Abstract
AIM: To clarify the mechanism underlying the anti-mutagenic and anti-cancer activities of Scorpio water extract (SWE).
METHODS: Human hepatoma HepG2 cells were incubated with various concentrations of SWE. After 24-h incubation, cytotoxicity and apoptosis evaluations were determined by MTT and DNA fragmentation assay, respectively. After treatment with SWE, mitochondrial membrane potential (MMP) was determined by measuring the retention of the dye 3,3’-dihexyloxacarbocyanine (DiOC6(3)) and the protein expression including cytochrome C and poly-(ADP-ribose) polymerase (PARP) were measured by Western blotting. Caspase-3 and -9 enzyme activities were measured using specific fluorescence dyes such as Ac-DEVD-AFC and Ac-LEHD-AFC.
RESULTS: We found that treatment with SWE induced apoptosis as confirmed by discontinuous DNA fragmentation in cultured human hepatoma HepG2 cells. Our investigation also showed that SWE-induced apoptosis of HepG2 cells were associated with intracellular events including disruption of MMP, increased translocation of cytochrome C from mitochondria to cytosol, activation of caspase-3, and PARP. Pre-treatment of N-acetyl-Asp-Glu-Val-Asp-CHO (Ac-DEVD-CHO), a caspase-3 specific inhibitor, or cyclosporin A (CsA), an inhibitor of MMP disruption, completely abolished SWE-induced DNA fragmentation.
CONCLUSION: These results suggest that SWE possibly causes mitochondrial damage, leading to cytochrome C release into cytosol and activation of caspases resulting in PARP cleavage and execution of apoptotic cell death in HepG2 cells. These results further suggest that Scorpio may be a valuable agent of therapeutic intervention of human hepatomas.
Keywords: Scorpio, Human hepatoma HepG2 cell, Apoptosis
INTRODUCTION
Recently, some herbs have been shown to be potent cancer- protective or cancer- preventive agents against chemical-induced carcinogenesis model in vivo[1] and cancer cells in vitro[1-3]. The use of these herbs has attracted a great deal of attention as one of alternative cancer therapies from the viewpoint of less toxicity and cost benefit.
Scorpio is the whole body of Buthus martensii Karsch and belongs to Buthidae[4]. It is commonly used in alleviating pain and treating liver disease and cancer. Experimentally, it was reported that Scorpio has anti-tumor promoting activity in two-stage mouse skin and lung carcinogenesis induced by chemical carcinogens and cytotoxic effect on human hepatoma HepG2 cells[5,6]. However, the events leading to cell-death after Scorpio treatment are not well understood.
Apoptosis, or programed cell death, plays a central role in the development and homeostasis of all multicellular organisms[7,8] and is characterized by loss of plasma membrane phospholipid asymmetry, chromatin condensation, and discontinuous DNA fragmentation[9]. Suppression of the apoptotic machinery is a hallmark of cancer. Therefore, induction of apoptosis of cancer-cells is a useful method, as a valuable tool for cancer treatment[10].
Many investigators have demonstrated that mitochondria are key regulators of apoptosis[11,12]. Mitochondria have shown to be involved in integrating different pro-apoptotic pathways via release of cytochrome C into cytosol[11,13], which is associated with loss of mitochondrial membrane potential (MMP) and an increased production of reactive oxygen species[14]. The released cytochrome C induces the activation of a family of caspases (aspartate-specific cystein proteases)[15]. Caspase activity is responsible, either directly or indirectly, for cleavage of cellular proteins including poly-(ADP-ribose) polymerase (PARP)[16], nuclear lamin[17] and inhibitors of deoxyribonuclease (such as DFF45 or ICAD)[18] during apoptosis.
In the present study, we demonstrated that Scorpio water extract (SWE) could induce apoptosis of human hepatoma HepG2 cells. Additionally, to investigate the mechanisms, we measured the loss of MMP, cytochrome C release from mitochondria, activations of caspase-9 and -3 and the cleavage of PARP in SWE-treated HepG2 cells.
