Abstract
AIM: To evaluate the clinical utility of serum fibrosis markers, including YKL-40, in patients with HCV-associated liver disease.
METHODS: A total of 109 patients with HCV-associated liver disease were enrolled. We measured serum type IV collagen, amino-terminal peptide of type III procollagen (PIIIP), hyaluronic acid (HA), YKL-40 levels and biochemical. Parameters by RIA or ELISA. Eighty-eight patients underwent liver biopsy, and 67 of 109 patients received interferon (IFN) therapy. We also investigated the relationship between the concentrations of serum fibrosis markers and histological fibrosis scores (METAVIR), and evaluated the changes of the levels of fibrosis markers before and after the IFN therapy.
RESULTS: The increase in serum levels of all markers, particularly HA, was correlated with the progression of liver fibrosis (for type IV collagen, F = 9.076, P<0.0001; for PIIIP, F = 9.636, P<0.0001; for HA, F = 13.128, P<0.0001; and for YKL-40, F = 8.016, P<0.0001). YKL-40 had strong correlation with HA (r = 0.536, P<0.0001). Based on the receiver operating curve (ROC), the ability of serum HA exceeded the abilities of other serum markers to determine fibrosis score 4 from fibrosis score 0-3 (AUC = 0.854). While YKL-40 was superior to other fibrosis markers for predicting severe fibrosis (F2-F4) from mild fibrosis (F0-F1) (YKL-40, AUC = 0.809; HA, AUC = 0.805). After IFN therapy, only YKL-40 values significantly decreased not only in the responder group, but also in the nonresponder group (P = 0.03).
CONCLUSION: YKL-40 may be a useful non-invasive serum marker to estimate the degree of liver fibrosis and to evaluate the efficacy of IFN therapies in patients with HCV-associated liver disease.
Keywords: HCV, Liver fibrosis, YKL-40, Interferon
INTRODUCTION
Hepatic fibrosis is the most important factor for estimating clinical outcome and determining therapeutic strategy, especially interferon therapy, in patients with hepatitis C virus (HCV)-associated liver diseases. Liver biopsy is currently the most reliable standard for assessing hepatic fibrosis, necrosis and inflammation[1,2]. However, liver biopsy has potential complications[3], and so is not repeatedly performed. Thus, there is a need to establish noninvasive monitoring methods for assessing the severity of hepatic fibrosis.
Several attempts have been made to find accurate noninvasive markers of disease activity and fibrosis. To date, several laboratory markers, such as platelet counts, ALT/AST ratio[4], or levels of hyaluronic acid (HA)[5], N-terminal propeptide of type III collagen (P III P)[6,7]or type IV collagen[8], have been proposed to represent hepatic fibrosis, focusing particularly on the diagnosis of advanced hepatic fibrosis. Some have combined several biochemical and clinical markers with scoring systems to predict the presence or absence of fibrosis[9]. However these scoring systems are somewhat complicated, since some analyses are not routinely available and the calculation system can be complex.
Among the single fibrosis markers, HA and PIIIP levels have been well studied in patients with chronic liver diseases[10]. HA is a glucosaminoglycan synthesized by the mesenchymal cells and degraded by hepatic sinusoidal cells by a specific receptor-mediated process. Serum levels of HA are highly correlated with advanced fibrosis and liver cirrhosis[5,11,12]. However, serum HA alone has limited value in predicting histological change over a treatment period. PIIIP, a product of collagen synthesis, correlates better with histological inflammation than fibrosis[10]. Hence, there is a need to develop a simple, accurate and reliable noninvasive marker for evaluating the severity of fibrosis.
YKL-40 (chondrex, human cartilage glycoprotein-39) is a recently described glycoprotein that belongs to the chitinase family[13]. YKL-40 mRNA was strongly expressed in human liver and arthritic articular cartilage[13,14] and was elevated in the synovial fluid[15] and serum with active rheumatoid arthritis[16], severe osteoarthritis[17] and alcoholic liver disease[6,18]. Although its physiological function is unknown in detail, YKL-40 is thought to contribute to tissue remodeling or degradation of the extracellular matrix[19]. Previous reports have indicated that the YKL-40 is a growth factor for fibroblasts and that YKL-40 acts synergistically with insulin-like growth factor 1 in stimulating the growth of fibroblasts[20], YKL-40 is also a growth factor for chondrocytes and synovial cells[15] and acts as a chemo-attractant for endothelial cells and stimulates migration of these cells at a level comparable to that achieved by basic fibroblast growth factor[21]. Furthermore, YKL-40 modulates vascular endothelial cell morphology by promoting the formation of branching tubules, indicating that YKL-40 may play a role in angiogenesis by stimulating the migration and reorganization of vascular endothelial cells[21].
