Abstract
AIM: To explore the correlation between expression of somatostatin (SS), gastrin (GAS) and cell apoptosis regulation gene bcl-2/bax in large intestine carcinoma.
METHODS: Sixty-two large intestine cancer tissue samples were randomly and retrospectively selected from patients with large intestine carcinoma. Immunohistochemical staining for bcl-2, bax, GAS, SS was performed according to the standard streptavidin-biotin-peroxidase (S-P) method. According to the semi-quantitative integral evaluation, SS and GAS were divided into three groups as follows. Scores 1-3 were defined as the low expression group, 4-8 as the intermediate expression group, 9-16 as the high expression group. Bax and bcl-2 protein expressions in different GAS and SS expression groups of large intestine carcinoma were assessed.
RESULTS: The positive expression rate of bax had a prominent difference between SS and GAS high, intermediate and low expression groups (P<0.05, χ2SS = 9.246; P<0.05, χ2GAS = 6.981). The positive expression rate of bax in SS high (80.0%, 8/10) and intermediate (76.5%, 13/17) expression groups was higher than that in low expression group (40.0%, 14/35) (P<0.05, χ2high vs low = 5.242; P<0.05,χ2middle vs low = 6.097). The positive expression rate of bax in GAS high expression group (27.3%, 3/8) was lower than that in low expression group (69.4%, 25/36) (P<0.05, χ2 = 4.594). However, bax expression in GAS intermediate expression group (46.7%, 7/15) was lower than that in low expression group, but not statistically significant. The positive expression rate of bcl-2 had a prominent difference between SS and GAS high, intermediate and low expression groups (P<0.05, χ2SS = 7.178; P<0.05, χ2GAS = 13.831). The positive expression rate of bcl-2 in GAS high (90.9%, 10/11) and intermediate (86.7%, 13/15) expression groups was higher than that in low expression group (44.4%, 16/36) (P<0.05, χ2high vs low = 5.600; P<0.05, χ2middle vs low = 7.695). However, the positive expression rate of bcl-2 in SS high (40.0%, 4/10) and intermediate (47.1%, 8/9) expression groups was lower than that in low expression group (77.1%, 27/35) (P<0.05, χ2high vs low = 4.710; P<0.05, χ2middle vs low = 4.706). There was a significant positive correlation between the integral ratio of GAS to SS and the integral of bcl-2 (P<0.01, r = 0.340). However, there was a negative correlation between the integral ratio of GAS to the SS and bax the integral of (P<0.05, r = -0.299).
CONCLUSION: The regulation and control of gastrin, somatostatin in cell apoptosis of large intestine carcinoma may be directly related to the abnormal expression of bcl-2, bax.
Keywords: Large intestine carcinoma, Gastrin, Somatostatin, bcl-2 gene, Bax gene, Apoptosis
INTRODUCTION
Large intestine cancer is one of the commonest malignancies in the world including china. Although early diagnosis and treatment have somewhat improved outcomes of patients, large intestine carcinoma still remains the major killer among Chinese[1-4]. Previous studies have demonstrated that the occurrence of large intestine cancer is directly related to the abnormal expression of gastrointestinal hormones such as gastrin, somatostatin, etc[5]. Mean while, somatostatin is able to induce cell apoptosis of large intestine cancer and inhibit cell proliferation, but the function of GAS is opposite[6-8]. However, the detailed mechanism of gastrin and somatostatin in regulation and control of cell apoptosis of large intestine carcinoma remains unknown. We used immunohistochemical staining S-P method to detect the expression of GAS, SS, bcl-2, bax proteins in large intestine cancer tissue. The aim of this study was to explore whether GAS, SS could regulate and control cell apoptosis mainly via influencing the expression of bcl-2/bax proteins in large intestine cancer.
