Abstract
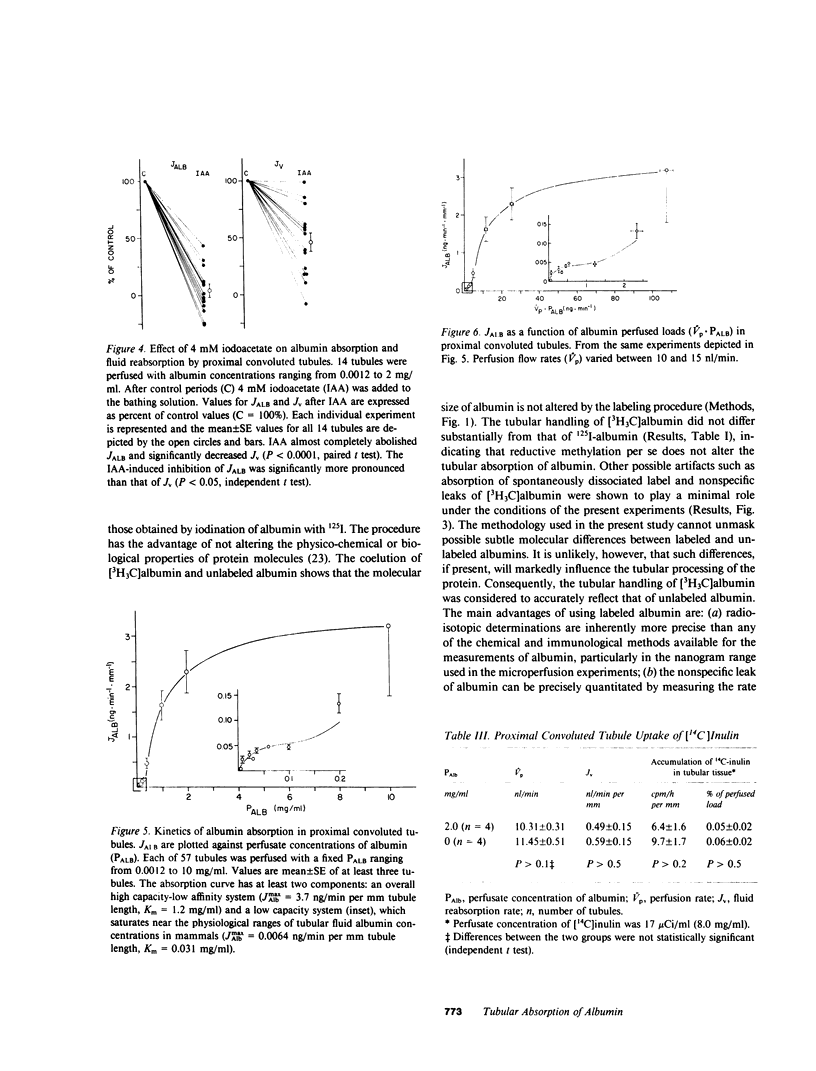
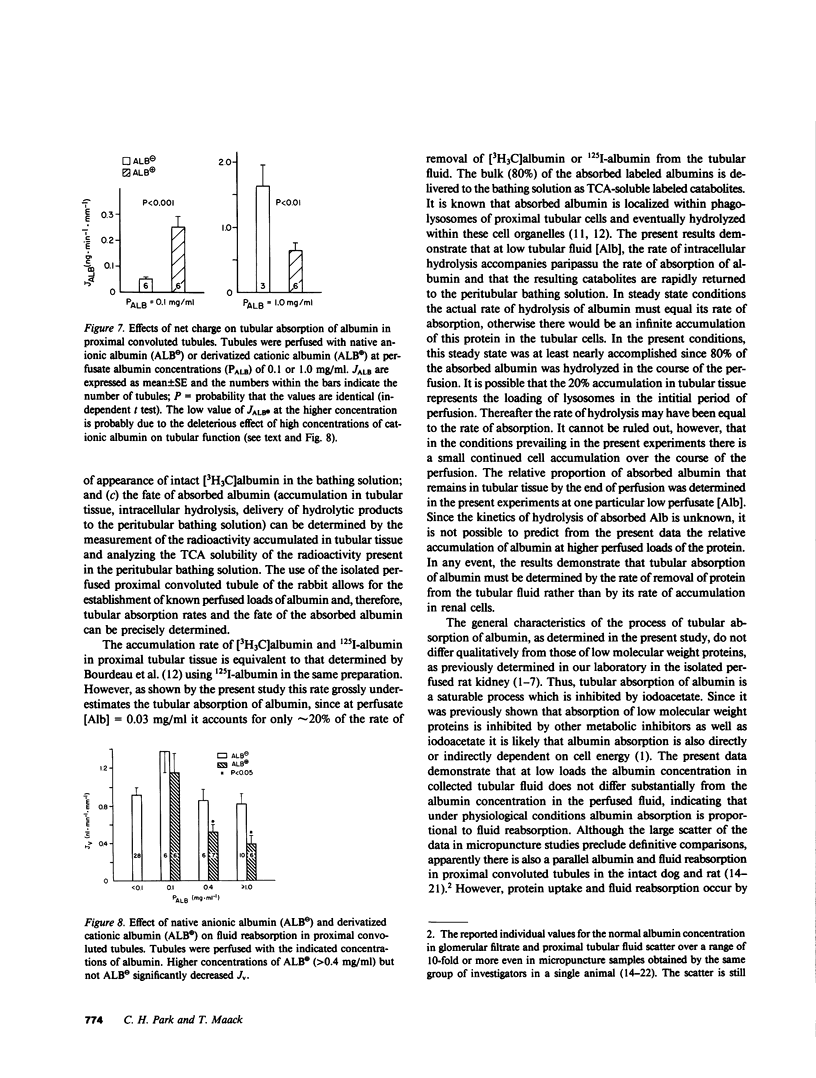
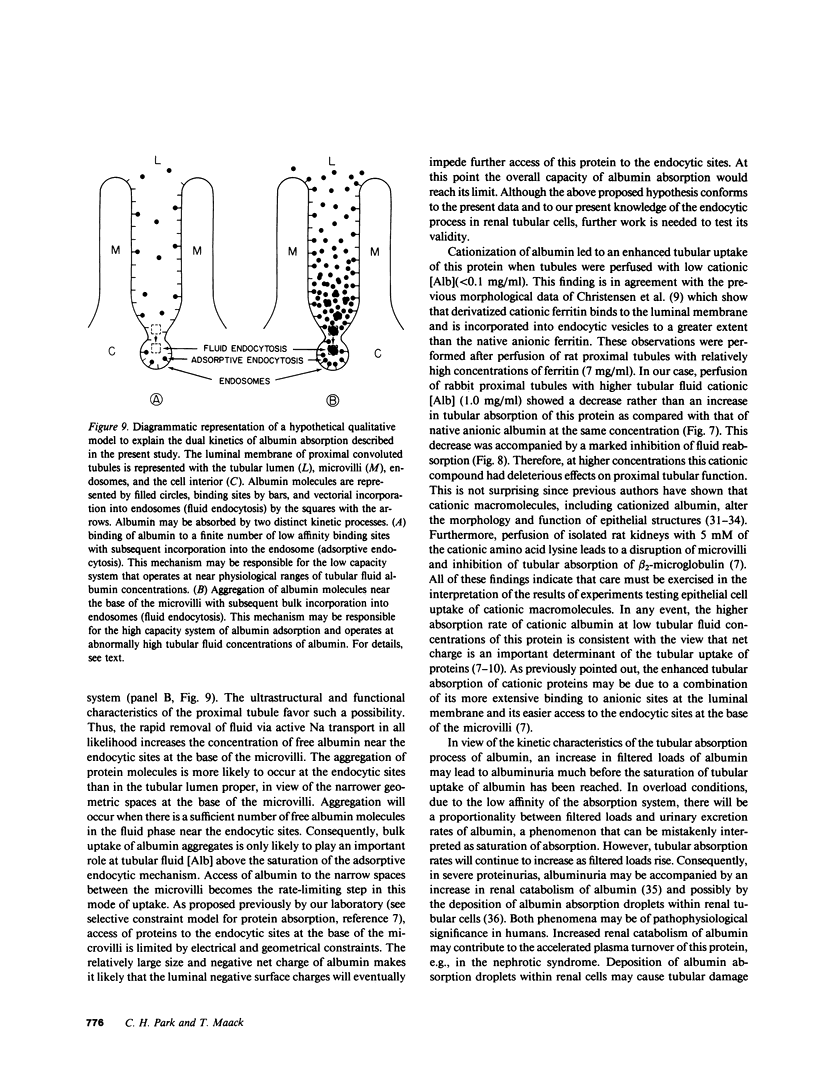
Overall characteristics and kinetics of tubular absorption of albumin (Alb) were studied in isolated perfused proximal convoluted tubules of the rabbit. The fate of absorbed Alb was determined in tubules perfused with low [Alb]. Alb was labeled with tritium by reductive methylation ( [3H3C]Alb). At [Alb] = 0.03 mg/ml, approximately 80% of the absorbed [3H3C]Alb was released to the peritubular bathing solution as catabolic products. Transcellular transport of intact [3H3C]Alb was negligible. Iodoacetate (IAA, 4 mM) inhibited albumin absorption (JAlb) by greater than 95% and fluid reabsorption (JV) by 55%. At [Alb] = 0.1 mg/ml the absorption rate of a derivatized cationic Alb (pI = 8.4) was fivefold greater (P less than 0.01) than that of anionic Alb. Higher cationic [Alb] had deleterious effects on tubular functions. Overall Alb absorption was of high capacity and low affinity (JmaxAlb = 3.7 ng/min per mm tubule length, apparent Michaelis constant (Km) = 1.2 mg/ml). A low capacity system that saturates at near physiological loads was also detected (JmaxAlb = 0.064 ng/min per mm, apparent Km = 0.031 mg/ml). High [Alb] did not alter the rate of endocytic vesicle formation as determined by the tubular uptake of [14C]inulin. Results show that Alb absorption is a saturable process that is inhibited by high IAA concentrations and is affected by the charge of the protein. Absorbed Alb is hydrolyzed by tubular cells and catabolic products are readily released to the peritubular side. The dual kinetics of Alb absorption may be due to a combination of adsorptive endocytosis (low capacity system) and fluid endocytosis of albumin aggregates (high capacity system). Results indicate that albuminuria occurs much before albumin absorption is saturated. The kinetic characteristics of the process of tubular absorption of albumin helps to explain the concomitance of albuminuria, increased renal catabolic rates of albumin, and renal cell deposition of protein absorption droplets in severe glomerular proteinurias.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeyer H. V., Van Liew J. B., Klassen J., Boylan J. W. Filtration of protein in the anti-glomerular basement membrane nephritic rat: a micropuncture study. Kidney Int. 1976 Dec;10(6):425–437. doi: 10.1038/ki.1976.129. [DOI] [PubMed] [Google Scholar]