However, clinical use of serum YKL-40 level for the assessment of hepatic fibrosis in patients with HCV-associated liver diseases has not been elucidated. Furthermore, the response of fibrosis markers after interferon (IFN) therapy, a potential anti-fibrosis therapy, is not well understood. We questioned whether the fibrosis-related markers type IV collagen, PIIIP, HA and YKL-40 could discriminate between histological fibrosis stages, and reflect the histological response of post-interferon therapy in patients with HCV-associated liver diseases.
MATERIALS AND METHODS
Patients
One hundred and nine patients with chronic liver disease were studied and all patients had detectable serum HCV-RNA (Amplicor HCV 2.0; Roche Diagnostics, Branchburg, NJ, USA). Eighty-eight patients underwent liver biopsies for histological examination of the liver. Twenty-one patients, whose diagnosis was based on clinical, biochemical and imaging findings, were classified as having liver cirrhosis, because they had much risk for the biopsies. None of the patients had other causes of chronic liver injury, a history of habitual alcohol consumption nor hepatocellular carcinoma. All tissue and serum samples were obtained with informed consent.
Liver histological findings
Eighty-eight patients underwent liver biopsies as a part of clinical standard management. Tissue sections from the patients were stained with hematoxylin and eosin and evaluated for the stage of liver fibrosis and the grade of liver activity.
The patients were sub-classified into five groups based on the stage of liver fibrosis according to the METAVIR classification: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with few septa; F3, numerous septa without cirrhosis; and F4, cirrhosis. The grade of liver activity was classified into four groups according to METAVIR classification: A0, no activity; A1, mild activity; A2, moderate activity; and A3, severe activity.
Serological and biochemical methods
We measured the levels of type IV collagen, PIIIP, HA and YKL-40 from patient serum samples that were stored at -20 °C. The concentration of type IV collagen was determined with a commercial RIA kit (Panassay IV. C, Daiichi Chemical Co. Ltd. Tokyo, Japan). PIIIP concentration was measured by RIA-gnost PIIIP kit (Hoechst, Tokyo, Japan). ELISA kits were used for determination of HA concentrations (Chugai Pharmaceuticals, Tokyo, Japan) and YKL-40 concentrations (Quidel Corporation, San Diego, USA). We determined the concentrations of these markers before and after interferon (IFN) treatment. Serum albumin and alanine aminotransferase (ALT) were measured by routine methods. All biochemical data were expressed as median (range).
Receiver operating curve
To assess the ability of the four serum fibrosis markers for differentiating chronic hepatitis (fibrosis score F0-F3) from liver cirrhosis (F4), and for differentiating mild hepatitis (F0-1) from severe hepatitis (F2-4), we calculated the sensitivity and the specificity for each value of each fibrosis marker and then constructed receiver operating curves (ROC) by plotting the sensitivity against the reverse specificity (1 minus specificity) at each value. The diagnostic value of each serum marker was assessed by the area under the ROC. An area under the curve (AUC) of 1.0 is characteristics of an ideal test, whereas 0.5 indicates a test of no diagnostic value. The nearer a curve shifts to the top left-hand corner of the graph, the more useful marker is for the diagnosis. We determined the turning point of the curve to the best cut-off value for the diagnosis, and it was also a maximal value at the sum of the sensitivity and specificity. The diagnostic accuracy was calculated by sensitivity, specificity, positive and negative predictive values, considering significant fibrosis of the disease.
Assessment of response to IFN treatment
We also evaluated whether a marker was an independent predictor of the response to interferon (IFN) treatment in chronic hepatitis C. Sixty-seven patients with chronic hepatitis C received a 6-mo course of interferon-based therapy. We determined serum levels for each fibrosis marker and determined HCV-RNA for each patient at 6 mo after the therapy Patients whose HCV-RNA became positive after IFN treatment were classified into the non-virological responser (NVR) group, and those whose HCV-RNA remained negative after IFN treatment were classified as the sustained-virological responser (SVR) group. The NVR group was sub-divided into those patients whose ALT remained below 664 nkat/L l after IFN treatment as the biochemical responders (BR) group.
Statistical analysis
Results of serum fibrosis markers were expressed as box plots. The statistical significance was calculated by one-way ANOVA. The correlations between four serum fibrosis markers and biochemical data were evaluated by analysis of person’s correlation coefficient. The levels of serum fibrosis markers in the patients with IFN treatment were analyzed by the paired t test. P values <0.05 were considered statistically significant.
RESULTS
Patient characteristics
Baseline demographic and laboratory values are summarized in Table 1 for the109 patients. The median age was 54 years, with a male predominance. The values of platelets and serum albumin decreased in the patients with HCV-associated liver disease according to the stage of liver fibrosis (F0-4) (Table 1).
Table 1.