MATERIALS AND METHODS
Large intestine carcinoma specimens
Sixty-two large intestine cancer tissue samples were randomly and retrospectively selected from patients with large intestine carcinoma in the First Affiliated Yijishan Hospital of Wannan Medical College from 2000 to 2002. Among them, 41 were cases of rectal cancer, 21 were cases of colorectal carcinoma. Twenty-two were females, 40 were males. The median age was 50.9±7.8 years, with a range of 28-77 years. The clinical stage was determined according to Dukes’ stage. Thirty-four were Dukes’ stages A, B and 28 were Dukes’ stages C, D. Histological grade of tumors was determined according to the WHO criteria, and 21 patients were grade I, 25 grade II, 16 grade III.
Main reagents
The polyclonal rabbit antibodies against human SS and GAS, monoclonal mouse antibodies against human bcl-2 and bax, and immunohistochemical staining kits were all purchased from Beijing Zhongshan Biological Technology Co, Ltd.
Immunohistochemical staining
Specimens obtained at surgery were routinely fixed in 10% neutral formalin and embedded in paraffin. Serial 4 μm thick sections were cut. Immunohistochemical staining for bcl-2, bax, GAS, SS was performed according to the standard streptavidin-biotin-peroxidase (S-P) method. The detailed manipulation was conducted according to the introductions for users. A previously known positive pancreatic tissue, stomach antrum mucous membrane, amygdala tissue, Hodgkin’s disease tissue were used as positive controls for GAS, SS, bcl-2, bax, respectively. PBS 0.01M wao uged as a negative control to replace the primary antibody.
Evaluation of scores
The standard positive SS and GAS expressions were stained brown-yellow mainly in cell plasma, partly in cell membranes. Both the extent and intensity of immunopositivity of SS and GAS expressions were scored according to Wu et al[8]. The intensity of positivity was scored as follows: 1, no staining; 2, light-yellow; 3, brown-yellow; 4, brown-black. The extent of positivity was scored as follows (one hundred cells were counted by two independent observers, who did not know the clinicopathological features of these large intestine cancers.): 1, ≤5%, 2, >5-10%, 3, >10-20%, 4, >20% of the tumor cells in the respective lesions. The final score was determined by multiplying the intensity with extent of positivity scores, yielding a range from 1 to 16. According to the semi-quantitative integral evaluation, SS and GAS were divided into three groups as follows. Scores 1-3 were defined as the low expression group, 4-8 as the intermediate expression group, 9-16 as the high expression group.
The standard positive bcl-2 and bax expressions were stained brown-yellow mainly in cell plasma. Both the extent and intensity of immunopositivity of bcl-2 and bax expressions were scored according to Fromowitz et al[9]. The intensity of positivity was scored as follows: 0, negative; 1, light-yellow; 2, brown-yellow; 3, brown-black. The extent of positivity was scored as follows: 1, ≤25%; 2, >25-50%; 3, >50-75%; 4, >75%. The final score was determined by adding the intensity to extent of positivity scores, yielding a range from 0 to 12. Scores 1-2 were defined as negative expression (-), 3 as weak staining pattern (+), 4 as moderate staining (++), ≥5 as strong staining (+++).
Statistical analysis
Statistical evaluation was performed using chi-square test to differentiate the rates of different groups and using Spearman test to analyze the correlation between the ratio of GAS to SS and the integral of bcl-2 and bax. P<0.05 was considered statistically significant. SPSS 10.0 software for Windows was employed to analyze all data.
RESULTS
Bax expression in GAS, SS high, intermediate, and low expression groups of large intestine carcinoma
The positive expression rate of bax had a prominent difference in SS and GAS high, intermediate and low expression groups of large intestine cancer (P<0.05, χ2SS = 9.246; P<0.05, χ2GAS = 6.981). The positive expression rate of bax in SS high (80.0%, 8/10) and intermediate (76.5%, 13/17) expression groups was higher than that in low expression group (40.0%, 14/35) (P<0.05, χ2high vs low = 5.242; P<0.05, χ2middle vs low = 6.097). The positive expression rate of bax in GAS high expression group (27.3%, 3/8) was lower than that in low expression group (69.4%, 25/36) (P<0.05, χ2 = 4.594). Bax expression in GAS intermediate expression group (46.7%, 7/15) was lower than that in low expression group, but without statistical significance (Table 1, Figure 1: A-C).
