Abstract
AIM: To detect the expression of CK20mRNA in peripheral blood of pancreatic cancer and evaluate its clinical significance.
METHODS: Expression of CK20mRNA in peripheral blood was detected by fluorogenic qualitative reverse transcription-polymerase chain reaction (RT-PCR) in 40 cases of pancreatic cancer at the night before operation, in 5 cases of benign pancreatic diseases, in 5 cases of healthy individuals. The relationships were investigated between CK20mRNA expression and the clinicopathological variables, and clinical follow-up outcome in those patients with pancreatic cancer having undergone radical resection.
RESULTS: Of the 40 patients with pancreatic cancer, 23 (57.5%) cases were positive for CK20mRNA expression. CK20mRNA expression was significantly correlated with lymphatic metastasis (P = 0.008), histopathological grading (P = 0.009), and pathological stage (P = 0.021); there was no significant correlation between CK20mRNA expression and age, gender, tumor diameter, and depth of invasion. The cumulative metastasis rates of patients with CK20mRNA expression were higher than those of patients with no CK20mRNA expression within 6 mo (34.7% vs 5.9%, P = 0.043) or 12 mo (73.9% vs 35.3%, P = 0.02) after operation. CK20mRNA expression in peripheral blood of pancreatic cancer indicated poorer prognosis. The survival rate of patients with CK20mRNA expression was lower than that of patients with negative CK20mRNA expression (Log-Rank = 13.31, P = 0.0003).
CONCLUSION: CK20mRNA is a sensitive and specific molecular marker for the detection of micrometastasis in peripheral blood of patients with pancreatic cancer. The CK20mRNA expression in peripheral blood is correlated with biological characteristic of pancreatic cancer. It can help to predict the prognosis of pancreatic cancer after operation, and to determine which patient will benefit from aggressive adjuvant therapies.
Keywords: CK20mRNA, Peripheral blood, Pancreatic cancer
INTRODUCTION
Pancreatic cancer is one of the most lethal malignancies with less than 3-5% of the overall five-year survival rate, and the patients normally die within six months after diagnosis. In United States, more than 30000 people were diagnosed and reported dead of pancreatic cancer in 2003, representing the fourth leading cause of cancer death[1]. In China, the incidence and mortality rates of pancreatic cancer have taken an upward trend countrywide. Based on the date of demography and death collected through Chinese Disease Surveillance Point System (DSPS) over the period of 1991-2000, the age-standardized mortality rate due to pancreatic cancer increased from 2.18 in 1991 to 3.26 in 2000 per 1000000 populations and the peak mortality of pancreatic cancer might reach China in the next few decades[2]. The disease is often advanced at first presentation, and only 10-20% patients undergoing surgery have a curative resection[3,4]. Metastasis and relapse are the common factors leading to death of patients[5]. During the development of pancreatic cancer, a few of tumor cells escape from the original focus and disseminate into the lymph system, blood circulation, bone marrow, liver, kidney and other organs, which is called micro-metastasis[6,7]. It cannot be detected by any routine biochemical and histopathological assays or any graphical methods such as X-ray, CT, MRI. At the same time, the patients may not have any obvious symptoms. Minute focus would grow rapidly and develop to metastasize and relapse. So, to develop an efficient diagnostic method for the detection of micrometastasis is of great clinical significance.
Cytokeratin (CK) is observed mainly in epithelial cells, and CK20mRNA was used to detect carcinoma cells in the blood samples in various tumors[8]. In this study, we used fluorogenic qualitative reverse transcriptase polymerase chain reaction (RT-PCR) technique to detect CK20mRNA expression in peripheral blood of patients with pancreatic cancer, and investigated the relationship between CK20mRNA expression and the patient’s clinicopathological variables and prognosis.
MATERIALS AND METHODS
Patients and specimens
Fifty specimens of peripheral blood were collected from forty patients with pancreatic cancer and five patients with benign pancreatic diseases or five normal volunteers in the Department of Surgery of Zhejiang Cancer Hospital from January 2001 to December 2003. Among the 40 patients with pancreatic cancer, 35 cases underwent pancreaticoduodenectomy (Whipple’s operation), 5 cases underwent distal pancreatectomy. None of these patients had distant site metastases, and received either radiation or chemotherapy perioperatively. The final diagnoses of these patients were determined pathologically. All patients were diagnosed with pancreatic ductal adenocarcinoma. The blood specimens were collected on the night before operation, and anti-coagulated with heparin (5 u/mL).