MATERIALS AND METHODS
Materials
Culture medium RPMI 1640, fetal bovine serum and trypsin-EDTA were from Gibco Ltd. Paisley and Strathclyde were from UK. DiOC6(3) were from Molecular Probes (Eugene, Oregon, USA). Mouse IgG1 monoclonal antibodies against PARP and cytochrome C, N-acetyl-Asp-Glu-Val-Asp-AFC (Ac-DEVD-AFC), Acetyl-Leu-Glu-His-Asp-AFC (Ac-LEHD-AFC), N-acetyl-Asp-Glu-Val-Asp-CHO (Ac-DEVD-CHO) were from BD PharMingen (Franklin Lakes, NJ, USA). Cyclosporin A (CsA), anti-mouse IgG antibody conjugated with alkaline phosphatase were from Sigma (Saint Louis, Missouri, USA).
Cell culture
The human hepatoma HepG2 cell line was purchased from the American Type Culture Collection. Cells were placed into 75 cm2 tissue culture flasks and grown at 37 °C under a humidified, 50 mL/L CO2 atmosphere in RPMI 1640 medium (Gibco, BRL) supplemented with 10% fetal bovine serum and 2 mmol/L glutamine, 10000 units/mL of penicillin, 10 mg/mL of streptomycin, and 2.5 μg/mL of amphotericin B.
Preparation of Scorpio water extract (SWE)
The herb was identified as Scorpio by local experts. Voucher samples were preserved for reference in the herbarium of Department of Physiology, School of Oriental Medicine, Wonkwang University (Omcphy, 2001-40-d). For extraction, 200 g of Scorpio was added to 1800 mL of water and boiled for 2 h, filtered and then concentrated to 200 mL. The sterile extract (57.67 g) was stored at -20 °C.
MTT assay for cell viability
The viability of cultured cells was determined by assaying for the reduction of MTT to formazan[19]. In brief, after incubation with SWE, cells (104/well) in 96-well plates were washed twice with PBS and MTT (100 μg/0.1 mL of PBS) was added to each well. Cells were incubated at 37 °C for 1 h, and DMSO (100 μL) was added to dissolve the formazan crystals. Absorbance was measured at 570 nm with a spectrophotometer (model E-MAX, Molecular Devices, USA).
Measurement of mitochondrial membrane potential (MMP)
MMP was determined as described[20] by measuring the retention of the dye 3,3’-dihexyloxacarbocyanine (DiOC6(3). Briefly, cells (5×105 in 500 μL of complete RPMI 1640 medium) were loaded with 100 nmol/L DiOC6(3) during the last 30 min of treatment. The cells were then pelleted by centrifugation at 700 g for 10 min. The supernatant was removed and the pellet was re-suspended and washed twice in PBS. The pellet was then lysed by the addition of 600 μL of deionized water followed by homogenization. The concentration of retained DiOC6(3) was read on a spectrofluorometer (F-2500, Hitachi, Japan) with an excitation wavelength of 488 nm and an emission wavelength of 500 nm.
Detection of cytochrome C release
The release of mitochondrial cytochrome C was determined by Western blot[21]. Briefly, at the end of various designated treatments, cells (1.5×107) were washed with PBS, and re-suspended in ice-cold homogenizing buffer (250 mmol/L sucrose, 20 mmol/L Hepes-KOH (pH 7.5), 10 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L DTT, 1 mmol/L PMSF, 1 μg/mL aprotinin, and 1 μg/mL leupeptin). After 30-min incubation on ice, cells were homogenized with a glass Dounce homogenizer (30 strokes). The homogenate was subjected to a series of centrifugations at 100000 g for 60 min for the collection of mitochondrial pellets and cytosolic fraction. Thirty micrograms of protein was loaded on 15% SDS gel. After electrophoretic separation, the proteins were transferred to nitrocellulose membrane (Millipore, Bedford, MA, USA) using a semi-dry blotting apparatus (Bio-Rad, Munich, Germany), and the blot was incubated with mouse anti-cytochrome C antibody (Pharmingen, San Diego, CA, USA), followed by reaction with alkaline phosphatase conjugated secondary antibody.
Caspase activity assay
After treatment with SWE, cells were washed with ice-cold PBS and lysed in Triton X-100 buffer (5 mL/L Triton X-100, 10 mmol/L EDTA, and 10 mmol/L Tris–HCl, pH 7.5) for 30 min on ice. Cell lysates were mixed with caspase assay buffer (100 mL/L glycerol, 2 mmol/L DTT, and 20 mmol/L HEPES, pH 7.5) containing 20 μmol/L Ac-DEVD-AFC (specific for caspase-3) and 50 μmol/L Ac-LEHD-AFC (specific for caspase-9), caspase substrates, and incubated for 1 h at 37 °C. Enzyme catalyzed release of AFC was monitored using a spectrofluorometer (F-2500, Hitachi, Japan) with an excitation wavelength of 400 nm and an emission wavelength of 505 nm.
