Abstract
Rheumatic diseases affect a significant portion of the population and lead to increased health care costs, disability and even premature mortality; as such, effective preventive measures for these diseases could lead to substantial improvements in public health. Importantly, established and emerging data from natural history studies show that for most rheumatic diseases there is a period of ‘preclinical’ disease development during which abnormal biomarkers or other processes can be detected. These changes are useful to understand mechanisms of disease pathogenesis; in addition, they may be applied to estimate a personal risk of future disease, while individuals are still relatively asymptomatic. Based on this, a hope is to implement effective screening and preventive approaches for some rheumatic diseases, perhaps in the near future. However, a key part of such approaches is a deep understanding of the mechanisms of disease development as well as evidence-based and effective screening and preventive interventions that incorporate disease biology as well as ethical and public health concerns.
Keywords: Prevention, Rheumatic Diseases
Introduction
Across the multiple fields of medicine there is increasing interest in preventive approaches to disease. To help guide preventive approaches to disease, in the 1960's, the World Health Organization put forward recommendations for disease screening and prevention, as listed in Table 1 (1). Overall, these recommendations suggest that diseases targeted for screening and prevention should have an important impact on health, an identifiable asymptomatic (or minimally symptomatic period), during which individuals at high-risk for future disease can accurately be identified, and that there be available an effective means for preventing the further evolution of disease. Screening and prevention approaches that follow these guidelines are in action for many diseases. For example, across the globe there is considerable effort put forward to screen and prevent adverse outcomes from cardiovascular disease and many types of cancer, as well as programs to prevent many infectious diseases.
Table 1. WHO recommendations regarding screening and prevention for a disease.
|
Adapted from Wilson J, Jungner G. Principles and practice of screening for disease. WHO Public Health Papers 1968; 34: 1–163; with permission.
While most rheumatologists would agree that rheumatic diseases on the whole are important health problems and meet several of the other WHO criteria for screening, many key questions regarding prevention of rheumatic diseases are still unanswered. However, given the growing understanding of the etiologies rheumatic disease, and as discussed herein, a growing awareness that many rheumatic diseases have a period of relatively asymptomatic disease development during which there are abnormalities of biomarkers that can be used to predict future risk for disease (2), there is hope that rheumatic diseases could join the list of preventable diseases.
In this review, we will discuss some general principles of disease prevention applicable to rheumatic disease, and outline a potential research strategy for the development of effective preventive strategies that are able to reduce the adverse impact of these diseases.
General strategies for disease prevention
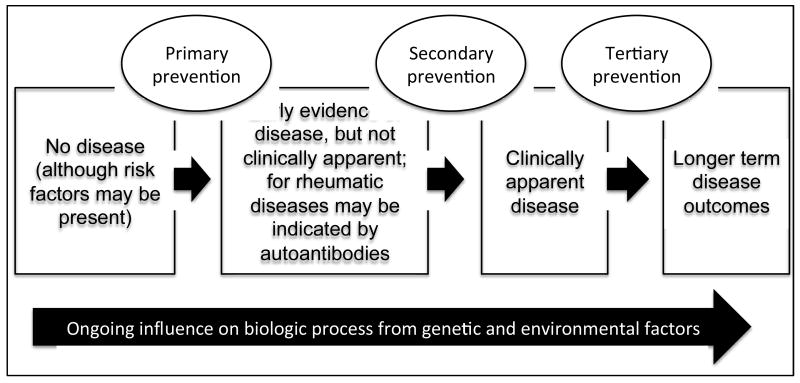
Prevention strategies are typically categorized into primary, secondary, or tertiary interventions (Figure 1) (3, 4). The aim of primary prevention is to avoid the development of disease by eliminating specific risk factors or increasing individual's resistance to the condition. An example of this type of approach is vaccines against infections. The aim of secondary prevention is to reduce the progression from a latent or asymptomatic phase of disease to symptomatic disease. Thus a secondary preventive intervention attempts to interrupt the mechanisms of disease development before they evolve into an apparent illness. Examples of this type of approach include early identification of cancers through programs such as mammograms and colonoscopies.
Figure 1. Natural history of rheumatic disease and possibilities for prevention.
This figure illustrates the natural history of rheumatic disease beginning on the left with no disease, although genetic and environmental factors may be present. Over time, there is early evidence of disease that is not clinically apparent. Examples of this are autoantibodies, elevated uric acid, or early cartilage injury. Later, clinically apparent disease develops that may be classifiable as a specific rheumatic disease. Once disease is clinically manifest, longer-term outcomes include issues such as response to therapy, disability. Throughout disease evolution, there are ongoing influences from genetic and environmental factors. Progression of rheumatic disease may be prevented at several points: prior to development of asymptomatic disease (primary prevention), during asymptomatic disease (secondary prevention), and after clinically-apparent disease has developed (tertiary prevention).
The aim of tertiary prevention is to delay or to limit the impact of an established disease (5). This is where most rheumatic diseases are currently dealt with, where rheumatologists are typically performing tertiary prevention by attempting to prevent progression of disease to disability or premature death after a patient present with clinically apparent disease (e.g. swollen joints in RA, or skin rash in SLE). However, rheumatologists are less used to carry out primary or secondary preventive interventions for rheumatic diseases. As knowledge of the risk factors for rheumatic diseases is growing (e.g. smoking for rheumatoid arthritis (RA))(6), primary prevention may become more of a priority for rheumatic diseases.
Potential primary preventive strategies
Environmental risk factors are of great interest for a preventive strategy of rheumatic diseases, as they are potentially modifiable. In particular, lifestyle modifications are a common request from at risk populations; specifically, when individuals at high-risk for RA were interviewed about potential preventive interventions, the majority primarily mentioned lifestyle adjustments as approaches that they would be comfortable with (7).