Table 1.
Bax expression in SS and GAS high, intermediate, and low expression groups of large intestine carcinoma.
| Groups | n |
Bax |
Positive rate (%) | |
| Positive | Negative | |||
| SS | ||||
| High | 10 | 8 | 2 | 80.0 (8/10)a |
| Intermediate | 17 | 13 | 4 | 76.5 (13/17)a |
| Low | 35 | 14 | 21 | 40.0 (14/35) |
| GAS | ||||
| High | 11 | 3 | 8 | 27.3 (3/11)c |
| Intermediated | 15 | 7 | 8 | 46.7 (7/15) |
| Low | 36 | 25 | 11 | 69.4 (25/36) |
P<0.05 vs the SS low expression group;
P<0.05 vs GAS low expression group.
Figure 1.
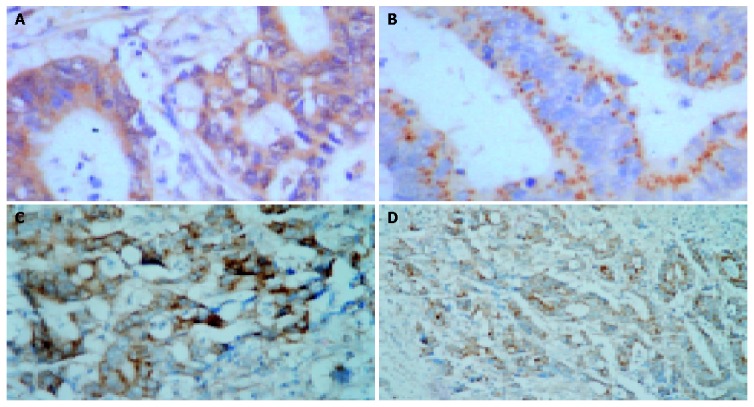
Strong expressions of GAS, SS, bcl-2 and bax in large intestine carcinoma tissue. A: Strong GAS expression in large intestine carcinoma tissue. S-P×400; B: Strong SS expression in large intestine carcinoma tissue. S-P×400; C: Strong bax expression in SS high expression group of large intestine carcinoma tissue. S-P×200; D: Strong bcl-2 expression in GAS high expression group of large intestine carcinoma tissue. S-P×200.
Bcl-2 expression in GAS, SS high, intermediate, and low expression groups of large intestine carcinoma
The positive expression rate of bcl-2 had a prominent difference in SS and GAS high, intermediate and low expression groups of large intestine cancer (P<0.05, χ2SS = 7.178; P<0.05, χ2GAS = 13.831). The positive expression rate of bcl-2 in GAS high (90.9%, 10/11) and intermediated (86.7%, 13/15) expression groups was higher than that in low expression group (44.4%, 16/36) (P<0.05, χ2high vs low = 5.600; P<0.05, χ2middle vs low = 7.695). However, the positive expression rate of bcl-2 in SS high (40.0%, 4/10) and intermediate (47.1%, 8/9) expression groups was lower than that in low expression group (77.1%, 27/35) (P<0.05, χ2high vs low = 4.710; P<0.05, χ2middle vs low = 4.706) (Table 2, Figure 1: D).
Table 2.
