Abstract
AIM: To investigate the therapeutic effect of somatostatin receptor type 2 (SSTR2) gene transfection on pancreatic carcinoma xenografts in vivo in experimental cancers.
METHODS: Human pancreatic cancer cell line Panc-1 was inoculated subcutaneously into the back of nude mice. When tumor nodules were grown as large as about 5 mm×5 mm days after inoculation, the mice were randomly divided into 3 groups (6 mice in each group). Group I served as untreated control group. Group II received an intratumoral injection of a combination of human cytomegalovirus promoter-6C (pCMV-6C) and lipofectamine 2000. Group III received an intratumoral injection of a combination of pCMV-6C-SSTR2 and lipofectamine 2000. The rate of tumor growth was compared among these three groups. The expression of SSTR2 in these tumors was detected by immunohistochemistry and Western-blot. Apoptosis index (AI) in these tumors was examined by using TUNEL in situ.
RESULTS: Intratumoral injection of a combination of pCMV-6C-SSTR2 and lipofectamine 2000 resulted in the expression of SSTR2 protein. The tumor size and weight in group III (0.318±0.098 cm3, and 0.523±0.090 g, respectively) were significantly lower than those in group I (2.058±0.176 cm3, and 1.412±0.146 g, respectively) and group II (2.025±0.163 cm3, and 1.365±0.116 g, respectively) (P<0.05) The AI in group III (1.47±0.13%) was significantly higher than that in group I (0.56±0.09%) and group II (0.57±0.11%) (P<0.05). But there were no significant differences between groups I and II.
CONCLUSION: Our data demonstrate that re-expression of SSTR2 gene has antitumor effects on experimental pancreatic cancer. Restoration of SSTR2 gene expression through gene transfer in vivo might be a potential gene therapy strategy for human pancreatic cancer.
Keywords: Pancreatic Cancer, Xenografts, Somatostatin receptor type 2, Transfection
INTRODUCTION
Pancreatic carcinoma is, at present, the fifth cause of cancer death in Western countries. The incidence of this disease is rising in China[1]. Up to now, the only possible curative treatment of pancreatic cancer is surgical resection. Unfortunately, because of late diagnosis due to a long silent clinical phase during tumor development and the absence of early specific and sensitive markers of this disease, surgery for curative purposes is possible in only 10-15% of cases[2]. The overall 5-year survival rate for pancreatic cancer is no more than 3%[2]. To improve the prognosis, studies focusing on this disease are to find new effective diagnostic techniques and to develop new modalities of treatment. Gene therapy strategy may provide therapeutic benefits with a more favorable risk: benefit ratio than the conventional treatment. Some experiments have involved suicide gene transfer, such as thymidine kinase/ganciclovir system[3-5], uracil phosphoribosyltransferase/5-fluorouracil system[5,6], or immunotherapy using interleukin 2 or interleukin 1β[7], or reintroduction of antioncogenic molecules such as p16INK4a or p53 tumor suppressor gene[8].
Somatostatin is a widely distributed peptide that inhibits the growth of multiple epithelial cell types[9]. The antiproliferative action of somatostatin (SS)/analogues is mediated by specific G-protein coupled receptors. Somatostatin receptor gene expression is a requirement for inhibition of pancreatic cancer growth by somatostatin and its analogues in vitro and in vivo. However, studies have revealed a specific loss of SSTR2 subtype gene expression in human pancreatic carcinoma and in most derived cell lines[10]. This may explain why recent clinical trials of somatostatin analogues in the adjuvant treatment of advanced pancreatic cancer have failed to demonstrate a response[11-14].
We proposed in the present study a new gene therapy for pancreatic cancer based on the antioncogenic properties of SSTR2 gene expression. In our study, we transfected somatostatin-receptor-negative pancreatic carcinoma xenografts in nude mice with SSTR2 gene, and observed their effects on tumors.