Multiple environmental and lifestyle factors have been identified for rheumatic diseases. In RA, tobacco smoking is the best-established risk factor and is responsible for one out of every 4 to 6 cases of RA (population attributable risk)(8). The effect of tobacco is dose-dependent and larger in shared-epitope positive individuals (8, 9). Other inhaled pollutants have also been implicated in the development of RA, such as silica dusts, factory dusts or exposure to traffic pollution (10, 11). Reproductive and hormonal factors also play a role in the development of RA and several other autoimmune diseases. Sex hormones have immunomodulatory effects, but the complex interactions among hormones are not fully understood. Oral contraceptive and hormone replacement therapy have been associated both with a lower RA risk, but not all studies have confirmed these findings. Several studies have found an increased risk of RA with obesity and with lower social class (12, 13). Dietary factors have generally given inconclusive results, but recently, high intake of soda and salt have been associated with an increased risk of RA (14, 15). On the contrary, a moderate alcohol consumption has consistently associated with a decreased risk of RA (16). In a similar way, tobacco smoking, occupational exposure to silica dust and exposure to sunlight have also been associated with an increased risk of SLE, while moderate alcohol intake appears to decrease the risk.(17) In some cases, specific disease triggers such as toxic oil, certain medications, or possibly exposure to certain mycotoxins may shed light on the pathophysiology of specific rheumatic diseases such as eosinophilic disease, drug-induced autoimmune syndromes, and potentially certain forms of osteoarthritis such as Kashin-Beck disease (18-21). Emerging data suggest that microorganisms may be implicated in the development of certain rheumatic diseases, such as Epstein-Barr Virus in SLE (22) or bacterial organisms in RA (23-25). If the infectious etiology for rheumatic diseases is confirmed, it could open the door to preventive strategies involving vaccines against causative organisms.
Risk factors for other rheumatic diseases are also known. For example, diet-related metabolic effects such as central obesity and diabetes as well as alcohol intake have been shown to be related to increased risk for gout (26). In addition, prior injury, obesity and abnormal joint mechanics are risk factors for OA (27). Many more environmental risk factors for rheumatic diseases certainly exist although more research is warranted to grasp the complex interactions between genetics and the environment, both to understand the etiology these diseases and to initiate preventive interventions.
While there are many environmental factors that have been associated with the development of rheumatic diseases, overall few of the identified environmental triggers for rheumatic diseases have enough supportive evidence and strong enough effect sizes to warrant altering a specific environmental risk factor on a population level. Even tobacco smoke, which as discussed above is one of the best-established environmental risk factor for RA, still only explains ∼30% of seropositive disease (28, 29). However, the effect of environmental risk factors may be much stronger in individuals with a certain genetic makeup (9, 30).
An interesting approach to identify individuals for whom environmental factor(s) or and/or lifestyle modifications may be most effective is to combine several environmental risk factors to identify individuals at very high risk for rheumatic disease. For example, a British study has proposed a risk score for inflammatory polyarthritis based solely on easily ascertained lifestyle factors(31). This lifestyle risk score simply combines pack-years of smoking (every 10 pack-years), alcohol consumption (units/day), occupational class (professional, manual, neither), obesity (BMI > 30), presence of diabetes, parity (≥2) and duration of breast-feeding in women (years). Based on a simple summation of these lifestyle factors, this risk score can identify individuals who have up to a six times higher risk of developing polyarthritis. In addition, using the United States-based Nurses Health Study, Karlson and colleagues have used a combination of family history, genetic and environmental factors to predict future risk for RA, with area under the curve (AUC) of >0.8 for their best predictive models for RA (32, 33). While targeting single environmental factors in the general population may not be a feasible strategy for relatively uncommon diseases, approaches combining genetic and environmental risk factors may be useful to detect specific individuals in whom a preventive intervention aimed at modifying lifestyle factors is most indicated.
Identification of preclinical phases of rheumatic disease
Because of the difficulty in identifying specific environmental risk factors for most rheumatic diseases as well as the relatively weak effect sizes of known environmental risk factors when applied on a population-basis (6), perhaps a more feasible approach to rheumatic disease prevention is to focus on interventions in individuals who are at a very early phase of rheumatic disease development prior to the development of significant tissue injury. This concept has gained traction over the past few years in large part due to a growing understanding of the natural history of rheumatic diseases. Specifically, many autoimmune rheumatic diseases are currently believed to result from multi-step processes, whereby an environmental trigger (or triggers) induces an immune reaction in genetically susceptible individuals. The genetic susceptibility may be assessed through a careful family history of disease or measured with specific genetic markers (2, 9, 34). Furthermore, in many rheumatic diseases including SLE (35), RA (36-40), and antineutrophil cytoplasmic antibody (ANCA) positive vasculitis (41, 42), disease specific autoantibodies may precede by several years the clinically apparent manifestations of disease, often termed ‘preclinical’ disease. Other rheumatic diseases such as gout and osteoarthritis also have ‘preclinical’ phases with abnormal biomarkers (e.g. uric acid s(43)) or early structural changes (e.g. hip dysplasia(44)), in absence of significant clinical symptoms. Thus, identifying a high risk population could be done either by identifying genetically susceptible individuals (i.e. genetic screening using genetic risk scores, or as a proxy - a family history of autoimmune disorders)(45), by detecting the presence of specific biomarkers (e.g. auto-antibodies), or by recognizing a set of highly relevant environmental exposures (31).
Potential secondary prevention strategies
While many rheumatic diseases may be identified in their preclinical phases, it is not clear how to safely and effectively prevent either the initiation of early autoimmunity, or the progression of early autoimmunity or other rheumatic disease mechanisms (e.g. high uric acid in gout, or early cartilage damage in OA) while the disease is in a relatively asymptomatic state. It is possible that environmental risk-factor modification could be effective to halt initiation of autoimmunity, or even progression of early autoimmunity to clinically apparent disease; however, we are still lacking knowledge of which factors act to initiate and propagate autoimmunity once it develops. Furthermore, modulating an environmental risk factor(s) may be effective to prevent the development and/or progression of rheumatic disease, but it may be difficult to measure its effect as the time between an intervention and the potential clinical benefit may be very long. For example, using data from the Nurses’ Health Study, Karlson and colleagues found that risk of RA remained elevated until 20+ years after smoking cessation (46); such an effect would be very difficult to measure in a clinical trial to show benefit in an evidence-based fashion. Tolerance-inducing regimens could also be an attractive approach for altering progression of autoimmunity; however, this approach is difficult to employ unless specific antigen targets and immune regulatory pathways are well understood.