Characteristics of subjects.
| Fibrosis | n | M/F | Age (yr) | Platelet (×104/mm3) | Albumin (g/L) | ALT (nkat/L) |
| F 0 | 5 | 3/2 | 35 | 19.9 | 42 | 182.6 |
| (29-39) | (14.4-26.0) | (3.2-4.6) | (7-84) | |||
| F 1 | 27 | 17/10 | 51 | 15.8 | 41 | 1477.4 |
| (31-73) | (10.3-25.2) | (3.6-4.7) | (20-267) | |||
| F 2 | 13 | 8/5 | 57 | 13 | 39 | 1543.8 |
| (50-64) | (10.0-15.1) | (3.5-4.5) | (31-235) | |||
| F 3 | 34 | 22/12 | 55 | 12.2 | 39 | 1062.4 |
| (30-68) | (8.3-19.1) | (3.3-4.3) | (23-295) | |||
| F 4 | 30 | 12/18 | 59 | 9.5 | 34 | 996 |
| (43-75) | (3.2-21.3) | (2.5-4.1) | (13-150) |
M: male, F: female.
Correlation between serum markers and the stage of liver fibrosis
The relation of serum concentrations of type IV collagen, PIIIP, HA and YKL-40 to the stage of liver fibrosis is illustrated in Figure 1. Serum levels of type IV collagen, PIIIP, HA and YKL-40 increased with the progression of liver fibrosis in patients with HCV-associated liver disease. The overall significances by ANOVA of the difference of these five groups were as follow: for type IV collagen, F = 9.076, P<0.0001; for PIIIP, F = 9.636, P<0.0001; for HA, F = 13.128, P<0.0001; and for YKL-40, F = 8.016, P<0.0001. Levels of each fibrosis marker had a strong correlation with the stage of liver fibrosis. HA values increased more significantly in F4 than other fibrosis markers. PIIIP and, particularly YKL-40, values increased according to the stage of liver fibrosis. The correlation between the grade of histological activity and serum fibrosis markers were evaluated with ANOVA. The overall significances in these four groups were as follows: for type IV collagen, F = 3.385, P<0.0219; for PIIIP, F = 0.991, P = 0.4011; for HA, F = 0.277, P = 0.8417; and for YKL-40, F = 0.246, P = 0.8638. Type IV collagen had a weak correlation with the grade of histological activity in liver histology, but other serum fibrosis markers did not show significant correlations.
Figure 1.

Serum levels of type IV collagen, PIIIP, HA and YKL-40 with respect to stage of liver fibrosis (F0-4). The box represents the interquartile range. The whiskers indicate the highest and lowest values, and the line across the box indicates the median value. Overall significance of differences among 5 groups was determined by ANOVA: for type IV collagen, F = 9.076, P<0.0001; for PIIIP, F = 9.636, P<0.0001; for HA, F = 13.128, P<0.0001; and for YKL-40, F = 8.016, P<0.0001.
Correlation between serum markers and biochemical parameters
The correlation between type IV collagen, PIIIP, HA, YKL-40 and biochemical liver function tests is shown in Table 2. Correlations were found between each fibrosis marker (P<0.001). There was a moderate correlation between type IV collagen and PIIIP (r = 0.432, P<0.0001). YKL-40 and HA showed the strongest correlation (r = 0.536, P<0.0001).
Table 2.
Correlation between type IV collagen, PIIIP, HA, YKL-40 and parameters of liver function.
| Type IV collagen | PIIIP | Hyaluronic acid | YKL-40 | |
| Type IV collagen | 0.432b | 0.25 | 0.258 | |
| PIIIP | 0.432b | 0.333 | 0.367 | |
| Hyaluronic acid | 0.25 | 0.333 | 0.536bd | |
| YKL-40 | 0.258 | 0.258 | 0.536bd | |
| Platelet | -0.453b | -0.105 | -0.494b | -0.478b |
| Albumin | -0.091 | 0.127 | -0.472b | -0.239 |
| ALT | 0.149 | -0.046 | -0.108 | 0.009 |
P<0.001,
P<0.0001.
Prediction of cirrhosis from chronic hepatitis
The ability of these serum markers to detect cirrhosis (F4) in patients with chronic hepatitis C (F0-3) was assessed using ROC. The best ROC derived from four serum fibrosis markers was applied by measuring the area under the curve (AUC), and the best ROC was that of HA (AUC = 0.854). Based on the ROC, the predictive ability of serum HA exceeded that of the other serum fibrosis markers (Figure 2). The selected cut-off values for diagnosing cirrhosis in patients with chronic hepatitis C was 183.5 ng/mL for serum HA with 80% sensitivity and 80% specificity (Table 3). The cut-off value for YKL-40 was 284.8 ng/mL with 80% sensitivity and 77% specificity, and that for PIIIP was 0.995 U/mL with 79% sensitivity and 66% specificity.
Figure 2.