MATERIALS AND METHODS
Materials
Panc-1, a human pancreatic cancer cell line, was purchased from Peking Union Medical College, Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) was purchased from Gibco BRL. SSTR2-pCMV-6C vector was kindly provided by G. I. Bell (Howard Hughes Medical Institute, The University of Chicago, Chicago, IL). Lipofectamine 2000 was purchased from Invitrogen (Invitrogen, San Diego, CA). Polyclonal antibody of SSTR2 and streptavidin peroxidase (SP) kit were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Peroxidase-conjugated rabbit anti-goat IgG and diaminobenzidine (DAB) were purchased from Beijing Zhongshan Biotechnology Inc. TUNEL reagent kit was purchased from Boehringer-Mannheim (Germany). Eighteen six-week-old BALB/C male nude mice were obtained from Hubei Center for Disease Control and Prevention.
Plasmid preparation
We transformed the colon bacillus (DH-5α) to amplify pCMV-6C-SSTR2 plasmid and pCMV-6C plasmid. The two plasmids were extracted by plasmid DNA purification system (Promega, USA) according to the manufacturer’s instructions, and kept at -20 °C.
Animal models and experimental protocol
Panc-1 cells were grown in DMEM supplemented with 100 mL/L FBS at 37 °C, in a humidified atmosphere containing 50 mL/L CO2. Cells were harvested from exponential phase growth, centrifuged and washed with phosphate-buffered saline (PBS, pH 7.4), re-suspended in serum-free media, and the concentration of cells was adjusted to 2×107/mL. Then each nude mouse received a subcutaneous injection containing 8×106 Panc-1 cells on the back. Nude mice were bred and maintained under pathogen-free conditions. After 7 d, when the tumor size was 5 mm×5 mm, the mice were divided randomly into three groups (6 mice/group). Group I served as an untreated control group, group II served as a vector control group receiving an intratumoral injection of a combination of pCMV-6C plasmid and lipofectamine 2000, and group III served as the experimental group receiving an intratumoral injection of a combination of pCMV-6C-SSTR2 plasmid and lipofectamine 2000. The injection containing 100 μL serum-free DMEM, 5 μg plasmid and 10 μL lipofectamine 2000, was given once a week for 4 wk. Subcutaneous tumors were externally measured in two dimensions using vernier calipers before each injection. Tumor volume was determined by equation V = W2 ×L/2, where W is the width and L is the length of the tumor. Tumors were dissected from mice under pentobarbital anesthesia on the thirty-second day of inoculation and kept at -80 °C immediately.
Immunohistochemistry
The expression of SSTR2 and the transfection efficiency in group III tumor cells were detected by using immunohistochemical SP methods. Four-micrometer-thick sections were prepared from formalin-fixed and paraffin-embedded specimens. Sections were deparaffinized, rehydrated, and treated with 30 mL/L H2O2 to block endogenous peroxidase activity. The sections were placed in 10 mmol/L sodium citrate buffer (pH 6.0) and heated three times for 5 min each at 15 min intervals using a microwave oven operating at 600 W. After washing three times with PBS (pH 7.4), sections were incubated for 20 min with goat serum solution to block the nonspecific antibody-bindings, then incubated with goat-anti-human polyclonal antibody diluted to 1:100, for 12 h at 4 °C, followed by immunoperoxidase immunostaining using streptavidin peroxidase/horse radish peroxidase (SP/HRP). Immunostaining was developed using DAB as chromogen. Slides were counter-stained with hematoxylin. Negative control slides were prepared by replacing the primary antibody with PBS.
Western blot
Frozen tumors were rinsed in ice-cold PBS, and homogenized in homogenization buffer containing 50 mmol/L Tris-HCL (pH 8.5), 150 mmol/L NaCL, 0.2 g/L NaN3, 0.1 g/L SDS, 100 μg /mL PMSF, 1 μg/mL aprotinin, 10 g/L NP-40, and 5 g/L sodium deoxycholate. The products were centrifuged at 14000 g for 15 min to remove cellular debris. Protein concentrations were determined by the Bradford methods. A total of 100 μg protein was separated by electrophoresis using 100 g/L sodium dodecyl sulfate-polyacrylamide gel, transferred to a Immun-Blot PVDF Membrane, blocked out with degreased milk for 12 h and hybridized with a polyclonal antibody for SSTR2 (1:1000 dilution) at 4 °C overnight. After washing three times, the membranes were incubated with rabbit anti-goat IgG at room temperature for 1 h. At last, the protein was visualized by a chemiluminescence detection kit.