Given the difficulties of modulating of environmental risk factors to prevent rheumatic disease, perhaps pharmacologic intervention using agents known to be effective for the treatment of established rheumatic diseases would be the best approach to prevent the progression of autoimmunity. In support of this approach, In animal models of autoimmune diseases, early therapeutic interventions are capable of averting the development of the clinical disease (47). However, to date, only indirect evidence supporting this hypothesis is available in humans: In the early stages of RA, a therapeutic “window of opportunity” appears to exists, where early anti-rheumatic therapy appears to modify the disease permanently in some patients (48)49-53. Furthermore, in the Dutch ‘PROMPT’ study, a limited course of methotrexate in patients with early undifferentiated arthritis initially delayed or prevented the onset of classifiable RA in a proportion of patients, especially those with seropositivity for antibodies to citrullinated proteins (49), although the effects of this intervention appeared to wane after 5 years of follow-up (50). Other rheumatic diseases such as SLE likely operate in a similar fashion (51). The exact mechanism for improved long-term outcomes is unclear, although several observations suggest that in the early stages of the disease process, the immune system might still be amendable to immunologic reprogramming. It has also been suggested that early intervention prevents the recruitment and/or evolution of effector cells such as synovial fibroblasts to a more pathogenic phenotype (52).
Based on these findings, drugs already known to be effective in clinically apparent rheumatic diseases could be applied in the preclinical phase to halt progression to a more damaging phase of disease. For example, in individuals who are at-risk for RA or SLE, drugs such as hydroxychloroquine, methotrexate or others could be applied in the preclinical phase of disease development. Furthermore, interventions at this early phase of disease may be more effective at altering autoimmunity because of less development of more persistent immune and inflammatory responses.
There is already limited evidence that such approaches may be effective in some rheumatic and other autoimmune diseases. In uncontrolled trials, use of hydroxychloroquine appears to reduce rates of progression from palindromic rheumatism (which may be a form of preclinical RA) to persistent inflammatory arthritis (53-55); in addition, in uncontrolled studies of SLE, early use of hydroxychloroquine appeared to delay the fulfillment of classification criteria for SLE and reduce the expansion of autoantibodies (51). Furthermore, a small trial tested a limited prevenitve intervention in postpartum females with presumed preclinical Graves disease based on high titers of thyroid antibodies, and suggested that a short term course of prednisolone may prevent the development of postpartum hypothyroidism (56). Finally, a clinical trial in The Netherlands is examining the efficacy and safety of rituximab to prevent the progression from systemic autoimmunity associated with RA (autoantibody positive individuals) to clinically classifiable RA (57). The results of this study could be highly informative about potential preventive approaches to RA, which could be applied to other rheumatic diseases as well.
Caveats to prevention
While alterations of environmental factors or pharmacologic approaches to prevention of rheumatic diseases are attractive, there are many caveats. Perhaps most importantly, a careful balance is needed between determining the risk of future disease and the potential adverse impact from screening and preventive interventions.
In terms of identifying individuals at risk for future rheumatic disease, there are several possibilities. Importantly, any approach to identify those at risk for future rheumatic disease would need to have sufficiently high predictive values for future disease that would allow for balancing risks for developing disease and the potential benefit of prevention, against the risks of preventive interventions, potential adverse effects from the test itself that could include emotional and physical harms, as well as inappropriate health-care costs that may from false positivity. Autoantibodies are known to be present prior to clinically apparent SLE and RA, and in some studies have high (>90%) positive predictive values (PPV) for future disease. For example, in case-control studies of RA-related autoantibodies, positivity for antibodies to citrullinated proteins in combination with rheumatoid factor were highly specific for RA, and had a PPV of close to 100% (39, 40). However, when the diagnostic accuracy of these autoantibodies is compared to population rates of RA of ∼1%, PPVs fall to ∼16%(39). If these autoantibodies were used in broad screening programs to identify subjects at high risk for future onset of disease, perhaps the absolute risk predicted by certain biomarkers would need to be higher in order to justify use of a potentially toxic medication. For diseases such as gout, high levels of uric acid may predict the future onset of clinically about gouty arthritis (58); however, is the risk of disease that can be estimated from an elevated level of uric acid high enough to justify the use of a medication such as allopurinol that has a low but real risk of serious adverse effects (59)?
In addition, screening strategies for rheumatic diseases should allow for the prediction of an individual's likelihood of developing future disease (i.e. will a person develop the disease?), as well as timing (i.e. when will they develop disease?). This is important both for an individual, when contemplating preventive interventions, as well as for prevention trials, where it is highly important to identify the number of expected outcomes within a temporally limited period. In RA, several studies have found that a combination of autoantibodies, specific cyotkines and chemokines predicted the likelihood and timing of future RA (60, 61). A Dutch study has shown that a combination of these factors can be used in subjects with arthralgias, but without inflammatory arthritis, to predict the likelihood and the timing of future RA (62).
Another caveat to prevention of rheumatic diseases is that the specific mechanisms at play in the earliest phases of development of rheumatic diseases are largely unknown. An agent, such as anti-tumor necrosis factor alpha may not be effective in the preclinical phase of disease, where TNF may not yet be a major pathogenic factor. If this was the case, then the use of such an agent may not offer any preventive benefit, but only potential harm. In addition, the duration of a pharmacologic intervention in the preclinical phase to prevent progression to disease is unknown. Could a short-term intervention ‘reset’ the immune system and lead to permanent reduction of future risk for clinically apparent rheumatic disease? Or, would an intervention need to be continued indefinitely in order to prevent tissue injury? A Dutch trial using two doses of intramuscular corticosteroids was unsuccessfully to prevent progression to clinically classifiable RA (63). This may not have been the correct pharmagologic agent, or the duration of therapy may have been inadequate, but these results highlight that these issues need to be addressed in carefully designed clinical trials.
Finally, identifying and measuring important outcomes in prevention is a difficult issue. Should the goal of prevention be to prevent the clinical onset of classifiable disease? To that end, are currently available classification schemes for rheumatic diseases adequate outcome measures for disease prevention? What if subjects participating in rheumatic disease prevention had improvements of symptoms or findings that did not meet standardized classification criteria for disease? For example, in RA prevention, what if a subject had improved arthralgias from a preventive therapy for RA even in absence of developing clinically apparent synovitis? Are current systems for measuring such outcomes adequate for robust determination of effectiveness of preventive strategies? Furthermore, would other outcomes such as alterations of biomarkers be acceptable? Certainly rheumatologists may believe that an intervention that made a specific autoantibody disappear may be worthwhile, but unless this results in meaningful improvement in clinical outcomes, it may be less attractive to regulatory agencies or even individuals participating in prevention strategies.
