Abstract
AIM: To improve the immunogenicity of receptor binding site of hepatitis B virus (HBV) on preS1 antigen using HBV core antigen as an immuno-carrier.
METHODS: One to 6 tandem copies of HBV preS1 (21-47) fragment were inserted into HBcAg at the sites of aa 78 and 82, and expressed in E.coli. ELISA, Western blot and animal immunization were used to analyze the antigenicity and immmunogenicity of purified particulate antigens. The ability to capture HBV by antibodies elicited by chimeric particles was detected with immuno-capture PCR.
RESULTS: Recombinant antigens CI, CII, CIII carrying 1-3 copies of HBV preS1 (21-47) individually could form virus-like particles (VLPs), similar to HBcAg in morphology. But recombinant antigens carrying 4-6 copies of HBV preS1 (21-47) were poorly expressed in E.coli. Chimeric antigens were lacking of immunoreactivity with anti-HBc monoclonal antibodies (McAbs), but still reserved good immunoreactivity with anti-HBe McAbs. CI, CII, CIII could strongly react with anti-preS1 McAb, suggesting that preS1 (21-47) fragment was well exposed on the surface of chimeric VLPs. Three chimeric VLP antigens (CI, CII and CIII) could stimulate mice to produce high-level antibody responses, and their immunogenicity was stronger than non-particulate antigen 21-47*6, containing 6 copies of preS1 (21-47). Mouse antibodies to CI, CII and CIII were able to capture HBV virions in immuno-capture PCR assay in vitro.
CONCLUSION: Chimeric particulate antigens of receptor binding site-core antigen of HBV can elicit strong antibody responses to preS1. They have a potential to be developed into prophylactic or therapeutic vaccines against HBV infection.
Keywords: Hepatitis B Virus, Chimeric particulate antigens, preS1 antigen, HBcAg
INTRODUCTION
Chronic hepatic diseases and hepatocellular carcinoma (HCC) caused by HBV infection are the public health problem of worldwide importance[1-3]. It is estimated that there are 350 million HBV carriers worldwide[4]. Though prophylactic vaccines against HBV have been proved to be a great success, approximately 5-10% of individuals are non-responsive or poorly responsive to conventional HB vaccines[5-9]. Meanwhile, there are still more than 350 million adult chronic HBV carriers and tens of million patients with chronic hepatic diseases in the world. If they could not be treated, it means that tens of million people would die of hepatocirrhosis or HCC in the next 20-30 years[10]. Unfortunately, there are no satisfactory measures to treat HBV infection so far[11-15]. It is necessary to develop new anti-virus methods, such as therapeutic vaccines.
Previous studies have shown that HBV preS1 (21-47) region is assigned to the hepatocyte receptor binding site, and antibody to preS1 (21-47) is virus-neutralizing[16-21]. The anti-preS1 antibody could represent an early serological marker for HBV clearance and health improvement or recovery from the disease[22,23]. It seems reasonable that incorporation of preS1 (21-47) into prophylactic or therapeutic vaccines might be a potential strategy to overcome non-responsiveness to HBsAg vaccines or to develop new therapeutic vaccines against HBV[24]. Some reports have mentioned the effect of vaccines carrying preS1, but the antibody responses against preS1 are very weak[25,26]. The introduction of preS1 did not lead to obvious improvements in the present HBsAg vaccines[27].
HBcAg is often used as an immuno-carrier to develop immunogens and vaccines because it could auto-assemble into VLPs in vitro[28-31]. The region between positions 78 and 83 of HBcAg (major immunodominant region, MIR) is surface accessible[32,33]. After foreign peptides are introduced into this position, the assembly of HBcAg is unaffected and inserting peptide could be exposed on the surface of VLPs[34-36]. Thus the position is an ideal site for foreign epitope inserting. Some reports have mentioned the features of chimeric HBc-preS1 VLPs, and shown that the immunogenicity of preS1 epitope could be improved when fused in MIR of HBc[37-39]. Based on these advantages, several peptide fragments containing different copies of preS1 (21-47) were inserted into MIR of HBcAg to further improve the immunogenicity of preS1 (21-47).
MATERIALS AND METHODS
Bacterial strains and plasmids
E.coli strain ER2566 and pMD 18-T vector were purchased from New England Biolabs Inc. and TAKARA Co. respectively. Expression vector pTO-T7 was constructed in our laboratory[40]. Clones containing genes of HBV adw2 strain (GenBank accession number: AF233236) in vector pMD 18-T were preserved in our laboratory. They were plasmid pT-C183 containing full-length HBV core gene; plasmids pT-21-47*1, pT-21-47*2, pT-21-47*3, pT-21-47*4, pT-21-47*5, pT-21-47*6 containing one to six copies of HBV preS1 (21-47) individually; and tandem preS1 (21-47) fragments spaced by GS-encoding linkers.