- Bourdeau J. E., Carone F. A., Ganote C. E. Serum albumin uptake in isolated perfused renal tubules. Quantitative and electron microscope radioautographic studies in three anatomical segments of the rabbit nephron. J Cell Biol. 1972 Aug;54(2):382–398. doi: 10.1083/jcb.54.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M. B., Orloff J. Control of fluid absorption in the renal proximal tubule. J Clin Invest. 1968 Sep;47(9):2016–2024. doi: 10.1172/JCI105888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen E. I., Carone F. A., Rennke H. G. Effect of molecular charge on endocytic uptake of ferritin in renal proximal tubule cells. Lab Invest. 1981 Apr;44(4):351–358. [PubMed] [Google Scholar]
- Cojocel C., Franzen-Sieveking M., Beckmann G., Baumann K. Inhibition of renal accumulation of lysozyme (basic low molecular weight protein) by basic proteins and other basic substances. Pflugers Arch. 1981 Jun;390(3):211–215. doi: 10.1007/BF00658263. [DOI] [PubMed] [Google Scholar]
- DIRKS J. H., CLAPP J. R., BERLINER R. W. THE PROTEIN CONCENTRATION IN THE PROXIMAL TUBULE OF THE DOG. J Clin Invest. 1964 May;43:916–921. doi: 10.1172/JCI104977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbach G. M., Liew J. B., Boylan J. W., Manz N., Muir P. Effect of angiotensin on the filtration of protein in the rat kidney: a micropuncture study. Kidney Int. 1975 Aug;8(2):80–87. doi: 10.1038/ki.1975.83. [DOI] [PubMed] [Google Scholar]
- Friedman P. A., Figueiredo J. F., Maack T., Windhager E. E. Sodium-calcium interactions in the renal proximal convoluted tubule of the rabbit. Am J Physiol. 1981 Jun;240(6):F558–F568. doi: 10.1152/ajprenal.1981.240.6.F558. [DOI] [PubMed] [Google Scholar]
- Galaske R. G., Baldamus C. A., Stolte H. Plasma protein handling in the rat kidney: micropuncture experiments in the acute heterologous phase of anti-GBM-nephritis. Pflugers Arch. 1978 Aug;375(3):269–277. doi: 10.1007/BF00582441. [DOI] [PubMed] [Google Scholar]