While there are caveats to prevention of rheumatic diseases, it must also be considered that the potential benefits of prevention approaches may extend beyond the disease it addressing. For example, perhaps strategies for RA prevention will prevent joint damage, but potentially also prevent cardiovascular disease associated with autoimmunity (64-67). Perhaps lowering uric acid with an agent such as allopurinol will prevent gouty arthritis, but also improve cardiovascular disease risk, and all-cause mortality as emerging data suggests it might (68, 69). These issues are difficult to define, but they will need to be considered when assessing the risks as potential benefits of preventive approaches in rheumatic diseases.
Personalizing approaches to prevention
Any preventive intervention for rheumatic disease will involve an individual choosing to participate in screening and preventive activities. The factors that may influence this choice to participate in screening and prevention include perceived personal risk for disease, familiarity with the illness and expected personal benefit (7, 70). The characteristics of the preventive approach (administration mode, duration of the preventive therapy, adverse event profile) may modulate their decision to participate in prevention (47).
An individual's perception of personal risk may be based on numbers that are provided to individuals by the health care community regarding risk (e.g. positive predictive value of a test), but may also involve personal characteristics such as underlying tendency to trust health care information or acknowledge their own personal risk for a disease (71). In addition, an individual's familiarity with the disease may further influence their decision to participate in preventive strategies. For example, an individual whose mother had severe RA, or whose father had severe gout, may approach prevention very differently than someone who has never known anyone with the disease. The perceived benefits of a preventive intervention may also be difficult to ascertain and explain to individuals. For example, someone who is asymptomatic yet at risk for future rheumatic disease may have special requirements to convince them to participate in a strategy that will prevent an adverse health outcome in the distant future (72). These are important issues because individuals are less likely to be compliant with a therapeutic approach if they do not gain any perceived benefit (73). Moreover, the benefits of prevention will need to be explained carefully to subjects, as the perceived harm of many rheumatic diseases is decreasing with modern therapies. Using RA as an example, it may not be fair to ‘scare’ someone into participating in disease prevention based their knowledge of a patient with very severe joint damage that developed in an era prior to effective disease-modifying therapy.
Furthermore, while the potential harms of pharmacologic agents may be readily ascertainable based on known side-effect profiles, other possible preventive interventions such as smoking cessation (RA) or weight loss (OA), may seem overall beneficial, but be intolerable or very difficult for some individuals. As an example, a youth with mild valgus deformity of the knee that increases his risk for future OA (74), may not be willing to avoid risky yet enjoyable behaviors, such as playing soccer, in order to avoid potential future symptomatic knee OA. Because these issues differ between diseases, acceptability of a preventive intervention needs to be appraised in each target population.
Highlighting some of these issues, in one of the possible target populations for a preventive strategy of RA, namely first degree relatives of patients with the condition, a qualitative study suggested that preventive interventions could meet the expectations of this population, given that the screening procedure used to identify at risk individuals is reliable and that the potential preventive therapy has only minimal constraints and a good safety profile.(7) In this study, the theoretical risk of developing RA in the next five years had to above 30% before the majority of the target population would consider taking a prophylactic treatment (Figure 2). Another study examined what factors persons at risk consider before taking a preventive treatment. Not unexpectedly, participants are more likely to consider taking a preventive treatment when the risk of developing the disease increases. The efficacy of the preventive intervention and the risk of serious adverse events were the most important attributes for choosing a preventive treatment for RA. It is noteworthy that individuals at risk of RA request considerable effectiveness from a potential preventive treatment, before they would consider taking such a therapy (75).
Figure 2. Theoretical 5-year risk of developing rheumatoid arthritis above which persons at risk are willing to take preventive medicine.
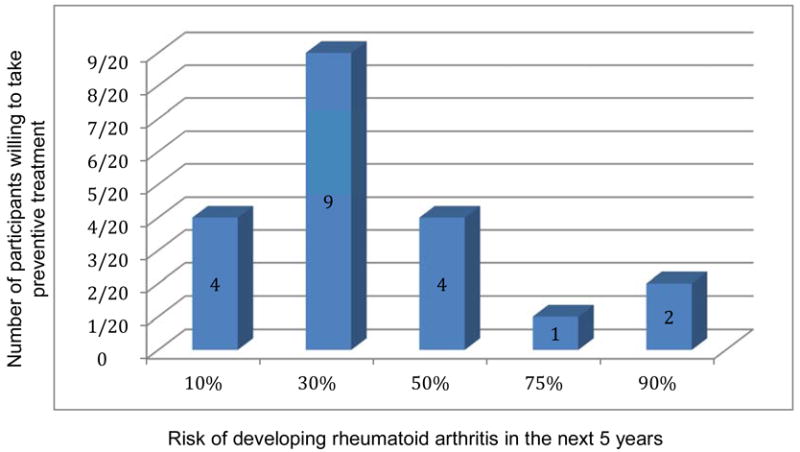
At a 30% hypothetical risk of developing RA within the next 5 years, the majority of first degree relatives of patients with RA were willing to take a limited preventive treatment. Adapted from Novotny F, Haeny S, Hudelson P, Escher M, Finckh A. Primary prevention of rheumatoid arthritis: A qualitative study in a high-risk population. Joint Bone Spine. 2013;80(6):673-4. doi: 10.1016/j.jbspin.2013.05.005. PubMed PMID: 23835304; with permission.
Even if we could adequately predict the future development of autoimmune disorders, not everyone will be eager to find out about their risk of future disease. In a prospective cohort study of first degree relatives of RA patients (76), participants could receive the results of their genetic and immunological tests. While most participants wanted to know, a significant proportion of participants opt not to receive the results of their biomarkers (Axel Finckh, personal communication).
The potential harms of screening have been largely debated in the oncology literature, in particular for breast cancer screening and the related risk of overtreatment (15). Thus, in the field of breast cancer, the trend is moving away from organized population screening programs towards a more personalized, risk-based approach (77). The ethical issues can become even harder with the advent of genetic testing, as we move away from conventional screening, aimed at detecting early-stage diseases, to probabilistic approaches. If this is applied to rheumatic diseases, we will have to interpret results of potential screening tests for rheumatic diseases in light of the individual's probability of disease (78). Furthermore, screening for disease before a valid treatment option becomes available, may not be ethical, as no preventive treatment can be offered to subjects identified at very high risk (79, 80), although to develop preventive strategies, there will need to be some initial steps to test interventions without knowing their full effects. For rheumatologists, the coming era of prevention will certainly require considerable counseling talents and communication skills, as probabilities are often not well understood by patients and the issues complex (81).