Immunological reagents
Recombinant protein 21-47*6, composed of six tandem copies of HBV preS1 (21-47), was prepared in our laboratory. McAbs to HBcAg and HBeAg were provided by Beijing Wantai Biological Medcine Co. Goat anti-mouse IgG-alkaline phosphatase conjugate and substrates BCIP, NBT were purchased from Boehinger Mannheim. McAb (4D11) to HBV preS1 (21-47) and HRP-conjugated 4D11 were prepared in our laboratory.
A synthetic peptide encompassing the 21-47 amino acids of HBV adw2 preS1 (PLGFFPDHQLDPAFG ANSNNPDWDFNP) was synthesized by MMG Co. (Germany), purified on a C18 column by HPLC, lyophilized and freshly resuspended in PBS before use. The peptide composition was confirmed by amino acid analysis. The purity of the peptide was higher than 95%.
Plasmid construction
Plasmid pC149/wt was constructed by cloning the N-terminus of HBcAg (aa1-149) encoding sequence into pTO-T7 at the BamH I/EcoRI sites. Site-directed mutagenesis was performed by PCR using Taq DNA polymerase, pC149/wt and the following primers: 5’-GTGGTACTGGATCCGGTGGTGGAGGTTCAGGAGGAGGTGGTAGGGAATTAGTAGTCAG-3’ (sense) and 5’-CCGGATCCAGTACCACCACCTCCAGAACCACCTCCACCATCTTCCAAATTACTTCC-3’ (anti-sense), and two HBc terminal primers 5’-GGATCCCATATGGACATTGACCCA and 5’-GAATTCTTAAAAACAGTAGTTTCCGG-3’. The amino acid-encoding sequence of PCR product (C149/mut) was HBc (1-78)-G4SG4T-GS-G4SG4-HBc (82-149). In fragment C149/mut, amino acid 79-81-encoding sequence of HBcAg was replaced by a linker (G4SG4T-GS-G4SG4), in which GS-encoding sequence was a BamH I site. Plasmid pC149/mut was constructed by cloning fragment C149/mut into pTO-T7 at the Nde I/EcoR I sites.
Plasmids pC149/21-47*1, pC149/21-47*2, pC149/21-47*3, pC149/21-47*4, pC149/21-47*5 and pC149/21-47*6 were constructed by cloning the Bgl II/BamH I digested fragment of pT-21-47*1, pT-21-47*2, pT-21-47*3, pT-21-47*4, pT-21-47*5 and pT-21-47*6 into pc149/mut at the BamH I sites. The construction method of each of the recombinant plasmids pC149/21-47*6 is shown in Figure 1.
Figure 1.
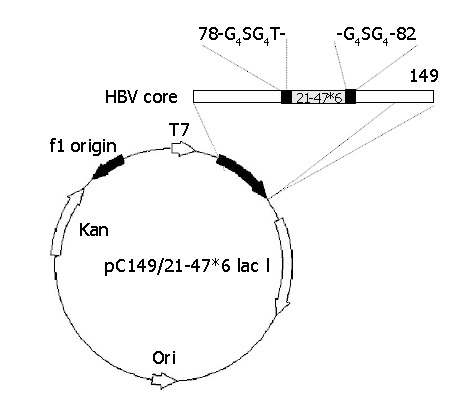
Construction of plasmid pC149/21-47*6. The horizontal bar represents the chimeric HBc-preS1 (21-47) protein. The insertion foreign DNA designed such that the 21-47*6 sequence, was flanked on both sides by glycine-rich linkers, and inserted into the major immunodominant region (MIR) of truncated, assembly-competent core protein derivative core 1-149. The authentic amino acids of 79-81 were removed.
Expression of recombinant HBV proteins in E.coli
The fresh overnight culture of ER2566 (DE3) (2 mL) carrying recombinant expression plasmids was diluted with 200 mL of fresh LB medium in the presence of 100 mg/L kanamycin and grown to A600 = 0.8 at 37 °C at a shaking speed of 210 r/min. The culture was induced by adding IPTG to a final concentration of 0.2 mmol/L at 32 °C for 3 h. The culture (1 mL) was harvested by centrifugation, cell pellets were resuspended in 400 μL SDS-PAGE loading buffer, aliquots were run on 13.5% SDS-PAGE gels.
Purification of recombinant HBV proteins
The harvested bacterial pellets were resuspended in PBS. After ultrasonication and high speed centrifugation, supernatants were collected and precipitated by 40% saturated ammonium sulfate. Precipitates were resuspended with PBS (pH 7.4) and then dialyzed four times in PBS. The obtained proteins were further purified with TSK-GEL SW3000 column for gel filtration and then with DEAE-5PW column (TOSOH) for anion exchange chromatography. The concentration of proteins was determined by Bradford assay. The purified recombinant proteins were stored at -20 °C prior to use.