TUNEL
Sections were deparaffinized and rehydrated, then rinsed three times with PBS and incubated with blocking solution (30 mL/L H2O2 in methanol) for 10 min at room temperature. Slides were rinsed three times with PBS and incubated with TUNEL reaction mixture in a humidified chamber for two hours at 37 °C, with goat serum in a humidified chamber for 20 min to avoid nonspecific staining, with peroxidase-labeled anti-fluorescein antibody in a humidified chamber for 30 min at 37 °C, respectively. After washing three times with PBS, slides were counter-stained with hematoxylin, and mounted under microscope. Some sections stationed with no TUNEL mixture were used as negative controls.
Statistical analysis
The results were expressed as mean±SD. Student’s t test was used to compare data. P<0.05 was considered statistically significant.
RESULTS
Re-expression of SSTR2 after transfection
Immunohistochemistry and Western blot analysis confirmed the absence of SSTR2 in groups I and II, and the expression of SSTR2 in tumors transfected with the SSTR2 gene (group III) (Figures 1, 2). SSTR2 protein expression could only be detected on the surface of SSTR2 transfected tumor cells (group III). These proved that the transfection succeeded in causing Panc-1 xenografts to re-express cell-surface SSTR2 protein.
Figure 1.
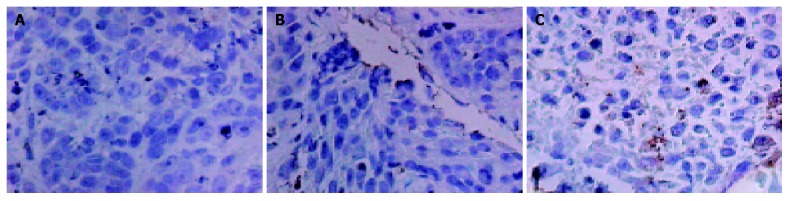
Immunohistochemical staining of SSTR2 expression in groups I (A), II (B), and III (C) (SP, magnification ×400).
Figure 2.
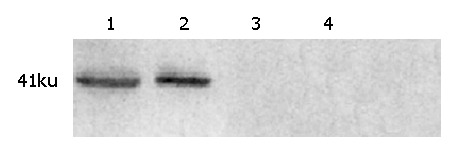
Western blot analysis for SSTR2 expression. Lanes 1 and 2: experimental group; lanes 3 and 4: control groups.
In vivo gene transfer efficiency detected by immunohistochemistry
To examine the efficiency of in vivo gene transfer into tumors by lipofectamine 2000, we selected two immunohistochemical sections per nude mouse tumor, and counted at least 5000 cells per section at high magnification (×40) on continuous fields. At last, quantification revealed that about 2% of tumor cells in group III had positive staining for SSTR2 (Figure 1C).
Expression of SSTR2 inhibited growth of pancreatic cancer xenografts
As shown in Figures 3 and 4, tumors in groups I and II grew rapidly. In contrast, the growth of SSTR2 tumors was dramatically inhibited. After 32 d, the tumor volume in groups I, II and III was 2.058±0.176 cm3, 2.025±0.163 cm3 and 0.318±0.098 cm3 respectively. The tumor weight in groups I, II and III was 1.412±0.146 g, 1.365±0.116 g and 0.523±0.090 g, respectively. There was a statistical significance between groups III and I or II (P<0.05), but no statistical significance between groups I and II.
Figure 3.
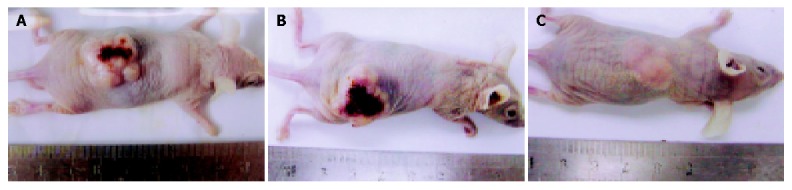
Pancreatic xenografts in the nude mice. Tumors in groups I (A) and II (B) were bigger than that in group III (C), and dark parts display distinct necrosis.