Bcl-2 expression in SS and GAS high, intermediate, and low expression groups of large intestine carcinoma.
| Groups | n |
Bcl-2 |
Positive rate (%) | |
| Positive | Negative | |||
| SS | ||||
| High | 10 | 4 | 6 | 40.0 (4/10)a |
| Intermediated | 17 | 8 | 9 | 47.1 (8/17)a |
| Low | 35 | 27 | 8 | 77.1 (27/35) |
| GAS | ||||
| High | 11 | 10 | 1 | 90.9 (10/11)c |
| Intermediated | 15 | 13 | 2 | 86.7 (13/15)c |
| Low | 36 | 16 | 20 | 44.4 (16/36) |
P<0.05 vs the SS low expression group;
P<0.05 vs the GAS low expression group.
Correlation between the integral ratio of GAS to SS and the integral of bcl-2, bax
There was a significant positive correlation between the integral ratio of GAS to SS and the integral of bcl-2 (P<0.01, r = 0.340). However, there was a negative correlation between the integral ratio of GAS to SS and the integral of bax (P<0.05, r = -0.299).
DISCUSSION
Previous studies have shown that tissue growth is regulated by hormones, and their tumor growth and development are still controlled by hormones[10]. Gastrointestinal hormones such as gastrin and somatostatin regulate the secretion, motility, absorption, blood flow and cell nutrition of the digestive tract. Abnormality of their secretion often affects the normal functions of digestive tract, even causes clinical symptoms or syndromes[11,12]. Some studies have demonstrated that there is a high correlation between the abnormal expressions of GAS, SS and the occurrence and development of large intestine cancer[13-15]. Recent studies have shown that the abnormal expressions of GAS and SS are closely related to cell apoptosis of large intestine cancer. Gastrin could promote cell proliferation and inhibit cell apoptosis. However, the action of somatostatin is opposite in large intestine carcinoma[8,16,17].
Apoptosis can not only maintain the body in well stable condition, but also plays an important role in regulating and controlling tumor occurrence, development and treatment[18]. It has been proved that occurrence of cancer is due to the loss of control of normal apoptosis and the disturbance of balance between cell proliferation and apoptosis[19,20]. Apoptosis related genes such as bcl-2 family are divided into two categories: pro-apoptosis genes and anti-apoptosis genes. Bcl-2 is an important apoptosis repressor, while Bax is one of the most important apoptosis promoters. The protein it encodes could combine with Bcl-2 to form compounds, which resist the action of apoptosis repression. But it has a positive regulatory action[21-24]. Recent data indicate that the regulation and control of cell apoptosis by bax and bcl-2 genes are not only based on the level of the two regulatory proteins but also based on their ratio. When the ratio is high, cells undergo apoptosis, otherwise, they proliferate[19,25,26].
Gastrin is mainly secreted from gastrin secreting cells (G cells) in antrum mucosa or upper small intestine, large intestine. Medulla oblongata and dorsal nuclei of vagus nerves in central nervous system also secrete gastrin[27]. Some studies indicate that external gastrin is able to inhibit apoptosis of MKN45 cells by inducing over-expression of anti-apoptosis gene bcl-2, and proglumide could block these effects of gastrin[28]. Zhang et al[29] found that gastrin was able to increase the threshold of apoptosis by upregulating bcl-2 gene expression in human cholangiocarcinoma cells, but it had no effect on the expression of bax gene. However, Hartwich et al[30] found that gastrin was able to restrain the apoptosis of tumor cells by inducing over-expression of bcl-2 and inhibiting the bax gene activity. Whether gastrin can regulate and control cell apoptosis mainly by affecting the bax gene expression, needs further studies. In this study, we found that GAS protein expression and the positive expression rate of bcl-2 were higher in large intestine carcinoma, however the expression of bax protein was opposite. These results accord with those of foreign reports and indicate that GAS regulation and control of cell apoptosis and proliferation of large intestine cancer can induce over-expression of bcl-2 protein and down-regulate bax gene activity.