Western blot analysis of recombinant proteins
Total cell lysates were run on SDS-PAGE gels and transferred electrophoretically to nitrocellulose membrane for 1 h under the voltage of 100 V. Then the membrane was incubated in blocking solution (5% skim milk in Tris-buffered saline, TBS) for 1 h at room temperature followed by incubation at room temperature for 2 h with anti-preS1 (21-47) McAb 4D11 that was prediluted to 1:200 with blocking solution. The membrane was washed three times with TTBS (0.05% Tween 20 in TBS) for 10 min, and alkaline phosphatase-labeled goat anti-human IgG antibodies diluted in TTBS (1:2000) were exposed to the membrane at room temperature for 1 h, and washed three times for 10 min with TTBS, and the membrane was visualized with a substrate solution of BCIP and NBT.
Electron microscopy
After high speed centrifugation, the purified recombinant proteins were analyzed by negative staining electron microscopy. Samples were absorbed to 200 mesh copper grids for 5 min. The grids were washed twice with ddH2O and air-dried. Specimens were negatively contrasted using 1.5% uranyl acetate for 5 min.
Immunizations
Eighteen female BALB/c mice aged from 7 to 12 wk were randomly assigned to one of groups, 3 each group. Four groups were immunized with 20 μg of CI, CII, CIII or 21-47*6 absorbed onto the Freund’s adjuvant at wk 0 and 5. The other two groups were immunized with 20 μg of CII or 50 μg of 21-47*6 without adjuvant at wk 0, 5 and 16. Sera were collected by tail bleeding and assayed for immuno-PCR or anti-preS1 (21-47) and anti-HBc by ELISA.
ELISA
Five VLP antigens (C149/wt, C149/mut, CI, CII and CIII) were coated to microplates in a quantity of 1 μg each well in 50 mmol/L carbonate buffer (pH9.6) for 2 h at 37 °C and overnight at 4 °C. Lysate of E.coli ER2566 carrying pGFP was used as a negative coating sample (10 μg/well). Plates were washed with PBS containing 0.05% Tween 20 and blocked with blocking buffer (0.05% Tween 20 and 1% bovine serum albumin in PBS) for 2 h at 37 °C. Three McAbs to HBe and 3 McAbs to HBc (1:1000) were applied for 30 min at 37 °C. Peroxidase-conjugated goat anti-human IgG used as secondary antibody was incubated for 30 min at 37 °C and visualized with o-phenyl-diamine-2HCl (10 g/L in 5 mmol/L Tris-HCl, pH7.0). Between each step wells were washed 5 times with PBST (0.05% Tween 20 in PBS). The reaction was stopped with 50 μL of 2 mol/L H2SO4. Absorption was measured at A450/620.
Synthetic peptide of preS1 (21-47) was used as a coating antigen (0.3 μg/well), and HRP-conjugated goat anti-mouse IgG was used as secondary antibody to develop indirect ELISA for detecting antibodies to preS1 in the pooled sera. A positive result was defined as an absorbance value of great than 2-fold the absorbance of negative control with a cut-off of 0.05. Anti-preS1 titers for a group of animals were expressed as geometric mean titers (GMT) of individual animal values, which were themselves the average of triplicate assays. Seroconversion was defined as a titer ≥100.
HBcAb in the pooled sera was detected with HBV ELISA kit (Beijing Wantai Biological Medicine Co.).
Immuno-capture PCR
Virus capture capability of antibodies in immune sera was carried out by immuno-capture PCR, based on the method of affinity capture detection of HCV with a few modifications. Briefly, 0.5 mL microcentrifuge tubes were coated with 25 μL goat anti-mouse IgG incubated at room temperature for 1 h. After blocking with 5% skim milk in PBST for 15 min at 37 °C and overnight at 4 °C, tubes were aspirated and washed with 250 μL PBST. The mouse antisera were adjusted to same titer of 1:100 by dilution with PBS, to avoid variations of the results caused by different antibody titers in different samples. Tubes were subsequently loaded with 25 μL properly diluted mouse immune antisera and 25 μL purified HBV particles (1×107 HBV genome Equivalent per mL, purchased from Institute of Virology, Wuhan, China) diluted 1:50 in PBS. The mixtures were mixed gently and incubated for 1 h at room temperature and overnight at 4 °C. Tubes were then washed five times with 250 μL PBST. Captured viruses were detected with HBV PCR which specifically amplifies a 360 bp fragment in the 5’-terminus of HBV core gene. HBV, which was captured directly by a McAb to HBsAg α epitope (kindly provided by Dr. Qiu, Beijing Wantai Biological Medcine Co.) was used as positive control.
RESULTS
Expression and purification of recombinant proteins
One to 6 tandem copies of HBV preS1 (21-47) fragment were inserted into HBcAg at the sites of aa 78 and 82. As a result, two plasmids containing HBV core gene and another six plasmids containing HBV core gene and preS1 (21-47) region were constructed (Table 1). The eight plasmids were transformed into E.coli ER2566 and induced with IPTG. SDS-PAGE showed five of them efficiently expressed (data of C149/wt not shown). The yields of them were about 20% in total bacterial proteins (Figure 2) and the productions were in soluble form (data not shown). The other three recombinant proteins CIV, CV and CVI could not be produced obviously.