Forty patients with pancreatic cancer consisted of 29 males and 11 females with the mean age of 56.5 years (range from 35-75 years). Histological classification of tumors was based on the World Health Organization (WHO) criteria. All tumors were staged according to the pTNM pathological classification of the UICC (International Union Against Cancer)[9]. Eleven cases were classified as stage II, 29 cases as stage III.
Fluorogenic qualitative RT-PCR
CR20mRNA fluorogenic qualitative RT-PCR Detection kit were purchased from Shanghai Jaochen Biotechnology CO. Total RNA was extracted from the peripheral cells with TRIzol reagent (Gibco, USA) and dissolved in non-sterile DEPC water, stored in ethanol at -20 °C according to manufacturer’s instructions. RT-PCR analysis was performed as follows: The extracted RNA was incubated at 70 °C for 5 min and chilled to 4 °C immediately before reverse transcribing. The RT mixture (5 μL) was incubated for 30 min at 37 °C with 18 μL reverse transcriptase buffer, 1 μg MMLV reverse transcriptase, 1 μ RNasin. For polymerase chain reaction (PCR), 43 μg of PCR buffer, 2 μL TaqMan polymerase, and 5 μL RNA mixture in a total volume of 50 μL were added. The primers of CK20mRNA and TaqMan were synthesized by Life Technology, Shanghai. CK20mRNA upstream primer: 5’ GAGGCACACGGTGAACTATGG 3’, downstream primer: 5’ CATCAGCTTCCACTGTTAGCG 3’; TaqMan fluorogenic probe: 5’ CAGATGGGAACTTCTAGTA 3’. The amplification reaction mixture was amplified through 40 cycles. For each cycle, the amplification reaction mixture was first heated at 95 °C for 5 min to terminate the reverse transcription reaction, and carried out at 95 °C for 30 s, at 62 °C for 30 s, at 72 °C for 30 s, followed by an incubation at 72 °C for 10 min, and then slowly cooled to 4 °C. The same procedure was performed with standard agent, positive control, negative control replaced of the RT mixture in each cycle. According to manufacturer’s instructions, the standard agent must be diluted as 4×108 copy/mL, 4×107 copy/mL, 4×106 copy/mL, and 4×105 copy/mL for each cycle. In each cycle, the amplified products were analyzed by PE7700 (Applied Biosystem, USA). The standard curve of CK20mRNA can be constructed, and the software of Sequence Detector V1.6.3 calculated the copy numbers of samples.
Follow-up
All patients had systematic follow-up examinations. The follow-up ended on Jun 2004. The median duration of follow-up was 17.5 mo (range 6-30 mo). Routine follow-up examinations included clinical examination, CA199, ultrasonography (BUS et al), CT (computed tomography), or SCT (spiral CT), X-ray and so on. The internal (exam?) between each clinical visit was set at 2 mo, but the internal was shortened according to clinical changes, such as CA199, BUS et al. In most patients, tumor metastases were diagnosed by abdominal SCT and CA199. Survival was considered from the day of surgery to the day of death or the most recent follow-up visit, the metastasis rate was computed from the day of surgery to the first follow-up visit that revealed tumor metastasis. Disease-free survival was defined as the combination of the two variables mentioned, considering the date of death or the date of the tumor metastasis as the time of terminal events.
Statistical analysis
Statistical analysis was performed by the SPSS10.0 software package. Associations between CK20mRNA expression and various clinicopathological variables were examined with Fisher’s exact test. Overall survival and disease-free survival were calculated by the Kaplan-Meier Estimate. The Log-Rank test was performed to compare survival time distributions between curves. P<0.05 was considered statistically significant.