The public health impact of prevention
In terms of potential impact, prevention of rheumatic diseases has a great potential of public health benefit, given the burden of these conditions in terms of disability and lost productivity. However, in spite of their impact, prevention of rheumatic diseases has not been a priority in most countries, possibly because these conditions are not immediately lethal. However, if rheumatic diseases are evaluated in aggregate, their overall impact on public health should make addressing preventive strategies a top priority for authorities.
If prevention of rheumatic conditions is to gain acceptance, it will have to meet certain criteria for primary prevention established by public health agencies. As mentioned in the Introduction to this chapter, as well as in the article by Dr. Ned Calonge included in this edition of Rheumatic Disease Clinics of North America, the WHO has presented guidelines for disease screening and prevention (Table 1), which apply to preventive approaches of rheumatic diseases. Importantly, any such approaches will require understanding the natural history these condition, from their asymptomatic phases to their clinically apparent phases. Notably, at present, of the rheumatic diseases, few meet these requirements, with the exception of osteoporosis, and potentially rheumatic fever, where treating patients with low bone density and Streptococcal pharyngeal infection, respectively, has demonstrated benefits (82). As for preventive strategies for other rheumatic diseases, arguably, the study of RA pathogenesis is probably the most advanced; and, its relatively high prevalence in relationship to other autoimmune rheumatic diseases makes it an attractive first target for a preventive intervention. However, while identifying preclinical RA has become a major scientific question, it still needs study before curing or preventing can become a reality. But, if success is met in one rheumatic disease, it could set the stage for preventive approaches for a host of immune-mediated conditions, ultimately leading to substantial benefits to public health.
Summary and future directions
Rheumatic diseases affect a large number of individuals and lead to significant morbidity, in some cases increased mortality, and high health care costs and loss of productivity. A growing understanding of the natural history of many of these diseases suggest that they could be approached in a preventive fashion to either stop the initial development of disease, or halt progression to disease during its preclinical phase. A better understanding of disease pathogenesis may lead to effective screening and prevention strategies for a broad range of rheumatic disease in the near future. Furthermore, studies of disease pathogenesis need to be paralleled by studies of the cost-effectiveness, feasibility and ethics of prevention strategies as well as subject-related factors that can influence participation in prevention.
Summary Points.
A growing understanding of a ‘preclinical’ period of many rheumatic diseases suggests that they could be approached in a preventive fashion.
Prevention of rheumatic diseases may be through ‘primary’ prevention of initial autoimmunity or tissue injury, or through ‘secondary’ prevention to halt progression of autoimmunity and/or tissue injury while subjects are still in an asymptomatic or minimally symptomatic phase.
Prevention may be approached through combinations of risk factor modification, induction of tolerance, or pharmacologic interventions.
Additional research is needed to identify effective biologic targets and methods for prevention of rheumatic diseases, as well as to learn how to apply effective screening and prevention strategies that able to improve public health in a cost-effective fashion.
Acknowledgments
Dr. Deane's work was supported by the National Institutes of Health (AI103023 and AI110503), the Rheumatology Research Foundation, and the Walter S. and Lucienne Driskill Foundation. Dr Finckh's work was supported by the Swiss National Science Foundation (SNSF: 32003B_120639) and by a grant from the Geneva University Hospital.
Footnotes
Conflicts of interest: The authors declare no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson JM, Jungner YG. Principles and practice of mass screening for disease. Boletin de la Oficina Sanitaria Panamericana Pan American Sanitary Bureau. 1968;65(4):281–393. Epub 1968/10/01. [PubMed] [Google Scholar]
- 2.Deane KD, El-Gabalawy H. Pathogenesis and prevention of rheumatic disease: focus on preclinical RA and SLE. Nat Rev Rheumatol. 2014;10(4):212–28. doi: 10.1038/nrrheum.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Public health measures in disease prevention. Science. 2012;337(6101):1468–70. doi: 10.1126/science.337.6101.1468-b. [DOI] [PubMed] [Google Scholar]
- 4.Ash C, Kiberstis P, Marshall E, Travis J. Disease prevention. It takes more than an apple a day. Introduction. Science. 2012;337(6101):1466–7. doi: 10.1126/science.337.6101.1466. [DOI] [PubMed] [Google Scholar]
- 5.Katz DL, Ali A. Commissioned for the IOM Summit on Integrative Medicine and the Health of the Public. Washington, DC: Institute of Medicine (IOM); 2009. Preventive Medicine, Integrative Medicine & The Health Of The Public. [Google Scholar]
- 6.Karlson EW, Deane K. Environmental and gene-environment interactions and risk of rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(2):405–26. doi: 10.1016/j.rdc.2012.04.002. Epub 2012/07/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novotny F, Haeny S, Hudelson P, Escher M, Finckh A. Primary prevention of rheumatoid arthritis: A qualitative study in a high-risk population. Joint Bone Spine. 2013;80(6):673–4. doi: 10.1016/j.jbspin.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Kallberg H, Ding B, Padyukov L, Bengtsson C, Ronnelid J, Klareskog L, Alfredsson L, Group ES. Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis. 2011;70(3):508–11. doi: 10.1136/ard.2009.120899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, Ronnelid J, Harris HE, Ulfgren AK, Rantapaa-Dahlqvist S, Eklund A, Padyukov L, Alfredsson L. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54(1):38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 10.Stolt P, Yahya A, Bengtsson C, Kallberg H, Ronnelid J, Lundberg I, Klareskog L, Alfredsson L. Silica exposure among male current smokers is associated with a high risk of developing ACPA-positive rheumatoid arthritis. Ann Rheum Dis. 2010;69(6):1072–6. doi: 10.1136/ard.2009.114694. [DOI] [PubMed] [Google Scholar]