Table 1.
Source of inserted fragment of expression vectors.
| Number | Plasmid | Recom binant antigen | Insertion fragment |
| 1 | pC149/wt | C149/wt | - |
| 2 | pC149/mut | C149/mut | - |
| 3 | pC149/21-47*1 | CI | Single copy of preS1 (21-47) |
| 4 | pC149/21-47*2 | CII | Two copies of preS1 (21-47) |
| 5 | pC149/21-47*3 | CIII | Three copies of preS1 (21-47) |
| 6 | pC149/21-47*4 | CIV | Four copies of preS1 (21-47) |
| 7 | pC149/21-47*5 | CV | Five copies of preS1 (21-47) |
| 8 | pC149/21-47*6 | CVI | Six copies of preS1 (21-47) |
Figure 2.
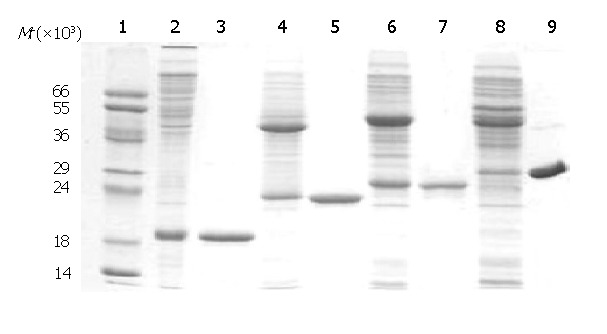
SDS-PAGE analysis of purified recombinant antigens. 1: protein MW marker; 2: C149/mut; 3: purified C149/mut; 4: CI; 5: purified CI; 6: CII; 7: purified CII; 8: CIII; 9: purified CIII.
Recombinant proteins C149/mut, CI, CII and CIII in the supernatants were precipitated by 40% saturated ammonium sulfate, and further purified by using TSK-GEL columns and with DEAE-5PW columns. Finally, the degrees of purity of all the proteins were over 85% (Figure 2).
The five putative particles C149/wt, C149/mut, CI, CII and CIII were demonstrated by negative-stain electron microscopy. Numerous empty core-like particles with an average diameter of 30 nm were observed (Figure 3). All of them looked much more uniform and regular.
Figure 3.
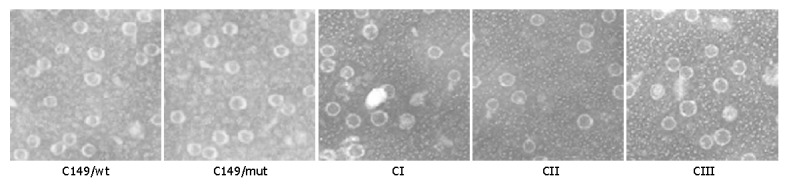
Transmission electron microscopy of VLP antigens purified from E.coli. The VLPs were stained with 1.5% uranyl acetate and photographed at 67000 magnification.
Immunoreactivity with anti-HBc, HBe and preS1 McAbs
To test the immunoreactivity of five VLP antigens with anti-HBc, HBe and preS1, ELISA was performed as described in “Materials and Methods”. The negative control (E.coli lysates containing GFP protein) did not react with all seven McAbs (Figure 4). All of five VLP antigens had a good reactivity with three anti-HBe McAbs, but their immunoreactivity with anti-HBc McAbs was very different. Native HBc VLPs (C149/wt) reacted strongly with all three anti-HBc McAbs. However, variant HBc VLPs (C149/mut) only reacted weakly with one of the anti-HBc McAbs. The three HBc-preS1 (21-47) chimeric VLPs also reacted weakly with one of the anti-HBc McAbs similar to C149/mut. Above results indicated that the dominant B cell epitope of HBc was destroyed because of the deletion of MIR and further destroyed by insertion of foreign epitopes. Three HBc-preS1 (21-47) chimeric VLPs had good immunoactivity with anti-preS1 McAb, indicating that foreign epitope preS1 (21-47) could be displayed on the surface of HBV core-like particles.
Figure 4.
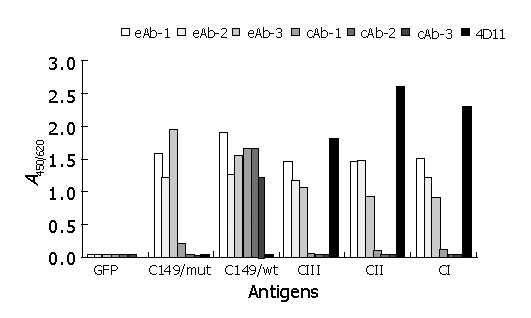
Reactivity of recombinant antigens with different HBV McAbs by ELISA. GFP: cell lysates containing recombinant GFP protein at 100 mg/L as negative coating control; recombinant proteins C149/wt, C149/mut, CI, CII and CIII at 10 mg/L as coating antigens; eAb-1, eAb-2, and eAb-3: three HBe-specific McAbs; cAb-1, cAb-2, and cAb-3: three HBc-specific McAbs; 4D11: anti-preS1 McAb.