Figure 4.
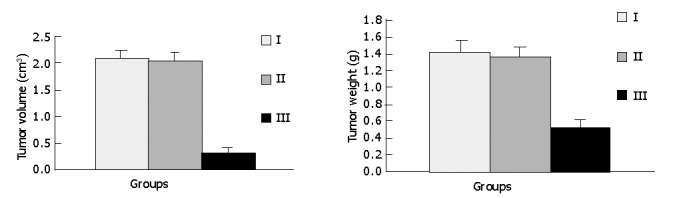
Effect of SSTR2 gene transfection on Panc-1 cell xenografts.
Apoptosis index
As shown in Figure 5, positive staining located in the nuclei of apoptotic cells. Quantitative analysis showed that AI significantly increased in group III (1.47±0.13%) compared with group I (0.56±0.09%) and group II (0.57±0.11%) (P<0.05). No significant differences were observed in AI between groups I and II.
Figure 5.
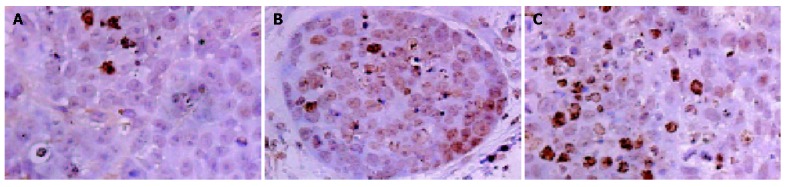
AI detected by TUNEL reaction in groups I (A), II (B), and III (C) (magnification ×400).
DISCUSSION
Since there is no satisfactory therapeutic method for pancreatic cancer, developing new therapeutic strategies have become a key point in this field. Gene therapy strategies may provide more favorable therapeutic benefits than the conventional treatments. Studies have indicated that SSTR2 gene is one of the candidate antioncogenes for therapy of pancreatic cancer. Re-expression of SSTR2 gene could exert therapeutic effects on tumor cell growth through different mechanisms[2,15-18]. We transferred SSTR2 gene into wild Panc-1 cell xenografts by lipofectamine through repeated intratumoral injections at different sites. The growth of tumor in experimental group was significantly inhibited. The AI of tumor cells significantly increased in SSTR2-expressed tumors. These data indicate that the therapeutic effect evoked by our means is remarkable.
At present, it is impossible to transfer therapeutic genes to all tumor cells for gene therapy of solid cancers. Bystander killing effect on nontransduced cells is thus required for the success of cancer gene therapy. Nontransduced cells could be suppressed or/and killed through the bystander killing effects comprising several mechanisms, including intercellular transfer of toxic metabolites via gap junctions or apoptotic vesicles[19], uptake by nearby untransduced cells of apoptotic vesicles containing hydrolases or enzymes triggered by programmed cell death[20], or active local cell-mediated immune response[21].
In our study, we observed that the therapeutic effect was remarkable despite a low ratio of SSTR2-expressed cells of tumors in experimental group. In a similar study, Vernejoul et al[2] observed a significant inhibition of tumor growth and a marked increase in AI in SSTR2-transduced tumors . Since AI of tumor cells in different groups is not parallel to the extent of SSTR2 expression, apoptosis could not be attributed solely to SSTR2-expressed cells entering into programmed cell death, suggesting strongly a bystander killing effect evoked by SSTR2-transduced cells on untransduced cells. We put forward here some hypotheses about the bystander killing effect. The first explanation is that apoptosis could contribute to it. A significant increase in apoptosis of tumor cells in experimental group was measured under the condition of limited transfection efficiency. We, therefore, conclude that apoptosis could diffuse from transduced cells to untransduced neighboring tumor cells. However, mechanisms other than apoptosis might be involved in the bystander killing effect. Rochaix et al[18] detected a marked decrease in Ki67 proliferative index in tumors expressing SSTR2. We know that Ki67 is a nuclear protein expressed only during G2-M phase of mitosis. Vernejoul et al[2] observed that SSTR2 gene transfer was accompanied with a significant decrease in the proliferating cell nuclear antigen (PCNA) index. The decreased degrees of these two proliferative indexes are not obviously parallel to the efficiency of SSTR2 transduction. Pages et al[22] have also confirmed that SSTR2 could mediate cell cycle arrest. Thus we conclude that SSTR2-transduced cells could suppress untransduced neighboring tumor cells.