RESULTS
Fluorogenic qualitative analysis of RT-PCR
According to the manufacturer’s instructions of the CK20mRNA kit, the PE7700 sequence detector allowed measurement of the fluorescent spectra of all 96 wells of the thermal cycle in realtime. Using the fluorescent emission data collected during the PCR amplification, the software constructed amplification plots. The measurements from 3 until 15 cycles were considered the baseline and its standard deviation was calculated. Ct (threshold cycle) values were calculated by determining the point at which the fluorescence exceeded a threshold limit. With the standard curve (Figure 1), the copy numbers of the samples were calculated. Among 40 patients with pancreatic cancer, 23 (57.5%) cases were positive for CK20mRNA expression in peripheral blood. The quantitative of CK20mRNA were 6017.58±4993.34 copy/mL. There was no CK20mRNA expression in benign pancreatic disease or healthy individuals.
Figure 1.
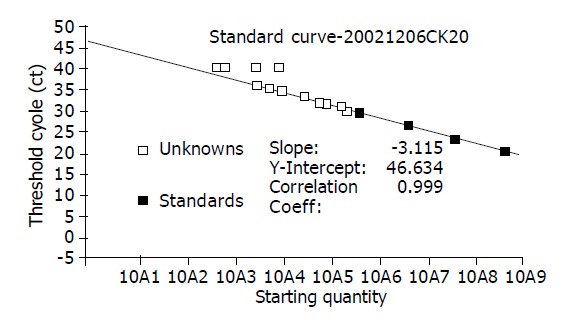
CK20mRNA Standard Curve: 3 until 15 cycles were the baseline and its standard deviation was calculated. Ct (threshold cycle) values were calculated by determining the point at which the fluorescence exceeded a threshold limit.
Correlations between CK20mRNA expression and clinicopathological variables
Correlations between RT-PCR CK20mRNA in peripheral blood and various clinicopathological features were summarized in Table 1. There were significant correlations between CK20mRNA expression and histopathological grading (P = 0.009), lymph node metastasis (P = 0.008), post-operative pathological stage (P = 0.021). No significant correlation was found between CK20mRNA expression and age, gender, tumor diameter and depth of invasion.
Table 1.
Associations between CK20mRNA and clinicopathologic variables in pancreatic cancer (Fisher’s exact test).
| Variable | n | CK20 (+) | CK20 (-) | P-value |
| Sex | ||||
| Male | 28 | 16 | 12 | 0.612 |
| Female | 12 | 7 | 5 | |
| Age (yr) | ||||
| ≥65 | 19 | 9 | 10 | 0.218 |
| <60 | 21 | 14 | 7 | |
| Tumor size | ||||
| ≥3 cm | 30 | 17 | 13 | 0.577 |
| <3 cm | 10 | 6 | 4 | |
| Tumor differentiation | ||||
| High differentiation | 14 | 4 | 10 | 0.009 |
| Moderate+poor | 26 | 19 | 7 | |
| Lymph node metastasis | ||||
| Negative | 10 | 2 | 8 | 0.008 |
| Positive | 30 | 21 | 9 | |
| Tumor stage (TNM) | ||||
| II | 11 | 3 | 8 | 0.021 |
| III | 29 | 20 | 9 | |
| Depth of tumor invasion | ||||
| T1-T2 | 12 | 5 | 7 | 0.164 |
| T3-T4 | 28 | 18 | 10 |
Correlations between CK20mRNA expression and tumor metastases after operation
At the end of follow-up, 8 (34.7%) of 23 patients with positive CK20mRNA expression developed distant metastases (mainly liver metastasis and retroperitoneal lymph node metastasis) within 6 mo after operation; 1 (5.9%) of 17 patients with negative CK20mRNA expression developed liver metastasis within 6 mo after operation. There was a significant difference between the two groups (P = 0.043). Within 12 mo after operation, the cumulative metastasis rates of positive and negative CK20mRNA expression were 73.9%, 35.3% respectively (P = 0.02). Within 24 mo after operation, the cumulative metastasis rates of the two groups were 82.6% and 76.4% respectively (P = 0.63). There was no significant difference (Table 2).
Table 2.
