [DOI] [PubMed] [Google Scholar]
- 18.Liu SQ, Shiraiwa H, Kawai K, Hayashi H, Akaza H, Kim BS, Oki A, Nishida M, Kubo T, Hashizaki K, et al. Tumor-specific autologous cytotoxic T lymphocytes from tissue sections. Nat Med. 1996;2:1283. doi: 10.1038/nm1296-1283. [DOI] [PubMed] [Google Scholar]
- 19.Kim C, Matsumura M, Saijo K, Ohno T. In vitro induction of HLA-A2402-restricted and carcinoembryonic-antigen-specific cytotoxic T lymphocytes on fixed autologous peripheral blood cells. Cancer Immunol Immunother. 1998;47:90–96. doi: 10.1007/s002620050508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng BG, Liu SQ, Kuang M, He Q, Totsuka S, Huang L, Huang J, Lu MD, Liang LJ, Leong KW, et al. Autologous fixed tumor vaccine: a formulation with cytokine-microparticles for protective immunity against recurrence of human hepatocellular carcinoma. Jpn J Cancer Res. 2002;93:363–368. doi: 10.1111/j.1349-7006.2002.tb01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 22.McCune CS, O'Donnell RW, Marquis DM, Sahasrabudhe DM. Renal cell carcinoma treated by vaccines for active specific immunotherapy: correlation of survival with skin testing by autologous tumor cells. Cancer Immunol Immunother. 1990;32:62–66. doi: 10.1007/BF01741726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vollmer CM, Eilber FC, Butterfield LH, Ribas A, Dissette VB, Koh A, Montejo LD, Lee MC, Andrews KJ, McBride WH, et al. Alpha-fetoprotein-specific genetic immunotherapy for hepatocellular carcinoma. Cancer Res. 1999;59:3064–3067. [PubMed] [Google Scholar]
- 24.Meng WS, Butterfield LH, Ribas A, Dissette VB, Heller JB, Miranda GA, Glaspy JA, McBride WH, Economou JS. alpha-Fetoprotein-specific tumor immunity induced by plasmid prime-adenovirus boost genetic vaccination. Cancer Res. 2001;61:8782–8786. [PubMed] [Google Scholar]
- 25.Nestle FO, Banchereau J, Hart D. Dendritic cells: On the move from bench to bedside. Nat Med. 2001;7:761–765. doi: 10.1038/89863. [DOI] [PubMed] [Google Scholar]
- 26.Homma S, Toda G, Gong J, Kufe D, Ohno T. Preventive antitumor activity against hepatocellular carcinoma (HCC) induced by immunization with fusions of dendritic cells and HCC cells in mice. J Gastroenterol. 2001;36:764–771. doi: 10.1007/s005350170019. [DOI] [PubMed] [Google Scholar]
- 27.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 29.Falo LD, Kovacsovics-Bankowski M, Thompson K, Rock KL. Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nat Med. 1995;1:649–653. doi: 10.1038/nm0795-649. [DOI] [PubMed] [Google Scholar]
- 30.Harding CV, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994;153:4925–4933. [PubMed] [Google Scholar]
- 31.Rock KL, Clark K. Analysis of the role of MHC class II presentation in the stimulation of cytotoxic T lymphocytes by antigens targeted into the exogenous antigen-MHC class I presentation pathway. J Immunol. 1996;156:3721–3726. [PubMed] [Google Scholar]
- 32.De Bruijn ML, Jackson MR, Peterson PA. Phagocyte-induced antigen-specific activation of unprimed CD8+ T cells in vitro. Eur J Immunol. 1995;25:1274–1285. doi: 10.1002/eji.1830250522. [DOI] [PubMed] [Google Scholar]
- 33.De Bruijn ML, Peterson PA, Jackson MR. Induction of heat-stable antigen expression by phagocytosis is involved in in vitro activation of unprimed CTL by macrophages. J Immunol. 1996;156:2686–2692. [PubMed] [Google Scholar]
- 34.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]