- 11.Hart JE, Laden F, Puett RC, Costenbader KH, Karlson EW. Exposure to traffic pollution and increased risk of rheumatoid arthritis. Environ Health Perspect. 2009;117(7):1065–9. doi: 10.1289/ehp.0800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Symmons DP, Bankhead CR, Harrison BJ, Brennan P, Barrett EM, Scott DG, Silman AJ. Blood transfusion, smoking, and obesity as risk factors for the development of rheumatoid arthritis: results from a primary care-based incident case-control study in Norfolk, England. Arthritis Rheum. 1997;40(11):1955–61. doi: 10.1002/art.1780401106. [DOI] [PubMed] [Google Scholar]
- 13.Bengtsson C, Nordmark B, Klareskog L, Lundberg I, Alfredsson L, Group ES. Socioeconomic status and the risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis. 2005;64(11):1588–94. doi: 10.1136/ard.2004.031666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H, Costenbader KH, Hu F, Karlson EW, Lu B, Solomon DH, editors. Sugar-Sweetened Soft Drink Consumption and risk of developing Rheumatoid Arthritis. American College of Rheumatology 2013 Annual Meeting; San Diego. Arthritis Rheum; 2013. [Google Scholar]
- 15.Sundström B, Johansson I, Rantapää Dahlqvist S, editors. Dietary Sodium Increases The Risk For Rheumatoid Arthritis Among Smokers – Results From a Nested Case-Control Study. American College of Rheumatology 2013 Annual Meeting; San Diego. Arthritis Rheum; 2013. [Google Scholar]
- 16.Maxwell JR, Gowers IR, Moore DJ, Wilson AG. Alcohol consumption is inversely associated with risk and severity of rheumatoid arthritis. Rheumatology (Oxford) 2010;49(11):2140–6. doi: 10.1093/rheumatology/keq202. [DOI] [PubMed] [Google Scholar]
- 17.Takvorian S, Merola J, Costenbader K. Cigarette smoking, alcohol consumption and risk of systemic lupus erythematosus. Lupus. 2014;23(6):537–44. doi: 10.1177/0961203313501400. [DOI] [PubMed] [Google Scholar]
- 18.Patterson R, Germolec D. Review article toxic oil syndrome: review of immune aspects of the disease. Journal of immunotoxicology. 2005;2(1):51–8. doi: 10.1080/15476910590960143. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Alvarez R, Perez-de-Lis M, Ramos-Casals M, group Bs Biologics-induced autoimmune diseases. Curr Opin Rheumatol. 2013;25(1):56–64. doi: 10.1097/BOR.0b013e32835b1366. Epub 2012/11/02. [DOI] [PubMed] [Google Scholar]
- 20.Sudre P, Mathieu F. Kashin-Beck disease: from etiology to prevention or from prevention to etiology? International orthopaedics. 2001;25(3):175–9. doi: 10.1007/s002640000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Steensel MA. Why minocycline can cause systemic lupus - a hypothesis and suggestions for therapeutic interventions based on it. Medical hypotheses. 2004;63(1):31–4. doi: 10.1016/j.mehy.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 22.James JA, Robertson JM. Lupus and Epstein-Barr. Curr Opin Rheumatol. 2012;24(4):383–8. doi: 10.1097/BOR.0b013e3283535801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebringer A. Rheumatoid arthritis and proteus. London: Springer-Verlag; 2012. [Google Scholar]
- 24.Scher JU, Abramson SB. Periodontal disease, Porphyromonas gingivalis, and rheumatoid arthritis: what triggers autoimmunity and clinical disease? Arthritis Res Ther. 2013;15(5):122. doi: 10.1186/ar4360. Epub 2013/11/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson SB, Huttenhower C, Littman DR. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2:e01202. doi: 10.7554/eLife.01202. Epub 2013/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roddy E, Choi HK. Epidemiology of Gout. Rheum Dis Clin North Am. 2014;40(2):155–75. doi: 10.1016/j.rdc.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arden NK, Leyland KM. Osteoarthritis year 2013 in review: clinical. Osteoarthritis Cartilage. 2013;21(10):1409–13. doi: 10.1016/j.joca.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, Kumagai S. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2010;69(1):70–81. doi: 10.1136/ard.2008.096487. Epub 2009/01/29. doi: ard.2008.096487 [pii]10.1136/ard.2008.096487. [DOI] [PubMed] [Google Scholar]
- 29.Costenbader KH, Kim DJ, Peerzada J, Lockman S, Nobles-Knight D, Petri M, Karlson EW. Cigarette smoking and the risk of systemic lupus erythematosus: a meta-analysis. Arthritis Rheum. 2004;50(3):849–57. doi: 10.1002/art.20049. [DOI] [PubMed] [Google Scholar]
- 30.Keenan BT, Chibnik LB, Cui J, Ding B, Padyukov L, Kallberg H, Bengtsson C, Klareskog L, Alfredsson L, Karlson EW. Effect of interactions of glutathione Stransferase T1, M1, and P1 and HMOX1 gene promoter polymorphisms with heavy smoking on the risk of rheumatoid arthritis. Arthritis Rheum. 2010;62(11):3196–210. doi: 10.1002/art.27639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lahiri M, Luben RN, Morgan C, Bunn DK, Marshall T, Lunt M, Verstappen SM, Symmons DP, Khaw KT, Wareham N, Bruce IN. Using lifestyle factors to identify individuals at higher risk of inflammatory polyarthritis (results from the European Prospective Investigation of Cancer-Norfolk and the Norfolk Arthritis Register--the EPIC-2-NOAR Study) AnnRheumDis. 2013 doi: 10.1136/annrheumdis-2012-202481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karlson EW, Ding B, Keenan BT, Liao K, Costenbader KH, Klareskog L, Alfredsson L, Chibnik LB. Association of environmental and genetic factors and gene-environment interactions with risk of developing rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013 doi: 10.1002/acr.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sparks JA, Chen CY, Jiang X, Askling J, Hiraki LT, Malspeis S, Klareskog L, Alfredsson L, Costenbader KH, Karlson EW. Improved performance of epidemiologic and genetic risk models for rheumatoid arthritis serologic phenotypes using family history. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-205009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemminki K, Li X, Sundquist J, Sundquist K. Familial associations of rheumatoid arthritis with autoimmune diseases and related conditions. Arthritis Rheum. 2009;60(3):661–8. doi: 10.1002/art.24328. Epub 2009/02/28. [DOI] [PubMed] [Google Scholar]