Somatostatin is distributed in human hypothalamus and other sites of the brain, peripheral nerve and gastrointestinal tract. In the digestive system, for example, somatostatin is secreted from somatostatin secreting cells (D cells). D cells are distributed mainly in intestinal nerve plexus, stomach and pancreas. Somatostatin acts as an inhibitory peptide of various secretory and proliferative responses. It has been found that its effects are mediated by a family of G-protein-coupled receptors (sst1-5)[31-34]. The mechanisms of the inhibition are the combined interaction of somatostatin and its analogs with SST1-5R in tumor tissues, either inhibiting division and proliferation of tumor cells or the activities of growth factors such as vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), etc[35-37], thus counteracting tumorigenesis and tissue proliferation[38]. Recent data have shown that somatostatin is not only able to restrain cell proliferation, but also to induce tumour cell apoptosis. However, the underlying mechanisms have not been elucidated. Sharma et al[39] reported that somatostatin analogs (SSa) octreotide (OCT) could elicit cytotoxic response in MCF-7 human breast cancer cells, leading to apoptosis which is associated with a rapid, time-dependent induction of wild-type p53 and an increase of bax. Kang et al[40] demonstrated that apoptosis by somatostatin might occur due to bax- and NO-independent p53 accumulation, and through Fas and caspase-8 activation pathways in peritoneal macrophages. Yuan et al[41] found that somatostatin analogs (SSa) were able to induce the apoptosis of pancreatic acinar cells. The mechanisms of apoptosis are probably correlated with the expression of apoptosis-regulated gene bax, but have no relationship with the expression of p53. In a word, somatostatin and its analogues could induce cell apoptosis. In this study, we found that the higher the integral of SS was, the higher expression of bax protein, but the expression of bcl-2 protein was lower. Our data indicate that SS can promote cell apoptosis and restrain cell proliferation of large intestine carcinoma. The mechanism is through up-regulation of bax gene expression and inhibition of the activity of anti-apoptosis gene bcl-2.
In the present study, we found that the ratio of GAS to SS had an effect on biological characteristics such as malignant type, tissue differentiation and clinical stages of large intestine cancer. The ratio of GAS to SS was increased, which is of significance in large intestine cancer occurrence and development[10]. Our results show that there is a positive correlation between the ratio of GAS to SS and the semi-quantitative integral of bcl-2 expression, and a negative correlation between GAS/SS and bax. Furthermore, the expression of GAS and SS proteins has a direct relation with the expression of bax and bcl-2.
In conclusion, abnormal expression of GAS and SS can lead to abnormal expression of bcl-2 and bax in large intestine carcinoma.
Footnotes
Supported by National Natural Science Foundation of China, No.39270769, and Natural Science Foundation of Anhui Province, No.03043704, and Natural Science Foundation of Education Bureau of Anhui Province, No.2002kj307
Edited by Wang XL and Zhu LH
References
- 1.Zhang ZS, Zhang YL. To study evolvement of the large intestine cancer in china. Shijie Huaren Xiaohua Zazhi. 2001;9:489–494. [Google Scholar]