CK20mRNA expression and cumulative tumor metastasis after operation (Fisher’s exact test).
| CK20mRNA expression | n | Within 6 mo | Within 12 mo | Within 24 mo |
| Positive | 23 | 8 (34.7%) | 17 (73.9%) | 19 (82.6%) |
| Negative | 17 | 1 (5.9%) | 6 (35.3%) | 13 (76.5%) |
| P-value | 0.043 | 0.02 | 0.631 |
CK20mRNA expression and survival
At the end of follow-up, the median disease-free survival time for the patients with positive CK20mRNA expression was 10 mo after operation, 16 mo for negative CK20mRNA expression; the median overall survival time of positive CK20mRNA expression was 15 mo after operation, 23 mo for negative CK20mRNA expression (Table 3). The overall survival curve was shown in Figure 2. The overall survival rate was significantly correlated with CK20mRNA expression in the peripheral blood of pancreatic cancer (Log-Rank = 13.31, P = 0.0003).
Table 3.
CK20mRNA expression and survival time after operation.
| CK20mRNA expression |
Disease-free survival time |
Overall survival time |
||
| Median (mo) | 95%CI | Median (mo) | 95%CI | |
| Positive | 10 | 5.97-14.21 | 15 | 13.93-16.07 |
| Negative | 16 | 11.36-20.64 | 23 | 17.15-28.85 |
Kaplan-Meier method, 95%CI (95% confidence interval).
Figure 2.
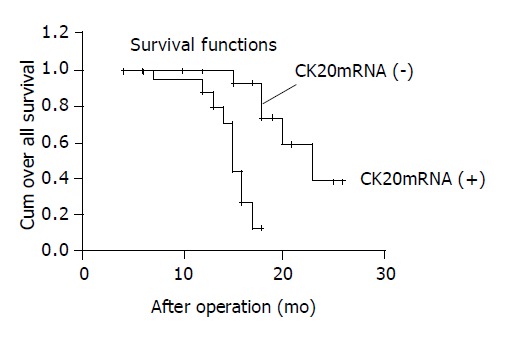
Kaplan-Meier analysis for overall survival according to CK20mRNA expression in patients with pancreatic cancer (log-rank = 13.31, P = 0.0003).
DISCUSSION
CK is a component of cell skeleton, which distributes in cells deriving from ectoderm. CK20, a member of this family, has been found to have a stricter selectivity to epithelial tissue. CK20mRNA could not be detected from the peripheral blood circulation of healthy people. If CK20mRNA was detected from the peripheral blood circulation of patients, there were live cells expression of CK20mRNA existing in blood specimens, hemocytes came from mesoderm and there were no epithelial cells in blood circulation. So, when CK20mRNA was amplified from blood circulation, there must be tumor cells in the specimens. If the specimens were extracted from pancreatic cancer patients, the tumor cells must be pancreatic cancer cells. It has been reported that CK20mRNA was extensively used as a probe to detect the presence of circulating tumor cells in the blood samples of various epithelial tumors such as gastrointestinal cancer[10,11], colorectal cancer[12], breast carcinoma[13], oral cavity, pharynx and larynx cancer[14], renal neoplasm[15], hepatocellular carcinoma[16], thyroid tumor[17]. Meanwhile, it has been confirmed that CK20mRNA showed relatively high sensitivity, specificity and positive predictive value to detect tumor micrometastasis.
Traditional methods to detect micrometastasis in the pancreatic cancer such as cell morphology, flow cytometer and cytogenetics were not so sensitive. In recent years, fluorogenic quantitative RT-PCR was invented and it has a high efficiency in detecting the micrometastasis of peripheral blood circulation, bone morrow, lymph tract and peritoneal cavity. The method has a high sensitivity, precision, reproducibility and without being false positive or false negative. It has been presently regarded as the most effective method to detect tumor micrometastasis[18-21]. In this study, we used fluorogenic qualitative RT-PCR method to detect CK20mRNA expression in circulating blood of 40 patients with pancreatic cancer. We found the CK20mRNA expression very frequently, in 57.5% (23/40) of pancreatic cancer, and no CK20mRNA expression in normal or benign pancreatic diseases. In the present study, we evaluated the relationships between CK20mRNA expression in peripheral blood and the clinicopathological variables of pancreatic cancer, and found CK20mRNA expression was a significant correlation with tumor staging, tumor differentiation, and lymph node metastasis. It seems likely that CK20mRNA expression might be relevant to neoplastic progression. A similar correlation was reported in gastric carcinoma[11], colorectal cancer[11], and breast cancer[13].