- 35.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–33. doi: 10.1056/NEJMoa021933. Epub 2003/10/17. doi: 10.1056/NEJMoa021933 349/16/1526 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Aho K, Palosuo T, Heliovaara M. Predictive significance of rheumatoid factor. J Rheumatol. 1995;22(11):2186–7. [PubMed] [Google Scholar]
- 37.Aho K, von Essen R, Kurki P, Palosuo T, Heliovaara M. Antikeratin antibody and antiperinuclear factor as markers for subclinical rheumatoid disease process. J Rheumatol. 1993;20(8):1278–81. [PubMed] [Google Scholar]
- 38.Del Puente A, Knowler WC, Pettitt DJ, Bennett PH. High incidence and prevalence of rheumatoid arthritis in Pima Indians. Am J Epidemiol. 1989;129(6):1170–8. doi: 10.1093/oxfordjournals.aje.a115238. [DOI] [PubMed] [Google Scholar]
- 39.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, Sundin U, van Venrooij WJ. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 40.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50(2):380–6. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 41.McAdoo SP, Hall A, Levy J, Salama AD, Pusey CD. Proteinase-3 antineutrophil cytoplasm antibody positivity in patients without primary systemic vasculitis. J Clin Rheumatol. 2012;18(7):336–40. doi: 10.1097/RHU.0b013e31826d2005. [DOI] [PubMed] [Google Scholar]
- 42.Olson SW, Owshalimpur D, Yuan CM, Arbogast C, Baker TP, Oliver D, Abbott KC. Relation between asymptomatic proteinase 3 antibodies and future granulomatosis with polyangiitis. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(8):1312–8. doi: 10.2215/CJN.10411012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen JH, Pan WH, Hsu CC, Yeh WT, Chuang SY, Chen PY, Chen HC, Chang CT, Huang WL. Impact of obesity and hypertriglyceridemia on gout development with or without hyperuricemia: a prospective study. Arthritis Care Res (Hoboken) 2013;65(1):133–40. doi: 10.1002/acr.21824. [DOI] [PubMed] [Google Scholar]
- 44.Laborie LB, Engesaeter IO, Lehmann TG, Eastwood DM, Engesaeter LB, Rosendahl K. Screening strategies for hip dysplasia: long-term outcome of a randomized controlled trial. Pediatrics. 2013;132(3):492–501. doi: 10.1542/peds.2013-0911. [DOI] [PubMed] [Google Scholar]
- 45.Chibnik LB, Keenan BT, Cui J, Liao KP, Costenbader KH, Plenge RM, Karlson EW. Genetic risk score predicting risk of rheumatoid arthritis phenotypes and age of symptom onset. PLoS One. 2011;6(9):e24380. doi: 10.1371/journal.pone.0024380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119(6):503 e1–9. doi: 10.1016/j.amjmed.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 47.Bowman MA, Leiter EH, Atkinson MA. Prevention of diabetes in the NOD mouse: implications for therapeutic intervention in human disease. Immunol Today. 1994;15(3):115–20. doi: 10.1016/0167-5699(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 48.Finckh A, Liang MH, van Herckenrode CM, de Pablo P. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: A meta analysis. Arthritis Rheum. 2006;55(6):864–72. doi: 10.1002/art.22353. Epub 2006/12/02. [DOI] [PubMed] [Google Scholar]
- 49.Van Dongen H, Van Aken J, Lard LR, Visser K, Ronday HK, Hulsmans HMJ, Speyer I, Westedt ML, Peeters AJ, Allaart CF, Toes RE, Breedveld FC, Huizinga TW. Efficacy of Methotrexate Treatment in Patients With Probable Rheumatoid Arthritis. A Double-Blind, Randomized, Placebo-Controlled Trial. Arthritis Rheum. 2007;56(5):1424–32. doi: 10.1002/art.22525. [DOI] [PubMed] [Google Scholar]
- 50.van Aken J, Heimans L, Gillet-van Dongen H, Visser K, Ronday HK, Speyer I, Peeters AJ, Huizinga TW, Allaart CF. Five-year outcomes of probable rheumatoid arthritis treated with methotrexate or placebo during the first year (the PROMPT study) Ann Rheum Dis. 2014;73(2):396–400. doi: 10.1136/annrheumdis-2012202967. [DOI] [PubMed] [Google Scholar]
- 51.James JA, Kim-Howard XR, Bruner BF, Jonsson MK, McClain MT, Arbuckle MR, Walker C, Dennis GJ, Merrill JT, Harley JB. Hydroxychloroquine sulfate treatment is associated with later onset of systemic lupus erythematosus. Lupus. 2007;16(6):401–9. doi: 10.1177/0961203307078579. Epub 2007/08/01. [DOI] [PubMed] [Google Scholar]
- 52.Arend WP, Firestein GS. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nat Rev Rheumatol. 2012;8(10):573–86. doi: 10.1038/nrrheum.2012.134. [DOI] [PubMed] [Google Scholar]
- 53.Katz SJ, Russell AS. Palindromic rheumatism: a pre-rheumatoid arthritis state? J Rheumatol. 2012;39(10):1912–3. doi: 10.3899/jrheum.120995. [DOI] [PubMed] [Google Scholar]
- 54.Abraham RR. Palindromic rheumatism: strategies to prevent evolution to rheumatoid arthritis. South Med J. 2012;105(6):322. doi: 10.1097/SMJ.0b013e318257c53e. author reply 3. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez-Lopez L, Gamez-Nava JI, Jhangri G, Russell AS, Suarez-Almazor ME. Decreased progression to rheumatoid arthritis or other connective tissue diseases in patients with palindromic rheumatism treated with antimalarials. J Rheumatol. 2000;27(1):41–6. Epub 2000/01/27. [PubMed] [Google Scholar]
- 56.Tada H, Hidaka Y, Izumi Y, Takano T, Nakata Y, Tatsumi K, Amino N. A Preventive Trial of Short-Term Immunosuppressive Therapy in Postpartum Thyroid Dysfunction. Int J Endocrinol Metab. 2003;2:48–54. [Google Scholar]
- 57.Tak PP. Prevention of clinically manifest rheumatoid arthritis by B cell directed therapy in the earliest phase of the disease (PRAIRI) Nederlands Trials Register [Internet] 2010 Available from: http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2442.