Western blot analysis of PARP cleavage
Cell extract proteins were separated by SDS-PAGE. Subsequently the proteins were transferred onto a nitrocellulose membrane using a semi-dry blotting apparatus. Prior to incubation with antibodies against PARP, membranes were blocked with 20 g/L BSA for 30 min. After the membranes were washed, an alkaline-phosphatase coupled secondary antibody was added. The target proteins became visible following the addition of 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (BCIP/NBT), a substrate of alkaline phosphatase.
Detection of DNA fragmentation by gel electrophoresis
Cell pellets (3×106) were resuspended in 500 μL of lysis buffer (5 mL/L Triton X-100, 10 mmol/L EDTA, and 10 mmol/L Tris–HCl, pH 8.0) at room temperature for 15 min and centrifuged at 16000 g for 10 min. DNA was then extracted twice with phenol/chloroform (1:1), precipitated with ethanol, and resuspended in Tris/EDTA buffer (10 mmol/L Tris-HCl, pH 8.0, and 1 mmol/L EDTA). DNA was analyzed after separation by gel electrophoresis (20 g/L agarose).
Protein determination
The cytosolic protein concentration in HL-60 cells was determined by the method of Bradford[22] with bovine serum albumin as the standard. All the samples were assayed in triplicate.
Statistical analysis
Statistical analysis of the data was performed with Student’s t-test and ANOVA. Differences with P<0.05 were considered statistically significant.
RESULTS
Induction of apoptosis by SWE in HepG2 cells
The effect of SWE on the viability of HepG2 cells was examined using the MTT staining. SWE decreased cell viability in a dose-dependent manner as shown in Figure 1A. A significant cytotoxicity by SWE was started at the concentration of 1.0 mg/mL. The IC50 of SWE for HepG2 was less than 2.0 mg/mL. We further examined whether SWE induced apoptotic cell death in HepG2 cells. In DNA agarose gel electrophoresis, a ladder of fragmented DNA was detected 12 h after incubation and sustained until 24 h in 2.0 mg/mL SWE-treated HepG2 cells, as shown in Figure 1B. These results showed that SWE had an activity in inducing apoptosis of HepG2 human hepatoma cells.
Figure 1.
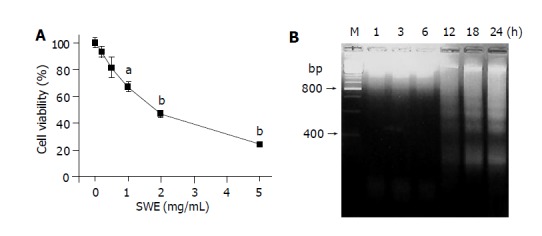
Effects of SWE on cell viability (A) and DNA fragmentation (B). A: Cells (1×104) treated with various concentrations of SWE for 24 h; B: Cells (3×106) treated with 2.0 mg/mL SWE for the indicated time periods. aP<0.05, bP<0.01 vs control.
Role of mitochondrial alteration in SWE-treated HepG2 cells
Because mitochondria were known to play an important role in apoptosis, we examined whether SWE induced loss of mitochondrial membrane potential (MMP) assessed by DiOC6(3) fluorescence. Treatment with 2.0 mg/mL SWE represented a time- dependent loss of DiOC6(3) fluorescence, which was inhibited by preincubation with cyclosporin A (CsA), a known inhibitor of mitochondrial permeability transition (MPT) for 30 min in HepG2 cells (Figures 2A and B). The MPT released cytochrome C from mitochondria into cytosol. SWE-treated HepG2 cells produced a release of cytochrome C to the cytosol and a concomitant decrease in the content of cytochrome C in the mitochondria, which was almost prevented by pretreatment with CsA (Figure 2C).
Figure 2.
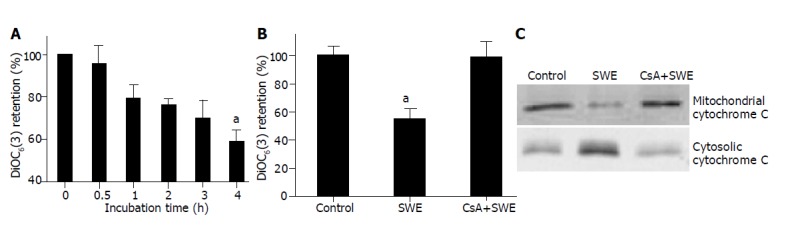
Role of mitochondrial alterations in SWE-treated HepG2 cells. A: Cells (5×105) treated with 2.0 mg/mL SWE for the indicated time periods; B and C: Cells pretreated for 30 min with 5 μmol/L CsA and then treated with 2.0 mg/mL SWE for 6 h. aP<0.05 vs control.