Great progress has been made in the early diagnosis and treatment of pancreatic cancer in recent years, but the survival of patients with pancreatic cancer even after a potentially curative resection remains poor, and patients generally succumb to metastatic diseases. Pancreatic cancer has a high potential for liver metastasis or other distant metastases. Michael reported that 16 (73%) of 22 patients with pathologic Stage I pancreatic adenocarcinoma, which suggested metastases not detected by routine histopathology, had occult metastases[22]. In our study, we found high incidence of CK20mRNA expression in peripheral blood of Stage II or stage III pancreatic cancer. The high incidence of CK20mRNA expression in peripheral blood might be one of the mechanisms contributing to the dismal outcome of patients with completely resected pancreatic cancer. Consistent with this hypothesis, we found that the cumulative metastasis rates of the patients with positive CK20mRNA expression were significantly higher than those of negative CK20mRNA expression within 6 or 12 mo after operation. Moreover, the overall survival rate of patients with positive CK20mRNA expression was significantly lower than that of patients with negative CK20mRNA expression. Our ability to detect gross metastatic diseases at the time of operation was good, but the detection of micrometastases and circulating cancer cells is not part of clinical practice. Detection of CK20mRNA expression in peripheral blood seems to be somewhat useful to predict the occurrence of micrometastases in pancreatic cancer, and to determine which patients would benefit from aggressive adjuvant therapies[23].
In conclusion, CK20mRNA is a sensitive and specific molecular marker for the detection of micrometastasis in peripheral blood of patients with pancreatic cancer. Fluorogenic Quantitative RT-PCR has a high sensitivity, precision, and reproducibility. The CK20mRNA expression in peripheral blood is correlated with biological characteristics of pancreatic cancer. CK20mRNA expression in peripheral blood may help to predict the prognosis of pancreatic cancer after operation, and to determine aggressive adjuvant therapies.
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Yang GH, Lu XH, Huang ZJ, Li H. Pancreatic cancer mortality in China (1991-2000) World J Gastroenterol. 2003;9:1819–1823. doi: 10.3748/wjg.v9.i8.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhlmann KF, de Castro SM, Wesseling JG, ten Kate FJ, Offerhaus GJ, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549–558. doi: 10.1016/j.ejca.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Zhang QH, Ni QX. Clinical analysis of 2340 cases of pancreatic cancer. Zhonghua YiXue ZaZhi. 2004;84:214–218. [PubMed] [Google Scholar]
- 5.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirata H, Hisatomi H, Kawakita M, Nagao K, Yamamoto S, Hikiji K, Nakamoto T, Harasawa H, Kaneko N, Matsuda T, et al. Genetic detection for hematogenous micrometastasis in patients with various types of malignant tumors using Uroplakin II derived primers in polymerase chain reaction. Oncol Rep. 2003;10:963–966. [PubMed] [Google Scholar]
- 7.Yamaguchi K, Chijiiwa K, Torato N, Kinoshita M, Tanaka M. Ki-ras codon 12 point and P53 mutations: a molecular examination of the main tumor, liver, portal vein, peripheral arterial blood and para-aortic lymph node in pancreatic cancer. Am J Gastroenterol. 2000;95:1939–1945. doi: 10.1111/j.1572-0241.2000.02081.x. [DOI] [PubMed] [Google Scholar]
- 8.Ikeguchi M, Ohro S, Maeda Y, Fukuda K, Yamaguchi K, Shirai H, Kondo A, Tsujitani S, Kaibara N. Detection of cancer cells in the peripheral blood of gastric cancer patients. Int J Mol Med. 2003;11:217–221. [PubMed] [Google Scholar]