Studies have revealed that vascular endothelial growth factor (VEGF) plays a key role in tumor growth[23,24]. It has been reported that VEGF and its receptor are overexpressed in cancer cells, including pancreatic cancer cells[25,26]. Our previous studies[27-29] have confirmed that VEGF expression could be down-regulated in pancreatic cancer cells after transferring SSTR2 gene, suggesting the antiangiogenic effect of SSTR2 gene. Since tumor growth depends on blood supply, some tumor cell growth is suppressed, and then programmed cell death occurs. The expression of SSTR2 could also influence the expression of other subtypes of SSTR, such as up-regulation of SSTR1, which might contribute to inhibition of pancreatic cancer cell proliferation[30].
We adopted the way of repeated intratumoral injections at more sites to observe the prolonged effect of SSTR2 gene on pancreatic cancer. In order to promote the transfection effect before injection, we mixed plasmid and lipofectamine 2000 in 100 µL DMEM without serum. Tumor cells might interact with the plasmid more easily due to the local tension produced by the liquid. It might be beneficial for the plasmid to be infused with cell membranes or to be endocytosed by tumor cells.
In conclusion, re-expression of SSTR2 gene in vivo can inhibit the growth of pancreatic tumor by increasing apoptosis of tumor cells, and restoration of SSTR2 in pancreatic cancer cell line Panc-1 may offer an avenue for antitumor therapy.
Footnotes
Supported by National Natural Science Foundation of China, No. 30271473
Co-correspondents: Zhi-Yong Du and Ren-Yi Qin
Edited by Kumar M and Wang XL
References
- 1.Huizhen Y, Jiefu H. Pancreatic Carcinoma. 1st ed. Shanghai: Shanghai Science Technol Press; 2001. pp. 1–2. [Google Scholar]
- 2.Vernejoul F, Faure P, Benali N, Calise D, Tiraby G, Pradayrol L, Susini C, Buscail L. Antitumor effect of in vivo somatostatin receptor subtype 2 gene transfer in primary and metastatic pancreatic cancer models. Cancer Res. 2002;62:6124–6131. [PubMed] [Google Scholar]
- 3.DiMaio JM, Clary BM, Via DF, Coveney E, Pappas TN, Lyerly HK. Directed enzyme pro-drug gene therapy for pancreatic cancer in vivo. Surgery. 1994;116:205–213. [PubMed] [Google Scholar]
- 4.Wang J, Lu XX, Chen DZ, Li SF, Zhang LS. Herpes simplex virus thymidine kinase and ganciclovir suicide gene therapy for human pancreatic cancer. World J Gastroenterol. 2004;10:400–403. doi: 10.3748/wjg.v10.i3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajri A, Wack S, Lehn P, Vigneron JP, Lehn JM, Marescaux J, Aprahamian M. Combined suicide gene therapy for pancreatic peritoneal carcinomatosis using BGTC liposomes. Cancer Gene Ther. 2004;11:16–27. doi: 10.1038/sj.cgt.7700628. [DOI] [PubMed] [Google Scholar]
- 6.Kanai F, Kawakami T, Hamada H, Sadata A, Yoshida Y, Tanaka T, Ohashi M, Tateishi K, Shiratori Y, Omata M. Adenovirus-mediated transduction of Escherichia coli uracil phosphoribosyltransferase gene sensitizes cancer cells to low concentrations of 5-fluorouracil. Cancer Res. 1998;58:1946–1951. [PubMed] [Google Scholar]