Receiver operating curves of type IV collagen, PIIIP, HA and YKL-40 for predicting stages greater than F3. The predictive ability of serum HA exceeded that of another serum markers (AUC = 0.854). The ability to predict cirrhosis (F4) from chronic hepatitis C (F0-3) was AUC = 0.795 for YKL-40 and AUC = 0.790 for PIIIP.
Table 3.
Prediction of cirrhosis vs chronic hepatitis C by hyaluronic acid and YKL-40.
| Cut-off value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
| IV collagen (ng/mL) | 6.55 | 60 | 61 | 61 | 60 |
| PIIIP (U/mL) | 0.995 | 77 | 66 | 69 | 67 |
| HA (ng/mL) | 183.5 | 80 | 80 | 80 | 80 |
| YKL-40 (ng/mL) | 284.8 | 80 | 71 | 73 | 78 |
Prediction of severe stage of fibrosis from mild stage of fibrosis
The utility of these markers to differentiate severe stage of fibrosis (F2-4) from mild stage of fibrosis (F0-1) was evaluated using ROC (Figure 3). The best ROC derived from four serum fibrosis markers was applied by measuring the area under the curve (AUC), and the best ROC was that of YKL-40 and HA (YKL-40, AUC = 0.809; HA, AUC = 0.805), exceeding those of PIIIP and type IV collagen. The cut-off values of YKL-40 and HA were 186.4 ng/mL and 75.7 ng/mL, respectively (Table 4). The sensitivity and specificity of these concentrations were 80% and 81% for YKL-40 and 75% and 81% for HA, respectively.
Figure 3.
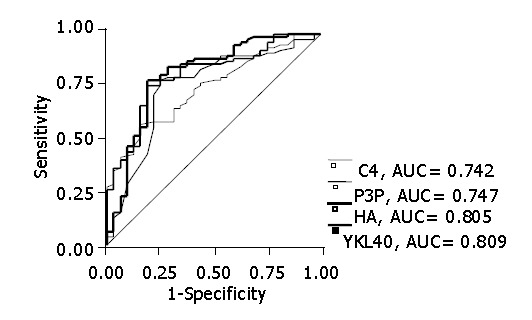
Receiver operating curves of type IV collagen, PIIIP, HA and YKL-40 for predicting stages greater than F1. YKL-40 and HA exceeded those of PIIIP and type IV collagen (YKL-40, AUC = 0.809; HA, AUC = 0.805).
Table 4.
Prediction of severe hepatitis C versus mild hepatitis C by hyaluronic acid and YKL-40.
| Cut-off value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
| IV collagen (ng/mL) | 5.75 | 65 | 69 | 67 | 66 |
| PIIIP (U/mL) | 0.835 | 78 | 75 | 76 | 77 |
| HA (ng/mL) | 75.7 | 75 | 81 | 79 | 76 |
| YKL-40 (ng/mL) | 186.4 | 78 | 81 | 80 | 79 |
Changes of serum markers after IFN therapy
The sixty-seven patients with HCV-associated liver disease received IFN therapy and were classified after therapy into NVR group (n = 44) and SVR group (n = 23). In SVR group, PIIIP, HA and YKL-40 levels significantly decreased after IFN treatment (for PIIIP, P<0.0001; for HA, P = 0.0077; for YKL-40, P = 0.0084). In contrast, in NVR group, PIIIP and HA levels did not change, whereas YKL-40 values significantly decreased (P = 0.03) (Figure 4). Furthermore, there were 9 patients in the BR group within the patients in NVR group (n = 44), and only one patient had normal ALT value before IFN therapy. Even in the BR group, only YKL-40 significantly decreased after IFN therapy (P = 0.0111). Moreover, there was a tendency for those in the SVR group to have lower YKL-40 values than those in the NVR group before IFN therapy (P = 0.084).
Figure 4.
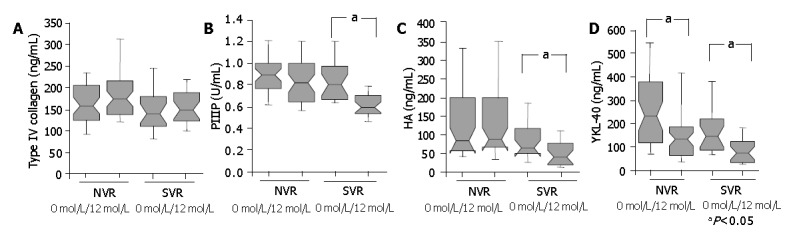
Serum levels of type IV collagen, PIIIP, HA and YKL-40 before and 6 months after IFN therapy in NVR and SVR patient groups. The box represents the interquartile range. The whiskers indicate the highest and lowest values, and the line across the box indicates the median value. The levels of all serum markers without type IV collagen 6 mo after IFN treatment were lower than those before IFN treatment in SVR group (aP<0.05), while only YKL-40 levels decreased in NVR group (aP<0.05).