- 9.Sobin LN, Wittekind CH, editors . UICC TMN classification of malignant tumors. 5th ed. New York: John Wiley and Sons, Inc; 1997. [Google Scholar]
- 10.Kim MA, Lee HS, Yang HK, Kim WH. Cytokeratin expression profile in gastric carcinomas. Hum Pathol. 2004;35:576–581. doi: 10.1016/j.humpath.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Huang P, Wang J, Guo Y, Xie W. Molecular detection of disseminated tumor cells in the peripheral blood in patients with gastrointestinal cancer. J Cancer Res Clin Oncol. 2003;129:192–198. doi: 10.1007/s00432-003-0425-y. [DOI] [PubMed] [Google Scholar]
- 12.Lee MJ, Lee HS, Kim WH, Choi Y, Yang M. Expression of mucins and cytokeratins in primary carcinomas of the digestive system. Mod Pathol. 2003;16:403–410. doi: 10.1097/01.MP.0000067683.84284.66. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto J, Ohshima K, Nabeshima K, Ikeda S, Iwasaki H, Kikuchi M. Comparative study of primary mammary small cell carcinoma, carcinoma with endocrine features and invasive ductal carcinoma. Oncol Rep. 2004;11:825–831. [PubMed] [Google Scholar]
- 14.Ueno T, Hoshii Y, Cui D, Kawano H, Gondo T, Takahashi M, Ishihara T. Immunohistochemical study of cytokeratins in amyloid deposits associated with squamous cell carcinoma and dysplasia in the oral cavity, pharynx and larynx. Pathol Int. 2003;53:265–269. doi: 10.1046/j.1440-1827.2003.01472.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim MK, Kim S. Immunohistochemical profile of common epithelial neoplasms arising in the kidney. Appl Immunohistochem Mol Morphol. 2002;10:332–338. doi: 10.1097/00129039-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Tickoo SK, Zee SY, Obiekwe S, Xiao H, Koea J, Robiou C, Blumgart LH, Jarnagin W, Ladanyi M, Klimstra DS. Combined hepatocellular-cholangiocarcinoma: a histopathologic, immunohistochemical, and in situ hybridization study. Am J Surg Pathol. 2002;26:989–997. doi: 10.1097/00000478-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz-Winnenthal FH, Weckauf H, Haufe S, Hinz U, Z'graggen K, Klar E, Büchler MW, Weber T. Detection and prognostic relevance of cytokeratin 20 in differentiated and anaplastic thyroid carcinomas by RT-PCR. Surgery. 2003;134:964–971; discussion 971-972. doi: 10.1016/s0039-6060(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 18.Lowe B, Avila HA, Bloom FR, Gleeson M, Kusser W. Quantitation of gene expression in neural precursors by reverse-transcription polymerase chain reaction using self-quenched, fluorogenic primers. Anal Biochem. 2003;315:95–105. doi: 10.1016/s0003-2697(02)00695-4. [DOI] [PubMed] [Google Scholar]
- 19.Höhne M, Schreier E. Detection and characterization of norovirus outbreaks in Germany: application of a one-tube RT-PCR using a fluorogenic real-time detection system. J Med Virol. 2004;72:312–319. doi: 10.1002/jmv.10573. [DOI] [PubMed] [Google Scholar]
- 20.Decaro N, Pratelli A, Campolo M, Elia G, Martella V, Tempesta M, Buonavoglia C. Quantitation of canine coronavirus RNA in the faeces of dogs by TaqMan RT-PCR. J Virol Methods. 2004;119:145–150. doi: 10.1016/j.jviromet.2004.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puig M, Mihalik K, Yu MY, Feinstone SM, Major ME. Sensitivity and reproducibility of HCV quantitation in chimpanzee sera using TaqMan real-time PCR assay. J Virol Methods. 2002;105:253–263. doi: 10.1016/s0166-0934(02)00119-2. [DOI] [PubMed] [Google Scholar]
- 22.Demeure MJ, Doffek KM, Komorowski RA, Wilson SD. Adenocarcinoma of the pancreas: detection of occult metastases in regional lymph nodes by a polymerase chain reaction-based assay. Cancer. 1998;83:1328–1334. [PubMed] [Google Scholar]
- 23.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]