Activation of caspase-3 and cleavage of PARP in SWE-treated HepG2 cells
Based on increased apoptosis of SWE-treated HepG2 cells, our next aim was to examine the involvement of caspases that play a major role in the execution of apoptotic events. As shown in Figures 3A and B, SWE caused concentration and time- dependent activation of caspase-3, but the activation of caspase-9 did not occur (data not shown). These results were well correlated with a decrease of viability and a discontinuous DNA fragmentation as depicted in Figure 1. Since PARP is one of the down stream substrates of caspase-3, we examined the effect of SWE-induced caspase-3 activation on PARP cleavage, which separates N-terminal DNA-binding domain from its C-terminal catalytic domain (85 ku). Our immunoblot analysis showed a time-dependent cleavage of PARP that corresponded to and further supported the possible involvement of caspase-3 activation in SWE-caused apoptosis of HepG2 cells (Figure 3C).
Figure 3.
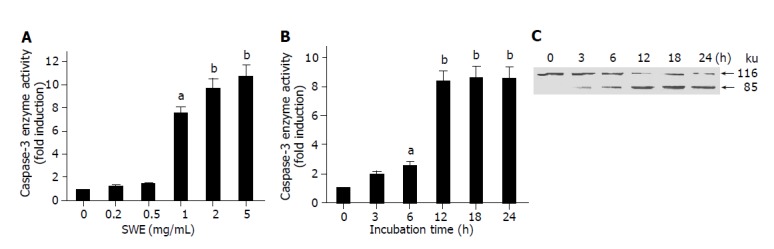
SWE induces the activation of caspase-3 and PARP cleavage in HepG2 cells. A: HepG2 cells (1.5×107) treated with 0-5.0 mg/mL of SWE for 24 h; B and C: HepG2 cells treated with 2.0 mg/mL SWE for the indicated time periods. aP<0.05, bP<0.01 vs control.
Inhibition of SWE-induced apoptosis by CsA and Ac-DEVD-CHO
To further confirm the involvement of caspase-3 activation and MMP loss in SWE-induced apoptosis of HepG2 cells, we employed pharmacological inhibitors such as Ac-DEVD-CHO, a specific inhibitor of caspase-3 activation, and CsA, an inhibitor of MMP loss. Pre-treatment with 25.0 μmol/L Ac-DEVD-CHO and 5.0 μmol/L CsA inhibited both the activation of caspase-3 and the cleavage of PARP by SWE in HepG2 cells (Figures 4A and B). Also, these inhibitors prevented SWE-induced DNA fragmentation, which is one of the characteristic signs of apoptosis (Figure 4C).
Figure 4.
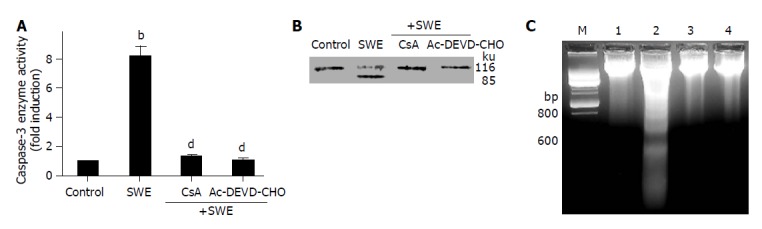
Inhibition of SWE-induced caspase-3 activation (A), PARP cleavage (B) and DNA fragmentation (C) by caspase-3 inhibitor or MPT inhibitor. HepG2 cells were pretreated with Ac-DEVD-CHO (25 μmol/L) for 1 h or CsA (5 μmol/L) for 30 min followed by treatment with 2.0 mg/mL SWE for further 24 h. Lane M: DNA marker, lane 1: control, lane 2: 2.0 mg/mL SWE, lane 3: 2.0 mg/mL SWE with 5 μmol/L CsA, lane 4: 2.0 mg/mL SWE with 25 μmol/L Ac-DEVD-CHO. bP<0.01 vs control; dP<0.01 vs SWE alone.