Western blot of chimeric VLP antigens with anti-preS1 McAb
Purified antigens CI, CII and CIII were separated by SDS-PAGE and transferred electrophoretically to nitrocellulose for immunoassay with HRP-conjugated McAb against preS1 (4D11). As shown in Figure 5, all three antigens could react with 4D11 under either reducing or non-reducing conditions. Under non-reducing conditions, there appeared two bands on the membrane indicating chimeric HBc-preS1 (21-47) dimer might be connected by disulfide bonds. The result illustrated that CI, CII and CIII had good immunoactivity specific to antigen preS1 (21-47).
Figure 5.
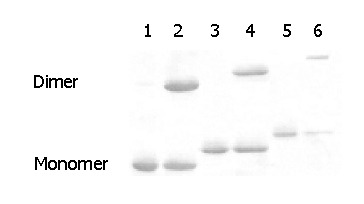
Western blot analysis of recombinant proteins CI, CII and CIII against anti-preS1 McAb (4D11) under reducing or nonreducing conditions. Lanes 1, 3, and 5: purified CI, CII and CIII treated with 500 mmol/L DTT; lanes 2, 4, and 6: purified CI, CII and CIII not treated with DTT.
Immunogenicity of chimeric VLP antigens in BALB/c
BALB/c mice were immunized with VLP antigens CI, CII, CIII or with non-VLP antigen 21-47*6. As Figure 6 shows, in contrast to 21-47*6, anti-preS1 antibody responses induced by CI, CII and CIII were rapid and strong. When Freund’s adjuvant was used, immunization of mice with VLP antigens gave 100% seroconversion (anti-preS1 titer>100) and these had high antibody titers (>600) at wk 1. While the titer of 21-47*6-injected mouse sera was lower than 1:100 at wk 1. When no adjuvant was used, CII gave anti-preS1 titer 200 times higher than 21-47*6 at wk 20 (P<0.05). The immunogenicity of three VLP antigens was similar, and CII was a little better than the others. It was noteworthy that anti-HBc antibodies were not detected in sera of the mice immunized with three chimeric VLPs during the whole observation.
Figure 6.
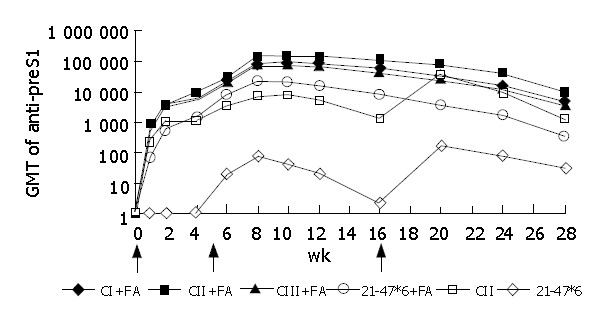
Dynamics of anti-preS1 responses of BALB/c mice immunized with CI, CII, CIII or 21-47*6 with Freund’s adjuvant (FA) at wk 0 and 5 (arrows) or immunized with CII and 21-47*6 without adjuvant at weeks 0, 5 and 16 (arrows). Mice were immunized by i.m. injection of proteins: 20 μg CI, CII, CIII or 50 μg 21-47*6.
Immuno-capture of anti-CII sera to HBV virions
To evaluate the specificity of anti-preS1 antibodies elicited by chimeric particles, immuno-capture PCR was applied (Figure 7). The results indicated that all the three sera from mice immunized with CII were able to capture HBV virions in vitro, while that of immunized with C149/mut could not capture HBV virions in vitro.
Figure 7.
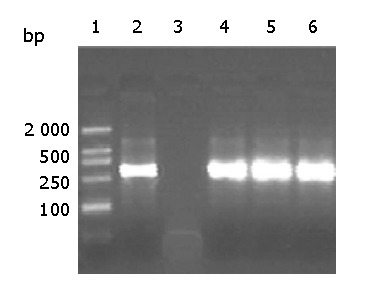
Detection of HBV captured by mouse antisera by PCR. After immuno-capture of HBV from purified HBV virions, by antisera from three mice immunized with CII alone, HBV DNA was detected by PCR. 1: molecular markers 2: the positive control using anti-HBs monoclonal antibody 3: mouse serum injected with C149/mut 4-6: mouse sera immunized with CII alone at wk 2, 10 and 28 respectively.