DISCUSSION
Hepatic fibrosis is the main determinant of clinical outcome and therapeutic efficacy in patients with HCV-associated liver disease. A single-pass liver biopsy is able to correctly diagnose the stage of fibrosis or presence of cirrhosis in 80% of patients[22]. However, liver biopsy is an invasive procedure with associated morbidity that carries a significant cost[3]. For these reasons, a reliable noninvasive fibrosis marker is required. In recent studies, simple noninvasive methods without biopsy to predict both significant fibrosis and cirrhosis have been investigated. Measurements of mixed parameters, such as the blood test[22], fibrotest[9] or aspartate aminotransferase (AST) to platelet ratio[4,23] indices, have been assessed as substitutes for liver biopsy, but these methods are difficult to calculate, do not reflect the mechanism of the liver fibrosis directly, and do not relate to the efficacy of IFN treatment in patients with HCV-associated liver disease.
In an attempt to search for more suitable markers for prediction of liver fibrosis severity, we investigated the novel marker, YKL-40, in addition to the well established fibrosis markers, type IV collagen, PIIIP and HA. In our study, YKL-40 and HA were found more useful than other markers for assessing the fibrosis stage. In particular, YKL-40 was most useful for monitoring the fibrosis of liver disease and for distinguishing extensive liver fibrosis from mild stage of liver fibrosis, enabling us to predict severe stage of fibrosis at 80% positive predictive value. HA appeared to be slightly better for prediction of cirrhosis (F4) from chronic hepatitis (F0-3) than YKL-40.
Type IV collagen is composed of a major triple-helix, an amino-terminal triple-helix (7S domain) and a carboxy-terminal globular domain[24]. We measured type IV collagen by RIA, which recognizes the 7S domain. The 7S domain is thought to be derived primarily from the degradation of already existing basement membrane, and it correlates better with liver fibrosis rather than intact type IV collagen[8]. PIIIP is a component of the extracellular matrix deposited in the space of Disse[25]. It is produced from type III procollagen in hepatic stellate cells and is released into the circulation in stoichiometric amounts[26]. PIIIP correlates better with inflammation and is thought to reflect primarily active hepatic fibrogenesis in chronic liver disease[27,28]. HA is a polysaccharide found in virtually all connective tissues, and in liver fibrosis[29], it is a component of the extracellular matrix[30]. In chronic hepatitis, HA is synthesized by the hepatic stellate cells and is metabolized in the liver endothelial cells[11]. With severe fibrosis in chronic hepatitis, increasing deposition of basement membrane components causes sinusoidal capillarization, diminishing HA clearance. HA levels increase, particularly in patients with cirrhosis[31]. Therefore, it is thought that type IV collagen and PIIIP levels reflect more fibrogenesis than fibrosis, whereas HA levels reflect fibrosis. YKL-40 is a mammalian 40 ku molecule[13] of the chitinase family. Though the biological function of YKL-40 is unknown, it is expressed in various diseases, such as liver disease with alcoholic cirrhosis[32], and recurrent breast cancer[33] or colorectal cancer[34]. YKL-40 is produced in a wide variety of cell types, including chondrocytes, synovial cells[15], activated macrophages[35], neutrophils[36], and in particular from cells located in tissues with increased remodeling/degradation or inflammation of the extracellular matrix, such as the hepatic stellate cells[37]. Based on our results and those from other groups[6], it appeared that serum levels of YKL-40 represented ongoing fibrosis like HA, in addition to fibrogenesis similar to type IV collagen and PIIIP of the liver disease. Serum YKL-40 levels were valuable for diagnosing mild stage of fibrosis (value < 186.4), severe stage of fibrosis (186.4 < value < 284.8) and F4 (284.8 < value) in our patients with HCV-associated liver disease. These results suggested that YKL-40 might be a new useful marker for monitoring liver fibrosis.
We also examined which fibrosis marker reflected the response to IFN therapy in patients HCV-associated liver disease. The natural course of fibrosis progression was studied based on a single biopsy and a suspected, rather than proven, duration of infection from the patient’s history[38]. The natural course of fibrosis progression rate in patients with chronic hepatitis C was 0.133 units/year of the fibrosis score[39]. In contrast, persistently normal ALT levels correlated with slow progression of liver fibrosis (0.05 units/year)[40]. The short-term effects of IFN therapy on histological improvement are well documented and most studies have shown histological improvement at the end of treatment and/or within 1-2 years of follow-up[41], even though hepatitis C virus was not completely eradicated. Meta-analysis of the current data showed a correlation between biochemical response to IFN (i.e., normalization of ALT) and histological improvement of inflammatory activity and showed a slower progression of the liver fibrosis in the NVR group than that in the untreated patients[42,43]. IFN treatment also reduced activity in the SVR group, and even in NVR patients at 6-12 mo after completion of IFN treatment.