- Heinemann H. O., Maack T. M., Sherman R. L. Proteinuria. Am J Med. 1974 Jan;56(1):71–82. doi: 10.1016/0002-9343(74)90752-9. [DOI] [PubMed] [Google Scholar]
- Hoare D. G., Koshland D. E., Jr A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967 May 25;242(10):2447–2453. [PubMed] [Google Scholar]
- Johnson V., Maack T. Renal extraction, filtration, absorption, and catabolism of growth hormone. Am J Physiol. 1977 Sep;233(3):F185–F196. doi: 10.1152/ajprenal.1977.233.3.F185. [DOI] [PubMed] [Google Scholar]
- KATZ J., BONORRIS G., SELLERS A. L. ALBUMIN METABOLISM IN AMINONUCLEOSIDE NEPHROTIC RATS. J Lab Clin Med. 1963 Dec;62:910–934. [PubMed] [Google Scholar]
- Kau S. T., Maack T. Transport and catabolism of parathyroid hormone in isolated rat kidney. Am J Physiol. 1977 Nov;233(5):F445–F454. doi: 10.1152/ajprenal.1977.233.5.F445. [DOI] [PubMed] [Google Scholar]
- Landwehr D. M., Carvalho J. S., Oken D. E. Micropuncture studies of the filtration and absorption of albumin by nephrotic rats. Kidney Int. 1977 Jan;11(1):9–17. doi: 10.1038/ki.1977.2. [DOI] [PubMed] [Google Scholar]
- Leber P. D., Marsh D. J. Micropuncture study of concentration and fate of albumin in rat nephron. Am J Physiol. 1970 Aug;219(2):358–363. doi: 10.1152/ajplegacy.1970.219.2.358. [DOI] [PubMed] [Google Scholar]
- Lewy J. E., Pesce A. Micropuncture study of albumin transfer in aminonucleoside nephrosis in the rat. Pediatr Res. 1973 Jun;7(6):553–559. doi: 10.1203/00006450-197306000-00002. [DOI] [PubMed] [Google Scholar]
- Maack T., Johnson V., Kau S. T., Figueiredo J., Sigulem D. Renal filtration, transport, and metabolism of low-molecular-weight proteins: a review. Kidney Int. 1979 Sep;16(3):251–270. doi: 10.1038/ki.1979.128. [DOI] [PubMed] [Google Scholar]
- Maack T. Renal handling of low molecular weight proteins. Am J Med. 1975 Jan;58(1):57–64. doi: 10.1016/0002-9343(75)90533-1. [DOI] [PubMed] [Google Scholar]
- Maunsbach A. B. Absorption of I-125-labeled homologous albumin by rat kidney proximal tubule cells. A study of microperfused single proximal tubules by electron microscopic autoradiography and histochemistry. J Ultrastruct Res. 1966 Jun;15(3):197–241. doi: 10.1016/s0022-5320(66)80108-9. [DOI] [PubMed] [Google Scholar]