- 2.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Konturek PC, Bielanski W, Konturek SJ, Hartwich A, Pierzchalski P, Gonciarz M, Marlicz K, Starzynska T, Zuchowicz M, Darasz Z, et al. Progastrin and cyclooxygenase-2 in colorectal cancer. Dig Dis Sci. 2002;47:1984–1991. doi: 10.1023/a:1019652224424. [DOI] [PubMed] [Google Scholar]
- 5.Saga T, Tamaki N, Itoi K, Yamazaki T, Endo K, Watanabe G, Maruno H, Machinami R, Koizumi K, Ichikawa T, et al. Phase III additional clinical study of 111In-pentetreotide (MP-1727): diagnosis of gastrointestinal hormone producing tumors based on the presence of somatostatin receptors. Kaku Igaku. 2003;40:185–203. [PubMed] [Google Scholar]
- 6.Sadji-Ouatas Z, Lasfer M, Julien S, Feldmann G, Reyl-Desmars F. Doxorubicin and octreotide induce a 40 kDa breakdown product of p53 in human hepatoma and tumoral colon cell lines. Biochem J. 2002;364:881–885. doi: 10.1042/BJ20020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson SA, Morris TM, McWilliams DF, Harris J, Evans S, Smith A, Clarke PA. Potential role of endocrine gastrin in the colonic adenoma carcinoma sequence. Br J Cancer. 2002;87:567–573. doi: 10.1038/sj.bjc.6600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu P, Tu JS, Riu J, Hang H, Hang WB, Yuan P. To study the correlation between expression of gastrin, somatostatin and cell proliferation, apoptosis in colorectal carcinoma. Zhonghua Shiyan Waike Zaizhi. 2003;20:947. [Google Scholar]
- 9.Fromowitz FB, Viola MV, Chao S, Oravez S, Mishriki Y, Finkel G, Grimson R, Lundy J. ras p21 expression in the progression of breast cancer. Hum Pathol. 1987;18:1268–1275. doi: 10.1016/s0046-8177(87)80412-4. [DOI] [PubMed] [Google Scholar]
- 10.Sereti E, Gavriil A, Agnantis N, Golematis VC, Voloudakis-Baltatzis IE. Immunoelectron study of somatostatin, gastrin and glucagon in human colorectal adenocarcinomas and liver metastases. Anticancer Res. 2002;22:2117–2123. [PubMed] [Google Scholar]
- 11.Larsson LI. Developmental biology of gastrin and somatostatin cells in the antropyloric mucosa of the stomach. Microsc Res Tech. 2000;48:272–281. doi: 10.1002/(SICI)1097-0029(20000301)48:5<272::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 12.Portela-Gomes GM, Albuquerque JP, Ferra MA. Serotonin and gastrin cells in rat gastrointestinal tract after thyroparathyroidectomy and induced hyperthyroidism. Dig Dis Sci. 2000;45:730–735. doi: 10.1023/a:1005495710741. [DOI] [PubMed] [Google Scholar]
- 13.Glover SC, Tretiakova MS, Carroll RE, Benya RV. Increased frequency of gastrin-releasing peptide receptor gene mutations during colon-adenocarcinoma progression. Mol Carcinog. 2003;37:5–15. doi: 10.1002/mc.10117. [DOI] [PubMed] [Google Scholar]
- 14.Carroll RE, Matkowskyj K, Saunthararajah Y, Sekosan M, Battey JF, Benya RV. Contribution of gastrin-releasing peptide and its receptor to villus development in the murine and human gastrointestinal tract. Mech Dev. 2002;113:121–130. doi: 10.1016/s0925-4773(02)00032-1. [DOI] [PubMed] [Google Scholar]
- 15.Tejeda M, Gaal D, Barna K, Csuka O, Kéri G. The antitumor activity of the somatostatin structural derivative (TT-232) on different human tumor xenografts. Anticancer Res. 2003;23:4061–4066. [PubMed] [Google Scholar]