DISCUSSION
After foreign epitopes were inserted into several areas of HBc shells, HBc could correctly fold and self-assemble into VLPs in all viable prokaryotic and eukaryotic expression systems[31,36]. Meanwhile, foreign epitopes were often exposed to the protruding spike of VLPs. HBc carrier could provide a high level of B cell and T cell immunogenicity for foreign epitopes. Being a display carrier for foreign epitopes, HBc has more advantages over other proposed particulate carriers[28]. HBc protein consists roughly of assembly domain (1-149) and RNA-binding domain (150-183)[29]. After deletion of RNA-binding domain, the rest of HBc still could assemble into VLPs. In this study, assembly domain-encoding (1-149) sequence of HBc was cloned and three amino acids were deleted (79-81) in the MIR in order to minimize the interfering of HBc MIR to foreign epitope-specific immunoresponse. The construct (antigen C149/mut) eliminates the major immunodominant epitope of HBcAg as well as the reactivity with three HBc-specific McAbs, while it still reserves the reactivity with HBe-specific McAbs.
One to 6 serial copies of HBV preS1 (21-47) fragment were inserted into the MIR-encoding region of pC149/mut and expressed in E.coli. Recombinant antigens carrying 1-3 copies of preS1 (21-47) (CI, CII, and CIII) could form VLPs analogous to native HBcAg morphologically, but the three recombinant antigens carrying 4-6 copies of preS1 (21-47) were expressed poorly. In one study of Kratze et al[34], after GFP was inserted into HBc MIR, the chimeric protein was still efficiently expressed in E.coli. Contrary to GFP of 238 amino acids in length, the full length of four preS1 (21-47) was only 164. These results reveal that HBc MIR has different capability of accepting different foreign epitope insertions.
In the Western blot analysis for VLP chimeric antigens, only the monomer band could be seen after treatment with DTT, while an additional band of dimer appeared in the absence of DTT. The observation suggested disulfide bonds might be responsible for the formation of dimers of chimeric HBcAg. This conclusion is in agreement with the previously reported results[41,42].
The fact that antigens CI, CII and CIII could be strongly recognized by anti-preS1 McAb indicates that epitope preS1 (21-47) can be well displayed. In this study, three VLP antigens could stimulate mice to produce high-level antibody responses, and their immunogenicity was prior to non-particulate antigen 21-47*6. Moreover, immuno-capture PCR assay shows multiclonal antibody elicited by chimeric VLP antigen (CII) could capture HBV virions in vitro. These data indicate that the three VLP antigens CI, CII and CIII have a potential to be developed into assistant prophylactic or therapeutic vaccines. It may be a feasible strategy for vaccine designs to improve the immunogenicity of specific peptides in virtue of the display capability of HBcAg.
Footnotes
Supported by the Excellent Scholar Incubation Plan of Ministry of Education, China
Edited by Wang XL and Xu CT
References
- 1.Worman HJ, Feng L, Mamiya N. Molecular biology and the diagnosis and treatment of liver diseases. World J Gastroenterol. 1998;4:185–191. doi: 10.3748/wjg.v4.i3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942–1956. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Merican I, Guan R, Amarapuka D, Alexander MJ, Chutaputti A, Chien RN, Hasnian SS, Leung N, Lesmana L, Phiet PH, et al. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000;15:1356–1361. doi: 10.1046/j.1440-1746.2000.0150121356.x. [DOI] [PubMed] [Google Scholar]
- 4.Kane MA. Global status of hepatitis B immunisation. Lancet. 1996;348:696. doi: 10.1016/S0140-6736(05)65598-5. [DOI] [PubMed] [Google Scholar]
- 5.Cassidy WM. Adolescent hepatitis B vaccination. A review. Minerva Pediatr. 2001;53:559–566. [PubMed] [Google Scholar]
- 6.Suzuki T, Yamauchi K, Kuwata T, Hayashi N. Characterization of hepatitis B virus surface antigen-specific CD4+ T cells in hepatitis B vaccine non-responders. J Gastroenterol Hepatol. 2001;16:898–903. doi: 10.1046/j.1440-1746.2001.02530.x. [DOI] [PubMed] [Google Scholar]
- 7.McDermott AB, Cohen SB, Zuckerman JN, Madrigal JA. Hepatitis B third-generation vaccines: improved response and conventional vaccine non-response--evidence for genetic basis in humans. J Viral Hepat. 1998;5 Suppl 2:9–11. doi: 10.1046/j.1365-2893.1998.0050s2009.x. [DOI] [PubMed] [Google Scholar]