DISCUSSION
Previously, SWE has been reported to have an anti-tumor activity. However, the mechanisms of these actions of SWE are completely unknown. In the present study, we examined the effect of SWE on the proliferation of human hepatoma cell lines, HepG2 cells. Our results demonstrated that SWE inhibited HepG2 cell growth in a concentration-dependent manner. Moreover, cultured HepG2 cells treated with SWE exhibited a characteristic pattern of apoptosis such as DNA ladder.
Many reports suggested that mitochondria play an important role in apoptosis. Disruption of MMP plays a pivotal role in the initiation of apoptotic induction and is related to the release of cytochrome C[11,13,23]. Mitochondrial permeability transition (MPT) causes loss of the MMP and cytochrome C release from mitochondria into cytosol[20]. The fluorescent dye DiOC6(3) localizes at mitochondria as a consequence of MMP, and MPT reduces accumulation of DiOC6(3)[24,25]. In HepG2 cells, SWE produced a time-dependent loss of DiOC6(3) (Figure 2A) and released cytochrome C from mitochondria into cytosol. Participation of the MPT in SWE-induced apoptosis of HepG2 cells was shown by the observation that CsA, a known inhibitor of MPT, prevented the discontinuous DNA fragmentation (Figure 4C), as well as the loss of MMP (Figure 2B) and cytochrome C release (Figure 2C).
Cytochrome C released from mitochondria during apoptosis promotes the activation of caspase-3[20,26]. Caspase (cystein aspartate-specific proteases) family has been shown to play a central role in the initiation and execution of apoptosis[27,28]. The caspase family of proteases consists of at least 14 mammalian members that are constitutively expressed in almost all cell types[29]. Among them, caspase-3 is activated during most apoptotic processes and is believed to be the main executor caspase. In SWE-induced apoptosis of HepG2 cells, caspase-3 enzyme was activated in a dose- and time-dependent fashion (Figures 3A and B). Also, using DNA fragmentation assay, we observed that HepG2 cell apoptosis could be prevented by inhibiting caspase activity with caspase-3 inhibitors such as Ac-DEVD-CHO (Figure 4C). Many intracellular stimuli can activate caspase-3 including apoptosis-inducing factor (AIF), heat shock proteins (HSPs)[28], direct IAP binding proteins such as DIABLO/Smac, and several procaspases. Although we confirmed that cytochrome C released from mitochondria could induce the activation of caspase-3, further studies may be needed about other factors involved in SWE-induced apoptotic cascade of HepG2 cells.
The activation of caspase-3 induces characteristic patterns of apoptosis such DNA fragmentation and chromatin condensation through activation or inactivation by the cleavage of cellular substrates including lamin, actin, α-fodrin, PARP, and ICAD (inhibitor of caspase activated DNase)[16,17]. The increased caspase-3 activity in SWE-treated cells was accompanied with a decrease of PARP (116 ku) and concomitant increase of 85 ku (Figure 3C). The cleavage of PARP was prevented by the presence of either Ac-DEVD-CHO or CsA, indicating an essential role of the activation of caspase-3 and the loss of MMP in SWE-induced apoptosis of HepG2 cells.
In conclusion, SWE induces apoptosis through the mechanisms involved in MMP loss, the release of cytochrome C from mitochondria, and activation of caspase-3 with the consequent degradation of PARP in HepG2 cells.