- 16.Yu HG, Schrader H, Otte JM, Schmidt WE, Schmitz F. Rapid tyrosine phosphorylation of focal adhesion kinase, paxillin, and p130Cas by gastrin in human colon cancer cells. Biochem Pharmacol. 2004;67:135–146. doi: 10.1016/j.bcp.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Wu H, Rao GN, Dai B, Singh P. Autocrine gastrins in colon cancer cells Up-regulate cytochrome c oxidase Vb and down-regulate efflux of cytochrome c and activation of caspase-3. J Biol Chem. 2000;275:32491–32498. doi: 10.1074/jbc.M002458200. [DOI] [PubMed] [Google Scholar]
- 18.Heinke MY, Yao M, Chang D, Einstein R, dos Remedios CG. Apoptosis of ventricular and atrial myocytes from pacing-induced canine heart failure. Cardiovasc Res. 2001;49:127–134. doi: 10.1016/s0008-6363(00)00242-x. [DOI] [PubMed] [Google Scholar]
- 19.Kanzler S, Galle PR. Apoptosis and the liver. Semin Cancer Biol. 2000;10:173–184. doi: 10.1006/scbi.2000.0318. [DOI] [PubMed] [Google Scholar]
- 20.Park YN, Chae KJ, Kim YB, Park C, Theise N. Apoptosis and proliferation in hepatocarcinogenesis related to cirrhosis. Cancer. 2001;92:2733–2738. doi: 10.1002/1097-0142(20011201)92:11<2733::aid-cncr10126>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Bold RJ, Virudachalam S, McConkey DJ. BCL2 expression correlates with metastatic potential in pancreatic cancer cell lines. Cancer. 2001;92:1122–1129. doi: 10.1002/1097-0142(20010901)92:5<1122::aid-cncr1429>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.Lowe SL, Rubinchik S, Honda T, McDonnell TJ, Dong JY, Norris JS. Prostate-specific expression of Bax delivered by an adenoviral vector induces apoptosis in LNCaP prostate cancer cells. Gene Ther. 2001;8:1363–1371. doi: 10.1038/sj.gt.3301531. [DOI] [PubMed] [Google Scholar]
- 23.Chan SL, Yu VC. Proteins of the bcl-2 family in apoptosis signalling: from mechanistic insights to therapeutic opportunities. Clin Exp Pharmacol Physiol. 2004;31:119–128. doi: 10.1111/j.1440-1681.2004.03975.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim LH, Nadarajah VS, Peh SC, Poppema S. Expression of Bcl-2 family members and presence of Epstein-Barr virus in the regulation of cell growth and death in classical Hodgkin's lymphoma. Histopathology. 2004;44:257–267. doi: 10.1111/j.0309-0167.2004.01829.x. [DOI] [PubMed] [Google Scholar]
- 25.Jo EH, Hong HD, Ahn NC, Jung JW, Yang SR, Park JS, Kim SH, Lee YS, Kang KS. Modulations of the Bcl-2/Bax family were involved in the chemopreventive effects of licorice root (Glycyrrhiza uralensis Fisch) in MCF-7 human breast cancer cell. J Agric Food Chem. 2004;52:1715–1719. doi: 10.1021/jf035012t. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura H, Kumei Y, Morita S, Shimokawa H, Ohya K, Shinomiya K. Antagonism between apoptotic (Bax/Bcl-2) and anti-apoptotic (IAP) signals in human osteoblastic cells under vector-averaged gravity condition. Ann N Y Acad Sci. 2003;1010:143–147. doi: 10.1196/annals.1299.023. [DOI] [PubMed] [Google Scholar]
- 27.Swatek J, Chibowski D. Endocrine cells in colorectal carcinomas. Immunohistochemical study. Pol J Pathol. 2000;51:127–136. [PubMed] [Google Scholar]
- 28.Wang HM, Cao XF, Huang SQ, Li YS, Yuan AH, Zhang QH, Zhang YL. Effect of external gastrin on apoptosis and expression of bcl-2 gene in gastric cancer cells. AiZheng. 2002;21:171–173. [PubMed] [Google Scholar]
- 29.Zhang FS, He ZP, Ma KS, Wang SG, Dong JH. Effects of gastrin on apoptotic pathways in human cholangiocarcinoma cell line QBC939. Zhonghua Putong Waike Zaizhi. 2002;17:588–589. [Google Scholar]