- 7.Motoi F, Sunamura M, Ding L, Duda DG, Yoshida Y, Zhang W, Matsuno S, Hamada H. Effective gene therapy for pancreatic cancer by cytokines mediated by restricted replication-competent adenovirus. Hum Gene Ther. 2000;11:223–235. doi: 10.1089/10430340050015978. [DOI] [PubMed] [Google Scholar]
- 8.Ghaneh P, Greenhalf W, Humphreys M, Wilson D, Zumstein L, Lemoine NR, Neoptolemos JP. Adenovirus-mediated transfer of p53 and p16(INK4a) results in pancreatic cancer regression in vitro and in vivo. Gene Ther. 2001;8:199–208. doi: 10.1038/sj.gt.3301394. [DOI] [PubMed] [Google Scholar]
- 9.Schally AV. Oncological applications of somatostatin analogues. Cancer Res. 1988;48:6977–6985. [PubMed] [Google Scholar]
- 10.Buscail L, Saint-Laurent N, Chastre E, Vaillant JC, Gespach C, Capella G, Kalthoff H, Lluis F, Vaysse N, Susini C. Loss of sst2 somatostatin receptor gene expression in human pancreatic and colorectal cancer. Cancer Res. 1996;56:1823–1827. [PubMed] [Google Scholar]
- 11.Klijn JG, Hoff AM, Planting AS, Verweij J, Kok T, Lamberts SW, Portengen H, Foekens JA. Treatment of patients with metastatic pancreatic and gastrointestinal tumours with the somatostatin analogue Sandostatin: a phase II study including endocrine effects. Br J Cancer. 1990;62:627–630. doi: 10.1038/bjc.1990.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canobbio L, Boccardo F, Cannata D, Gallotti P, Epis R. Treatment of advanced pancreatic carcinoma with the somatostatin analogue BIM 23014. Preliminary results of a pilot study. Cancer. 1992;69:648–650. doi: 10.1002/1097-0142(19920201)69:3<648::aid-cncr2820690308>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 13.Friess H, Büchler M, Beglinger C, Weber A, Kunz J, Fritsch K, Dennler HJ, Beger HG. Low-dose octreotide treatment is not effective in patients with advanced pancreatic cancer. Pancreas. 1993;8:540–545. doi: 10.1097/00006676-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Raderer M, Hamilton G, Kurtaran A, Valencak J, Haberl I, Hoffmann O, Kornek GV, Vorbeck F, Hejna MH, Virgolini I, et al. Treatment of advanced pancreatic cancer with the long-acting somatostatin analogue lanreotide: in vitro and in vivo results. Br J Cancer. 1999;79:535–537. doi: 10.1038/sj.bjc.6690084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celinski SA, Fisher WE, Amaya F, Wu YQ, Yao Q, Youker KA, Li M. Somatostatin receptor gene transfer inhibits established pancreatic cancer xenografts. J Surg Res. 2003;115:41–47. doi: 10.1016/s0022-4804(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 16.Delesque N, Buscail L, Estève JP, Saint-Laurent N, Müller C, Weckbecker G, Bruns C, Vaysse N, Susini C. sst2 somatostatin receptor expression reverses tumorigenicity of human pancreatic cancer cells. Cancer Res. 1997;57:956–962. [PubMed] [Google Scholar]
- 17.Fisher WE, Wu Y, Amaya F, Berger DH. Somatostatin receptor subtype 2 gene therapy inhibits pancreatic cancer in vitro. J Surg Res. 2002;105:58–64. doi: 10.1006/jsre.2002.6450. [DOI] [PubMed] [Google Scholar]