- 8.Kubba AK, Taylor P, Graneek B, Strobel S. Non-responders to hepatitis B vaccination: a review. Commun Dis Public Health. 2003;6:106–112. [PubMed] [Google Scholar]
- 9.Pumpens P, Grens E, Nassal M. Molecular epidemiology and immunology of hepatitis B virus infection - an update. Intervirology. 2002;45:218–232. doi: 10.1159/000067915. [DOI] [PubMed] [Google Scholar]
- 10.André F. Hepatitis B epidemiology in Asia, the Middle East and Africa. Vaccine. 2000;18 Suppl 1:S20–S22. doi: 10.1016/s0264-410x(99)00456-9. [DOI] [PubMed] [Google Scholar]
- 11.Lau GK. Use of immunomodulatory therapy (other than interferon) for the treatment of chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2000;15 Suppl:E46–E52. doi: 10.1046/j.1440-1746.2000.02102.x. [DOI] [PubMed] [Google Scholar]
- 12.Torresi J, Locarnini S. Antiviral chemotherapy for the treatment of hepatitis B virus infections. Gastroenterology. 2000;118:S83–103. doi: 10.1016/s0016-5085(00)70008-4. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Tang B. Effect on replication of hepatitis B virus by Chinese traditional medicine. Shijie Huaren Xiaohua Zazhi. 2000;8:945–946. [Google Scholar]
- 14.Kubba AK, Taylor P, Graneek B, Strobel S. Non-responders to hepatitis B vaccination: a review. Commun Dis Public Health. 2003;6:106–112. [PubMed] [Google Scholar]
- 15.Malik AH, Lee WM. Chronic hepatitis B virus infection: treatment strategies for the next millennium. Ann Intern Med. 2000;132:723–731. doi: 10.7326/0003-4819-132-9-200005020-00007. [DOI] [PubMed] [Google Scholar]
- 16.Bock CT, Tillmann HL, Manns MP, Trautwein C. The pre-S region determines the intracellular localization and appearance of hepatitis B virus. Hepatology. 1999;30:517–525. doi: 10.1002/hep.510300206. [DOI] [PubMed] [Google Scholar]
- 17.Neurath AR, Seto B, Strick N. Antibodies to synthetic peptides from the preS1 region of the hepatitis B virus (HBV) envelope (env) protein are virus-neutralizing and protective. Vaccine. 1989;7:234–236. doi: 10.1016/0264-410x(89)90235-1. [DOI] [PubMed] [Google Scholar]
- 18.Paran N, Geiger B, Shaul Y. HBV infection of cell culture: evidence for multivalent and cooperative attachment. EMBO J. 2001;20:4443–4453. doi: 10.1093/emboj/20.16.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neurath AR, Strick N, Sproul P, Ralph HE, Valinsky J. Detection of receptors for hepatitis B virus on cells of extrahepatic origin. Virology. 1990;176:448–457. doi: 10.1016/0042-6822(90)90014-i. [DOI] [PubMed] [Google Scholar]
- 20.Petit MA, Dubanchet S, Capel F. A monoclonal antibody specific for the hepatocyte receptor binding site on hepatitis B virus. Mol Immunol. 1989;26:531–537. doi: 10.1016/0161-5890(89)90004-7. [DOI] [PubMed] [Google Scholar]
- 21.Wei J, Liu XJ, Wang YQ, Lu ZM, Li GD, Wang Y, Zhang ZC. Development of the diagnostic immunoassay to detect anti-PreS1(21-47aa) antibody--a marker suggesting the health improvement of hepatitis B patients. Clin Chim Acta. 2002;317:159–169. doi: 10.1016/s0009-8981(01)00783-5. [DOI] [PubMed] [Google Scholar]
- 22.Mi Z, Feng F, Zhang X, Lian Z, Wang H, Tong Y. The clinical and epidemiological significance of serum PreS1/anti-PreS1 in HBV infection. Chin Med J (Engl) 1999;112:321–324. [PubMed] [Google Scholar]
- 23.Ryu CJ, Kim YK, Hur H, Kim HS, Oh JM, Kang YJ, Hong HJ. Mouse monoclonal antibodies to hepatitis B virus preS1 produced after immunization with recombinant preS1 peptide. Hybridoma. 2000;19:185–189. doi: 10.1089/02724570050031248. [DOI] [PubMed] [Google Scholar]
- 24.Milich DR. T- and B-cell recognition of hepatitis B viral antigens. Immunol Today. 1988;9:380–386. doi: 10.1016/0167-5699(88)91239-X. [DOI] [PubMed] [Google Scholar]
- 25.Madalinski K, Sylvan SP, Hellström U, Mikolajewicz J, Zembrzuska-Sadkowska E, Piontek E. Antibody responses to preS components after immunization of children with low doses of BioHepB. Vaccine. 2001;20:92–97. doi: 10.1016/s0264-410x(01)00312-7. [DOI] [PubMed] [Google Scholar]
- 26.Leroux-Roels G, Desombere I, Cobbaut L, Petit MA, Desmons P, Hauser P, Delem A, De Grave D, Safary A. Hepatitis B vaccine containing surface antigen and selected preS1 and preS2 sequences. 2. Immunogenicity in poor responders to hepatitis B vaccines. Vaccine. 1997;15:1732–1736. doi: 10.1016/s0264-410x(97)00118-7. [DOI] [PubMed] [Google Scholar]