We investigated the values of serum fibrosis markers before and 6 mo after IFN therapy. In the SVR group, the levels of PIIIP, HA and YKL-40 significantly decreased after IFN treatment. The YKL-40 levels lowered significantly not only in the BR group, but also in the NVR group after IFN therapy. These results in BR group, which were different from the previous report which demonstrated that plasma PIIINP was the only marker predicting treatment[7], suggested that the values of YKL-40 after IFN treatment might promptly reflect the improvement of liver inflammation, as well as fibrogenesis and tissue remodeling. We speculated that the serum YKL-40 changes might reflect the efficacy of the IFN treatment in the patients with HCV-associated liver disease more directly and dynamically than other fibrosis markers. In addition, YKL-40 might estimate the therapeutic effect before IFN treatment, since YKL-40 value was relatively lower in SVR group than that in NVR group. Additional studies will address these observations and the resulting YKL-40 characteristics may provide information about fibrosis status and fibrosis improvement.
In conclusion, our study demonstrates that serum YKL-40 reflects fibrosis and fibrogenesis in patients with HCV-associated liver disease. Furthermore, serum YKL-40 measurements may be a serological marker of liver fibrosis and may be used as a noninvasive marker for evaluating the efficacy of various therapies in these patients.
Footnotes
Assistant Editor Guo SY Edited by Kumar M and Ma JY
References
- 1.Forns X, Ampurdanès S, Sanchez-Tapias JM, Guilera M, Sans M, Sánchez-Fueyo A, Quintó L, Joya P, Bruguera M, Rodés J. Long-term follow-up of chronic hepatitis C in patients diagnosed at a tertiary-care center. J Hepatol. 2001;35:265–271. doi: 10.1016/s0168-8278(01)00088-5. [DOI] [PubMed] [Google Scholar]
- 2.Yano M, Kumada H, Kage M, Ikeda K, Shimamatsu K, Inoue O, Hashimoto E, Lefkowitch JH, Ludwig J, Okuda K. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23:1334–1340. doi: 10.1002/hep.510230607. [DOI] [PubMed] [Google Scholar]
- 3.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 4.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 5.Parés A, Deulofeu R, Giménez A, Caballería L, Bruguera M, Caballería J, Ballesta AM, Rodés J. Serum hyaluronate reflects hepatic fibrogenesis in alcoholic liver disease and is useful as a marker of fibrosis. Hepatology. 1996;24:1399–1403. doi: 10.1002/hep.510240615. [DOI] [PubMed] [Google Scholar]
- 6.Nøjgaard C, Johansen JS, Christensen E, Skovgaard LT, Price PA, Becker U. Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease. J Hepatol. 2003;39:179–186. doi: 10.1016/s0168-8278(03)00184-3. [DOI] [PubMed] [Google Scholar]
- 7.Nøjgaard C, Johansen JS, Krarup HB, Holten-Andersen M, Møller A, Bendtsen F. Effect of antiviral therapy on markers of fibrogenesis in patients with chronic hepatitis C. Scand J Gastroenterol. 2003;38:659–665. doi: 10.1080/00365520310002300. [DOI] [PubMed] [Google Scholar]
- 8.Murawaki Y, Ikuta Y, Koda M, Yamada S, Kawasaki H. Comparison of serum 7S fragment of type IV collagen and serum central triple-helix of type IV collagen for assessment of liver fibrosis in patients with chronic viral liver disease. J Hepatol. 1996;24:148–154. doi: 10.1016/s0168-8278(96)80023-7. [DOI] [PubMed] [Google Scholar]
- 9.Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–992. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 10.Guéchot J, Poupon RE, Giral P, Balkau B, Giboudeau J, Poupon R. Relationship between procollagen III aminoterminal propeptide and hyaluronan serum levels and histological fibrosis in primary biliary cirrhosis and chronic viral hepatitis C. J Hepatol. 1994;20:388–393. doi: 10.1016/s0168-8278(94)80013-8. [DOI] [PubMed] [Google Scholar]
- 11.Patel K, Lajoie A, Heaton S, Pianko S, Behling CA, Bylund D, Pockros PJ, Blatt LM, Conrad A, McHutchison JG. Clinical use of hyaluronic acid as a predictor of fibrosis change in hepatitis C. J Gastroenterol Hepatol. 2003;18:253–257. doi: 10.1046/j.1440-1746.2003.02930.x. [DOI] [PubMed] [Google Scholar]