- Nomiyama K., Foulkes E. C. Reabsorption of filtered cadmium-metallothionein in the rabbit kidney (39883). Proc Soc Exp Biol Med. 1977 Oct;156(1):97–99. doi: 10.3181/00379727-156-39883. [DOI] [PubMed] [Google Scholar]
- OLIVER J., MACDOWELL M., LEE Y. C. Cellular mechanisms of protein metabolism in the nephron. I. The structural aspects of proteinuria; tubular absorption, droplet formation, and the disposal of proteins. J Exp Med. 1954 Jun 1;99(6):589–604. doi: 10.1084/jem.99.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken D. E., Cotes S. C., Mende C. W. Micropuncture study of tubular transport of albumin in rats with aminonucleoside nephrosis. Kidney Int. 1972;1(1):3–11. doi: 10.1038/ki.1972.2. [DOI] [PubMed] [Google Scholar]
- Oken D. E., Flamenbaum W. Micropuncture studies of proximal tubule albumin concentrations in normal and nephrotic rats. J Clin Invest. 1971 Jul;50(7):1498–1505. doi: 10.1172/JCI106635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtell J. N., Pesce A. J., Clyne D. H., Miller W. C., Pollak V. E. Isoelectric point of albumin: effect on renal handling of albumin. Kidney Int. 1979 Sep;16(3):366–376. doi: 10.1038/ki.1979.139. [DOI] [PubMed] [Google Scholar]
- Quinton P. M., Philpott C. W. A role for anionic sites in epithelial architecture. Effects of cationic polymers on cell membrane structure. J Cell Biol. 1973 Mar;56(3):787–796. doi: 10.1083/jcb.56.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C. F., Jr, Rennke H. G., Humes H. D. Acute renal failure induced by diethylaminoethyl dextran: importance of cationic charge. Kidney Int. 1981 Mar;19(3):424–430. doi: 10.1038/ki.1981.35. [DOI] [PubMed] [Google Scholar]
- Sumpio B. E., Maack T. Kinetics, competition, and selectivity of tubular absorption of proteins. Am J Physiol. 1982 Oct;243(4):F379–F392. doi: 10.1152/ajprenal.1982.243.4.F379. [DOI] [PubMed] [Google Scholar]
- Tack B. F., Dean J., Eilat D., Lorenz P. E., Schechter A. N. Tritium labeling of proteins to high specific radioactivity by reduction methylation. J Biol Chem. 1980 Sep 25;255(18):8842–8847. [PubMed] [Google Scholar]
- Tate S. S., Meister A. Subunit structure and isozymic forms of gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2599–2603. doi: 10.1073/pnas.73.8.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]