- 27.Eyigün CP, Yilmaz S, Gül C, Sengül A, Hacibektasoglu A, Van Thiel DH. A comparative trial of two surface subunit recombinant hepatitis B vaccines vs a surface and PreS subunit vaccine for immunization of healthy adults. J Viral Hepat. 1998;5:265–269. doi: 10.1046/j.1365-2893.1998.00106.x. [DOI] [PubMed] [Google Scholar]
- 28.Pumpens P, Grens E. Hepatitis B core particles as a universal display model: a structure-function basis for development. FEBS Lett. 1999;442:1–6. doi: 10.1016/s0014-5793(98)01599-3. [DOI] [PubMed] [Google Scholar]
- 29.Böttcher B, Wynne SA, Crowther RA. Determination of the fold of the core protein of hepatitis B virus by electron cryomicroscopy. Nature. 1997;386:88–91. doi: 10.1038/386088a0. [DOI] [PubMed] [Google Scholar]
- 30.Zlotnick A, Cheng N, Conway JF, Booy FP, Steven AC, Stahl SJ, Wingfield PT. Dimorphism of hepatitis B virus capsids is strongly influenced by the C-terminus of the capsid protein. Biochemistry. 1996;35:7412–7421. doi: 10.1021/bi9604800. [DOI] [PubMed] [Google Scholar]
- 31.Seifer M, Standring DN. Assembly and antigenicity of hepatitis B virus core particles. Intervirology. 1995;38:47–62. doi: 10.1159/000150414. [DOI] [PubMed] [Google Scholar]
- 32.Zlotnick A, Cheng N, Stahl SJ, Conway JF, Steven AC, Wingfield PT. Localization of the C terminus of the assembly domain of hepatitis B virus capsid protein: implications for morphogenesis and organization of encapsidated RNA. Proc Natl Acad Sci USA. 1997;94:9556–9561. doi: 10.1073/pnas.94.18.9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conway JF, Cheng N, Zlotnick A, Stahl SJ, Wingfield PT, Belnap DM, Kanngiesser U, Noah M, Steven AC. Hepatitis B virus capsid: localization of the putative immunodominant loop (residues 78 to 83) on the capsid surface, and implications for the distinction between c and e-antigens. J Mol Biol. 1998;279:1111–1121. doi: 10.1006/jmbi.1998.1845. [DOI] [PubMed] [Google Scholar]
- 34.Kratz PA, Böttcher B, Nassal M. Native display of complete foreign protein domains on the surface of hepatitis B virus capsids. Proc Natl Acad Sci USA. 1999;96:1915–1920. doi: 10.1073/pnas.96.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pumpens P, Razanskas R, Pushko P, Renhof R, Gusars I, Skrastina D, Ose V, Borisova G, Sominskaya I, Petrovskis I, et al. Evaluation of HBs, HBc, and frCP virus-like particles for expression of human papillomavirus 16 E7 oncoprotein epitopes. Intervirology. 2002;45:24–32. doi: 10.1159/000050084. [DOI] [PubMed] [Google Scholar]
- 36.Pumpens P, Grens E. HBV core particles as a carrier for B cell/T cell epitopes. Intervirology. 2001;44:98–114. doi: 10.1159/000050037. [DOI] [PubMed] [Google Scholar]
- 37.Hui J, Li G, Kong Y, Wang Y. Expression and characterization of chimeric hepatitis B surface antigen particles carrying preS epitopes. J Biotechnol. 1999;72:49–59. doi: 10.1016/s0168-1656(99)00049-8. [DOI] [PubMed] [Google Scholar]
- 38.Hui J, Mancini M, Li G, Wang Y, Tiollais P, Michel ML. Immunization with a plasmid encoding a modified hepatitis B surface antigen carrying the receptor binding site for hepatocytes. Vaccine. 1999;17:1711–1718. doi: 10.1016/s0264-410x(98)00430-7. [DOI] [PubMed] [Google Scholar]
- 39.Xu X, Li GD, Kong YY, Yang HL, Zhang Z, Cao HT, Wang Y. A modified hepatitis B virus surface antigen with the receptor-binding site for hepatocytes at its C terminus: expression, antigenicity and immunogenicity. J Gen Virol. 1994;75(Pt 12):3673–3677. doi: 10.1099/0022-1317-75-12-3673. [DOI] [PubMed] [Google Scholar]
- 40.Luo WX, Zhang J, Yang HJ, Li SW, Xie XY, Pang SQ, Li SJ, Xia NS. Construction and application of an Escherichia coli high effective expression vector with an enhancer. ShengWu GongCheng XueBao. 2000;16:578–581. [PubMed] [Google Scholar]
- 41.Zhou S, Standring DN. Hepatitis B virus capsid particles are assembled from core-protein dimer precursors. Proc Natl Acad Sci USA. 1992;89:10046–10050. doi: 10.1073/pnas.89.21.10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng J, Schödel F, Peterson DL. The structure of hepadnaviral core antigens. Identification of free thiols and determination of the disulfide bonding pattern. J Biol Chem. 1992;267:9422–9429. [PubMed] [Google Scholar]