- 12.McHutchison JG, Blatt LM, de Medina M, Craig JR, Conrad A, Schiff ER, Tong MJ. Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus Interferon Study Group. J Gastroenterol Hepatol. 2000;15:945–951. doi: 10.1046/j.1440-1746.2000.02233.x. [DOI] [PubMed] [Google Scholar]
- 13.Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268:25803–25810. [PubMed] [Google Scholar]
- 14.Hu B, Trinh K, Figueira WF, Price PA. Isolation and sequence of a novel human chondrocyte protein related to mammalian members of the chitinase protein family. J Biol Chem. 1996;271:19415–19420. doi: 10.1074/jbc.271.32.19415. [DOI] [PubMed] [Google Scholar]
- 15.Johansen JS, Jensen HS, Price PA. A new biochemical marker for joint injury. Analysis of YKL-40 in serum and synovial fluid. Br J Rheumatol. 1993;32:949–955. doi: 10.1093/rheumatology/32.11.949. [DOI] [PubMed] [Google Scholar]
- 16.Peltomaa R, Paimela L, Harvey S, Helve T, Leirisalo-Repo M. Increased level of YKL-40 in sera from patients with early rheumatoid arthritis: a new marker for disease activity. Rheumatol Int. 2001;20:192–196. doi: 10.1007/s002960100115. [DOI] [PubMed] [Google Scholar]
- 17.Johansen JS, Hvolris J, Hansen M, Backer V, Lorenzen I, Price PA. Serum YKL-40 levels in healthy children and adults. Comparison with serum and synovial fluid levels of YKL-40 in patients with osteoarthritis or trauma of the knee joint. Br J Rheumatol. 1996;35:553–559. doi: 10.1093/rheumatology/35.6.553. [DOI] [PubMed] [Google Scholar]
- 18.Tran A, Benzaken S, Saint-Paul MC, Guzman-Granier E, Hastier P, Pradier C, Barjoan EM, Demuth N, Longo F, Rampal P. Chondrex (YKL-40), a potential new serum fibrosis marker in patients with alcoholic liver disease. Eur J Gastroenterol Hepatol. 2000;12:989–993. doi: 10.1097/00042737-200012090-00004. [DOI] [PubMed] [Google Scholar]
- 19.Johansen JS, Christoffersen P, Møller S, Price PA, Henriksen JH, Garbarsch C, Bendtsen F. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol. 2000;32:911–920. doi: 10.1016/s0168-8278(00)80095-1. [DOI] [PubMed] [Google Scholar]
- 20.Sztrolovics R, Recklies AD, Roughley PJ, Mort JS. Hyaluronate degradation as an alternative mechanism for proteoglycan release from cartilage during interleukin-1beta-stimulated catabolism. Biochem J. 2002;362:473–479. doi: 10.1042/0264-6021:3620473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malinda KM, Ponce L, Kleinman HK, Shackelton LM, Millis AJ. Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp Cell Res. 1999;250:168–173. doi: 10.1006/excr.1999.4511. [DOI] [PubMed] [Google Scholar]
- 22.Afdhal NH. Diagnosing fibrosis in hepatitis C: is the pendulum swinging from biopsy to blood tests? Hepatology. 2003;37:972–974. doi: 10.1053/jhep.2003.50223. [DOI] [PubMed] [Google Scholar]
- 23.Fontana RJ, Lok AS. Noninvasive monitoring of patients with chronic hepatitis C. Hepatology. 2002;36:S57–S64. doi: 10.1053/jhep.2002.36800. [DOI] [PubMed] [Google Scholar]
- 24.Murawaki Y, Ikuta Y, Koda M, Kawasaki H. Serum type III procollagen peptide, type IV collagen 7S domain, central triple-helix of type IV collagen and tissue inhibitor of metalloproteinases in patients with chronic viral liver disease: relationship to liver histology. Hepatology. 1994;20:780–787. doi: 10.1002/hep.1840200403. [DOI] [PubMed] [Google Scholar]
- 25.Smedsrød B. Aminoterminal propeptide of type III procollagen is cleared from the circulation by receptor-mediated endocytosis in liver endothelial cells. Coll Relat Res. 1988;8:375–388. doi: 10.1016/s0174-173x(88)80008-6. [DOI] [PubMed] [Google Scholar]
- 26.Takai KK, Hattori S, Irie S. Type V collagen distribution in liver is reconstructed in coculture system of hepatocytes and stellate cells; the possible functions of type V collagen in liver under normal and pathological conditions. Cell Struct Funct. 2001;26:289–302. doi: 10.1247/csf.26.289. [DOI] [PubMed] [Google Scholar]
- 27.Plebani M, Giacomini A, Floreani A, Chiaramonte M, Soffiati G, Naccarato R, Burlina A. Biochemical markers of hepatic fibrosis in primary biliary cirrhosis. Ric Clin Lab. 1990;20:269–274. doi: 10.1007/BF02900712. [DOI] [PubMed] [Google Scholar]