- 30.Hartwich J, Konturek SJ, Pierzchalski P, Zuchowicz M, Konturek PC, Bielański W, Marlicz K, Starzyńska T, Ławniczak M. Molecular basis of colorectal cancer - role of gastrin and cyclooxygenase-2. Med Sci Monit. 2001;7:1171–1181. [PubMed] [Google Scholar]
- 31.Zatelli MC, Piccin D, Tagliati F, Ambrosio MR, Margutti A, Padovani R, Scanarini M, Culler MD, degli Uberti EC. Somatostatin receptor subtype 1 selective activation in human growth hormone (GH)- and prolactin (PRL)-secreting pituitary adenomas: effects on cell viability, GH, and PRL secretion. J Clin Endocrinol Metab. 2003;88:2797–2802. doi: 10.1210/jc.2002-021825. [DOI] [PubMed] [Google Scholar]
- 32.Guillermet J, Saint-Laurent N, Rochaix P, Cuvillier O, Levade T, Schally AV, Pradayrol L, Buscail L, Susini C, Bousquet C. Somatostatin receptor subtype 2 sensitizes human pancreatic cancer cells to death ligand-induced apoptosis. Proc Natl Acad Sci USA. 2003;100:155–160. doi: 10.1073/pnas.0136771100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faiss S, Pape UF, Böhmig M, Dörffel Y, Mansmann U, Golder W, Riecken EO, Wiedenmann B. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors--the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689–2696. doi: 10.1200/JCO.2003.12.142. [DOI] [PubMed] [Google Scholar]
- 34.Benali N, Ferjoux G, Puente E, Buscail L, Susini C. Somatostatin receptors. Digestion. 2000;62 Suppl 1:27–32. doi: 10.1159/000051852. [DOI] [PubMed] [Google Scholar]
- 35.Hortala M, Ferjoux G, Estival A, Bertrand C, Schulz S, Pradayrol L, Susini C, Clemente F. Inhibitory role of the somatostatin receptor SST2 on the intracrine-regulated cell proliferation induced by the 210-amino acid fibroblast growth factor-2 isoform: implication of JAK2. J Biol Chem. 2003;278:20574–20581. doi: 10.1074/jbc.M210767200. [DOI] [PubMed] [Google Scholar]
- 36.Buscail L, Vernejoul F, Faure P, Torrisani J, Susini C. Regulation of cell proliferation by somatostatin. Ann Endocrinol (Paris) 2002;63:2S13–2S18. [PubMed] [Google Scholar]
- 37.Puente E, Saint-Laurent N, Torrisani J, Furet C, Schally AV, Vaysse N, Buscail L, Susini C. Transcriptional activation of mouse sst2 somatostatin receptor promoter by transforming growth factor-beta. Involvement of Smad4. J Biol Chem. 2001;276:13461–13468. doi: 10.1074/jbc.M010981200. [DOI] [PubMed] [Google Scholar]
- 38.Ferjoux G, Bousquet C, Cordelier P, Benali N, Lopez F, Rochaix P, Buscail L, Susini C. Signal transduction of somatostatin receptors negatively controlling cell proliferation. J Physiol Paris. 2000;94:205–210. doi: 10.1016/s0928-4257(00)00206-0. [DOI] [PubMed] [Google Scholar]
- 39.Sharma K, Srikant CB. Induction of wild-type p53, Bax, and acidic endonuclease during somatostatin-signaled apoptosis in MCF-7 human breast cancer cells. Int J Cancer. 1998;76:259–266. doi: 10.1002/(sici)1097-0215(19980413)76:2<259::aid-ijc14>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 40.Kang BN, Jeong KS, Park SJ, Kim SJ, Kim TH, Kim HJ, Ryu SY. Regulation of apoptosis by somatostatin and substance P in peritoneal macrophages. Regul Pept. 2001;101:43–49. doi: 10.1016/s0167-0115(01)00264-6. [DOI] [PubMed] [Google Scholar]
- 41.Yuan Y, Gong Z, Lou K, Tu S, Di Z, Xu J. Effects and mechanisms of somatostatin analogs on apoptosis of pancreatic acinar cells in acute pancreatitis in mice. J Gastroenterol Hepatol. 2001;16:683–688. doi: 10.1046/j.1440-1746.2001.02499.x. [DOI] [PubMed] [Google Scholar]