- 18.Rochaix P, Delesque N, Estève JP, Saint-Laurent N, Voight JJ, Vaysse N, Susini C, Buscail L. Gene therapy for pancreatic carcinoma: local and distant antitumor effects after somatostatin receptor sst2 gene transfer. Hum Gene Ther. 1999;10:995–1008. doi: 10.1089/10430349950018391. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Chiang Y, Lenz HJ, Danenberg KD, Spears CP, Gordon EM, Anderson WF, Parekh D. Intercellular communication mediates the bystander effect during herpes simplex thymidine kinase/ganciclovir-based gene therapy of human gastrointestinal tumor cells. Hum Gene Ther. 1998;9:719–728. doi: 10.1089/hum.1998.9.5-719. [DOI] [PubMed] [Google Scholar]
- 20.Sturtz FG, Waddell K, Shulok J, Chen X, Caruso M, Sanson M, Snodgrass HR, Platika D. Variable efficiency of the thymidine kinase/ganciclovir system in human glioblastoma cell lines: implications for gene therapy. Hum Gene Ther. 1997;8:1945–1953. doi: 10.1089/hum.1997.8.16-1945. [DOI] [PubMed] [Google Scholar]
- 21.Barba D, Hardin J, Ray J, Gage FH. Thymidine kinase-mediated killing of rat brain tumors. J Neurosurg. 1993;79:729–735. doi: 10.3171/jns.1993.79.5.0729. [DOI] [PubMed] [Google Scholar]
- 22.Pagès P, Benali N, Saint-Laurent N, Estève JP, Schally AV, Tkaczuk J, Vaysse N, Susini C, Buscail L. sst2 somatostatin receptor mediates cell cycle arrest and induction of p27(Kip1). Evidence for the role of SHP-1. J Biol Chem. 1999;274:15186–15193. doi: 10.1074/jbc.274.21.15186. [DOI] [PubMed] [Google Scholar]
- 23.Verheul HM, Pinedo HM. The role of vascular endothelial growth factor (VEGF) in tumor angiogenesis and early clinical development of VEGF-receptor kinase inhibitors. Clin Breast Cancer. 2000;1 Suppl 1:S80–S84. doi: 10.3816/cbc.2000.s.015. [DOI] [PubMed] [Google Scholar]
- 24.Sipos B, Weber D, Ungefroren H, Kalthoff H, Zühlsdorff A, Luther C, Török V, Klöppel G. Vascular endothelial growth factor mediated angiogenic potential of pancreatic ductal carcinomas enhanced by hypoxia: an in vitro and in vivo study. Int J Cancer. 2002;102:592–600. doi: 10.1002/ijc.10753. [DOI] [PubMed] [Google Scholar]
- 25.Korc M. Role of growth factors in pancreatic cancer. Surg Oncol Clin N Am. 1998;7:25–41. [PubMed] [Google Scholar]
- 26.Arii S, Mori A, Uchida S, Fujimoto K, Shimada Y, Imamura M. Implication of vascular endothelial growth factor in the development and metastasis of human cancers. Hum Cell. 1999;12:25–30. [PubMed] [Google Scholar]
- 27.Qin RY, Fang RL, Gupta MK, Liu ZR, Wang DY, Chang Q, Chen YB. Alteration of somatostatin receptor subtype 2 gene expression in pancreatic tumor angiogenesis. World J Gastroenterol. 2004;10:132–135. doi: 10.3748/wjg.v10.i1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar M, Liu ZR, Thapa L, Qin RY. Anti-angiogenic effects of somatostatin receptor subtype 2 on human pancreatic cancer xenografts. Carcinogenesis. 2004;25:2075–2081. doi: 10.1093/carcin/bgh216. [DOI] [PubMed] [Google Scholar]
- 29.Kumar M, Liu ZR, Thapa L, Wang DY, Tian R, Qin RY. Mechanisms of inhibition of growth of human pancreatic carcinoma implanted in nude mice by somatostatin receptor subtype 2. Pancreas. 2004;29:141–151. doi: 10.1097/00006676-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Kikutsuji T, Harada M, Tashiro S, Ii S, Moritani M, Yamaoka T, Itakura M. Expression of somatostatin receptor subtypes and growth inhibition in human exocrine pancreatic cancers. J Hepatobiliary Pancreat Surg. 2000;7:496–503. doi: 10.1007/s005340070021. [DOI] [PubMed] [Google Scholar]