Footnotes
Supported by Wonkwang University (2004)
References
- 1.Huerta S, Arteaga JR, Irwin RW, Ikezoe T, Heber D, Koeffler HP. PC-SPES inhibits colon cancer growth in vitro and in vivo. Cancer Res. 2002;62:5204–5209. [PubMed] [Google Scholar]
- 2.Iizuka N, Miyamoto K, Okita K, Tangoku A, Hayashi H, Yosino S, Abe T, Morioka T, Hazama S, Oka M. Inhibitory effect of Coptidis Rhizoma and berberine on the proliferation of human esophageal cancer cell lines. Cancer Lett. 2000;148:19–25. doi: 10.1016/s0304-3835(99)00264-5. [DOI] [PubMed] [Google Scholar]
- 3.Kao ST, Yeh CC, Hsieh CC, Yang MD, Lee MR, Liu HS, Lin JG. The Chinese medicine Bu-Zhong-Yi-Qi-Tang inhibited proliferation of hepatoma cell lines by inducing apoptosis via G0/G1 arrest. Life Sci. 2001;69:1485–1496. doi: 10.1016/s0024-3205(01)01226-7. [DOI] [PubMed] [Google Scholar]
- 4.Ou M. Chinese-english manual of common-used in traditional Chinese medicine. 2nd ed. Hong Kong: Joint Publishing Co Ltd; 2000. pp. 232–233. [Google Scholar]
- 5.Jeong IC, Yoon CH, Jeong JC. Effects of Buthus martensi Karsch on tumor promotion in two-stage carcinogenesis in mice. Korean J Orient Int Med. 2000;21:251–257. [Google Scholar]
- 6.Kim SH, Kim KS. The antimutagenic effect and genetic safety of Buthus martensi Karsch aqua-acupuncture solution. J Korean Acupuncture Moxibustion Soc. 2000;17:151–167. [Google Scholar]
- 7.Horvitz HR. Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res. 1999;59:1701s–1706s. [PubMed] [Google Scholar]
- 8.Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 9.Kidd VJ. Proteolytic activities that mediate apoptosis. Annu Rev Physiol. 1998;60:533–573. doi: 10.1146/annurev.physiol.60.1.533. [DOI] [PubMed] [Google Scholar]
- 10.Kornblau SM. The role of apoptosis in the pathogenesis, prognosis, and therapy of hematologic malignancies. Leukemia. 1998;12 Suppl 1:S41–S46. [PubMed] [Google Scholar]
- 11.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 12.Susin SA, Zamzami N, Kroemer G. Mitochondria as regulators of apoptosis: doubt no more. Biochim Biophys Acta. 1998;1366:151–165. doi: 10.1016/s0005-2728(98)00110-8. [DOI] [PubMed] [Google Scholar]
- 13.Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, Macho A, Haeffner A, Hirsch F, Geuskens M, Kroemer G. Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med. 1996;184:1155–1160. doi: 10.1084/jem.184.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiang J, Chao DT, Korsmeyer SJ. BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc Natl Acad Sci USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 16.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi A, Alnemri ES, Lazebnik YA, Fernandes-Alnemri T, Litwack G, Moir RD, Goldman RD, Poirier GG, Kaufmann SH, Earnshaw WC. Cleavage of lamin A by Mch2 alpha but not CPP32: multiple interleukin 1 beta-converting enzyme-related proteases with distinct substrate recognition properties are active in apoptosis. Proc Natl Acad Sci USA. 1996;93:8395–8400. doi: 10.1073/pnas.93.16.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459–470. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- 19.Oez S, Platzer E, Welte K. A quantitative colorimetric method to evaluate the functional state of human polymorphonuclear leukocytes. Blut. 1990;60:97–102. doi: 10.1007/BF01720515. [DOI] [PubMed] [Google Scholar]
- 20.Pastorino JG, Chen ST, Tafani M, Snyder JW, Farber JL. The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J Biol Chem. 1998;273:7770–7775. doi: 10.1074/jbc.273.13.7770. [DOI] [PubMed] [Google Scholar]
- 21.Kwon KB, Yang JY, Ryu DG, Rho HW, Kim JS, Park JW, Kim HR, Park BH. Vibrio vulnificus cytolysin induces superoxide anion-initiated apoptotic signaling pathway in human ECV304 cells. J Biol Chem. 2001;276:47518–47523. doi: 10.1074/jbc.M108645200. [DOI] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 23.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 24.Krippner A, Matsuno-Yagi A, Gottlieb RA, Babior BM. Loss of function of cytochrome c in Jurkat cells undergoing fas-mediated apoptosis. J Biol Chem. 1996;271:21629–21636. doi: 10.1074/jbc.271.35.21629. [DOI] [PubMed] [Google Scholar]
- 25.Vayssiere JL, Petit PX, Risler Y, Mignotte B. Commitment to apoptosis is associated with changes in mitochondrial biogenesis and activity in cell lines conditionally immortalized with simian virus 40. Proc Natl Acad Sci USA. 1994;91:11752–11756. doi: 10.1073/pnas.91.24.11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 27.Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 28.Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. EMBO J. 1999;18:2040–2048. doi: 10.1093/emboj/18.8.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Köhler C, Orrenius S, Zhivotovsky B. Evaluation of caspase activity in apoptotic cells. J Immunol Methods. 2002;265:97–110. doi: 10.1016/s0022-1759(02)00073-x. [DOI] [PubMed] [Google Scholar]