- 28.Ramadori G, Zöhrens G, Manns M, Rieder H, Dienes HP, Hess G, Meyer KH, Büschenfelde Z. Serum hyaluronate and type III procollagen aminoterminal propeptide concentration in chronic liver disease. Relationship to cirrhosis and disease activity. Eur J Clin Invest. 1991;21:323–330. doi: 10.1111/j.1365-2362.1991.tb01377.x. [DOI] [PubMed] [Google Scholar]
- 29.Murawaki Y, Ikuta Y, Idobe Y, Koda M, Kawasaki H. Molecular weight of hyaluronate in the serum of patients with chronic liver disease. Res Commun Mol Pathol Pharmacol. 1998;99:207–216. [PubMed] [Google Scholar]
- 30.Gressner AM, Bachem MG. Cellular sources of noncollagenous matrix proteins: role of fat-storing cells in fibrogenesis. Semin Liver Dis. 1990;10:30–46. doi: 10.1055/s-2008-1040455. [DOI] [PubMed] [Google Scholar]
- 31.McHutchison JG, Blatt LM, de Medina M, Craig JR, Conrad A, Schiff ER, Tong MJ. Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus Interferon Study Group. J Gastroenterol Hepatol. 2000;15:945–951. doi: 10.1046/j.1440-1746.2000.02233.x. [DOI] [PubMed] [Google Scholar]
- 32.Johansen JS, Møller S, Price PA, Bendtsen F, Junge J, Garbarsch C, Henriksen JH. Plasma YKL-40: a new potential marker of fibrosis in patients with alcoholic cirrhosis? Scand J Gastroenterol. 1997;32:582–590. doi: 10.3109/00365529709025104. [DOI] [PubMed] [Google Scholar]
- 33.Johansen JS, Cintin C, Jørgensen M, Kamby C, Price PA. Serum YKL-40: a new potential marker of prognosis and location of metastases of patients with recurrent breast cancer. Eur J Cancer. 1995;31A:1437–1442. doi: 10.1016/0959-8049(95)00196-p. [DOI] [PubMed] [Google Scholar]
- 34.Cintin C, Johansen JS, Christensen IJ, Price PA, Sørensen S, Nielsen HJ. Serum YKL-40 and colorectal cancer. Br J Cancer. 1999;79:1494–1499. doi: 10.1038/sj.bjc.6690238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renkema GH, Boot RG, Au FL, Donker-Koopman WE, Strijland A, Muijsers AO, Hrebicek M, Aerts JM. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur J Biochem. 1998;251:504–509. doi: 10.1046/j.1432-1327.1998.2510504.x. [DOI] [PubMed] [Google Scholar]
- 36.Volck B, Price PA, Johansen JS, Sørensen O, Benfield TL, Nielsen HJ, Calafat J, Borregaard N. YKL-40, a mammalian member of the chitinase family, is a matrix protein of specific granules in human neutrophils. Proc Assoc Am Physicians. 1998;110:351–360. [PubMed] [Google Scholar]
- 37.Johansen JS, Christoffersen P, Møller S, Price PA, Henriksen JH, Garbarsch C, Bendtsen F. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol. 2000;32:911–920. doi: 10.1016/s0168-8278(00)80095-1. [DOI] [PubMed] [Google Scholar]
- 38.Shiratori Y, Omata M. Predictors of the efficacy of interferon therapy for patients with chronic hepatitis C before and during therapy: how does this modify the treatment course? J Gastroenterol Hepatol. 2000;15 Suppl:E141–E151. doi: 10.1046/j.1440-1746.2000.02116.x. [DOI] [PubMed] [Google Scholar]
- 39.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 40.Mathurin P, Moussalli J, Cadranel JF, Thibault V, Charlotte F, Dumouchel P, Cazier A, Huraux JM, Devergie B, Vidaud M, et al. Slow progression rate of fibrosis in hepatitis C virus patients with persistently normal alanine transaminase activity. Hepatology. 1998;27:868–872. doi: 10.1002/hep.510270333. [DOI] [PubMed] [Google Scholar]
- 41.Omata M, Shiratori Y. Long-term effects of interferon therapy on histology and development of hepatocellular carcinoma in hepatitis C. J Gastroenterol Hepatol. 2000;15 Suppl:E134–E140. doi: 10.1046/j.1440-1746.2000.02115.x. [DOI] [PubMed] [Google Scholar]
- 42.Reichard O, Glaumann H, Frydén A, Norkrans G, Schvarcz R, Sönnerborg A, Yun ZB, Weiland O. Two-year biochemical, virological, and histological follow-up in patients with chronic hepatitis C responding in a sustained fashion to interferon alfa-2b treatment. Hepatology. 1995;21:918–922. [PubMed] [Google Scholar]
- 43.Manesis EK, Papaioannou C, Gioustozi A, Kafiri G, Koskinas J, Hadziyannis SJ. Biochemical and virological outcome of patients with chronic hepatitis C treated with interferon alfa-2b for 6 or 12 months: a 4-year follow-up of 211 patients. Hepatology. 1997;26:734–739. doi: 10.1002/hep.510260327. [DOI] [PubMed] [Google Scholar]