- 58.Singh JA, Reddy SG, Kundukulam J. Risk factors for gout and prevention: a systematic review of the literature. Curr Opin Rheumatol. 2011;23(2):192–202. doi: 10.1097/BOR.0b013e3283438e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SC, Newcomb C, Margolis D, Roy J, Hennessy S. Severe cutaneous reactions requiring hospitalization in allopurinol initiators: a population-based cohort study. Arthritis Care Res (Hoboken) 2013;65(4):578–84. doi: 10.1002/acr.21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deane KD, O'Donnell CI, Hueber W, Majka DS, Lazar AA, Derber LA, Gilliland WR, Edison JD, Norris JM, Robinson WH. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age - dependent manner. Arthritis & Rheumatism. 2010;62(11):3161–72. doi: 10.1002/art.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, Edison JD, Gilliland WR, Tibshirani RJ, Norris JM, Holers VM, Robinson WH. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7(5):e35296. doi: 10.1371/journal.pone.0035296. Epub 2012/06/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van de Stadt LA, Witte BI, Bos WH, van Schaardenburg D. A prediction rule for the development of arthritis in seropositive arthralgia patients. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2012-202127. Epub 2012/11/28. [DOI] [PubMed] [Google Scholar]
- 63.Bos WH, Dijkmans BA, Boers M, van de Stadt RJ, van Schaardenburg D. Effect of dexamethasone on autoantibody levels and arthritis development in patients with arthralgia: a randomised trial. Annals of the rheumatic diseases. 2010;69(3):571–4. doi: 10.1136/ard.2008.105767. Epub 2009/04/14. [DOI] [PubMed] [Google Scholar]
- 64.Hjeltnes G, Hollan I, Forre O, Wiik A, Mikkelsen K, Agewall S. Anti-CCP and RF IgM: predictors of impaired endothelial function in rheumatoid arthritis patients. Scand J Rheumatol. 2011;40(6):422–7. doi: 10.3109/03009742.2011.585350. Epub 2011/12/14. [DOI] [PubMed] [Google Scholar]
- 65.Bartoloni E, Alunno A, Bistoni O, Gerli R. How early is the atherosclerotic risk in rheumatoid arthritis? Autoimmun Rev. 2010;9(10):701–7. doi: 10.1016/j.autrev.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 66.Liang KP, Gabriel SE. Autoantibodies: innocent bystander or key player in immunosenescence and atherosclerosis? J Rheumatol. 2007;34(6):1203–7. [PubMed] [Google Scholar]
- 67.Aho K, Salonen JT, Puska P. Autoantibodies predicting death due to cardiovascular disease. Cardiology. 1982;69(3):125–9. doi: 10.1159/000173494. [DOI] [PubMed] [Google Scholar]
- 68.Grassi D, Desideri G, Ferri C. New Insight into Urate-Related Mechanism of Cardiovascular Damage. Current pharmaceutical design. 2014 doi: 10.2174/1381612820666140417095730. [DOI] [PubMed] [Google Scholar]
- 69.Dubreuil M, Zhu Y, Zhang Y, Seeger JD, Lu N, Rho YH, Choi HK. Allopurinol initiation and all-cause mortality in the general population. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harmsen CG, Stovring H, Jarbol DE, Nexoe J, Gyrd-Hansen D, Nielsen JB, Edwards A, Kristiansen IS. Medication effectiveness may not be the major reason for accepting cardiovascular preventive medication: a population-based survey. BMC medical informatics and decision making. 2012;12:89. doi: 10.1186/1472-6947-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Groopman J, Hartzband P. Your medical mind: how to decide what is right for you. New York: Penguin Books; 2011. [Google Scholar]
- 72.Hardcastle SJ, Legge E, Laundy CS, Egan SJ, French R, Watts GF, Hagger MS. Patients' Perceptions and Experiences of Familial Hypercholesterolemia, Cascade Genetic Screening and Treatment. International journal of behavioral medicine. 2014 doi: 10.1007/s12529-014-9402-x. [DOI] [PubMed] [Google Scholar]
- 73.Kucukarslan SN. A review of published studies of patients' illness perceptions and medication adherence: lessons learned and future directions. Research in social & administrative pharmacy : RSAP. 2012;8(5):371–82. doi: 10.1016/j.sapharm.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Hayashi D, Englund M, Roemer FW, Niu J, Sharma L, Felson DT, Crema MD, Marra MD, Segal NA, Lewis CE, Nevitt MC, Guermazi A. Knee malalignment is associated with an increased risk for incident and enlarging bone marrow lesions in the more loaded compartments: the MOST study. Osteoarthritis Cartilage. 2012;20(11):1227–33. doi: 10.1016/j.joca.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Finckh A, Liang M, Escher M, Bansback N, editors. Factors Involved in the Decision to Take Medications to Prevent Rheumatoid Arthritis in First Degree Relatives of Patients with RA. A Discrete Choice Experiment. Annual Scientific Meeting of the American College of Rheumatology (ACR); Chicago. Arthritis & Rheum; 2011. [Google Scholar]
- 76.Finckh A, Müller R, Möller B, Dudler J, Kyburz D, Walker U, Bas S, Liang M, Gabay C, editors. A novel screening strategy for preclinical rheumatoid arthritis (RA) in first degree relatives of patients with RA. Ann Rheum Dis.Annual European Congress of Rheumatology EULAR; London. 2011. [Google Scholar]
- 77.Vilaprinyo E, Forne C, Carles M, Sala M, Pla R, Castells X, Domingo L, Rue M, Interval Cancer Study G Cost-effectiveness and harm-benefit analyses of risk-based screening strategies for breast cancer. PLoS One. 2014;9(2):e86858. doi: 10.1371/journal.pone.0086858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Finckh A, Liang MH. Anti-Cyclic Citrullinated Peptide Antibodies in the Diagnosis of Rheumatoid Arthritis: Bayes Clears the Haze. Ann Int Med. 2007 Jun 5th;146(11) doi: 10.7326/0003-4819-146-11-200706050-00011. [DOI] [PubMed] [Google Scholar]
- 79.Notkins AL. New Predictors of Disease. Scientific American. 2007:72–9. [PubMed] [Google Scholar]
- 80.Organisation WH, editor. Preventing Chronic Disease: A vital investment. WHO Press; 2005. [Google Scholar]
- 81.Smerecnik CM, Mesters I, Verweij E, de Vries NK, de Vries H. A systematic review of the impact of genetic counseling on risk perception accuracy. Journal of genetic counseling. 2009;18(3):217–28. doi: 10.1007/s10897-008-9210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cunningham MW. Streptococcus and rheumatic fever. Curr Opin Rheumatol. 2012;24(4):408–16. doi: 10.1097/BOR.0b013e32835461d3. [DOI] [PMC free article] [PubMed] [Google Scholar]