Abstract
Demonstrating or verifying a current or past exposure to an environmental mitochondrial toxin or toxicant is extraordinarily difficult. Thus, there is a pressing need to develop a biomarker for exposure to environmental mitochondrial inhibitors. Rotenone, an environmental toxicant, is a potent inhibitor of the mitochondrial electron transfer chain. Rotenone specifically inhibits complex I throughout the body and brain, thereby producing systemic mitochondrial impairment. As such, rotenone is a prototypical clinically relevant, environmental mitochondrial toxicant that may be used as an ideal initial platform to develop accessible biomarkers of exposure. The over-arching goal of this work is to explore and validate peripheral (blood and skeletal muscle) DNA damage as a biomarker of mitochondrial toxicant exposure using the rat rotenone model. In this effort, we utilized an extremely sensitive quantitative polymerase chain reaction (QPCR)-based assay that simultaneously allows the assessment of multiple forms of mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) damage. We found mtDNA damage in blood is detected after subclinical rotenone exposure and the damage persists even after complex I activity has returned to normal. With a more sustained rotenone exposure, mtDNA damage is also detected in skeletal muscle, suggesting that mtDNA damage in this tissue simply lags behind blood. Using the QPCR-based assay, we have no evidence for nDNA damage in peripheral tissues after rotenone exposure either acutely or chronically. Overall, these data support the idea that mtDNA damage in peripheral tissues in the rotenone model may provide a biomarker of past or ongoing mitochondrial toxin exposure.
Keywords: DNA damage, rotenone, mitochondrial toxins, pesticides, biomarkers
In the normal course of their lives, humans are exposed to a wide range of environmental toxins or toxicants, some of which specifically impair mitochondrial function, including bacterial and fungal toxins, solvents, metals, and pesticides (both natural and synthetic). Sometimes such exposures lead to clinical symptoms or disease. For example, exposures to certain pesticides have been associated with an increase in many types of cancers and neurodegenerative diseases (Pogribny and Rusyn, 2013; Tanner et al., 2011). In light of the connection between pesticides and human disease, biomonitoring of past or present exposure is an important area of research. Additionally, numerous nuclear and mitochondrial mutations can disrupt mitochondrial function and produce disease. While it is clear that mitochondrial dysfunction is associated with a variety of syndromes and diseases, genetic mitochondrial disease remains complicated to accurately and efficiently diagnose (Liang et al., 2014; Rodenburg, 2011). Mitochondria may also be the target for a large number of environmental toxicants (Meyer et al., 2013). Demonstrating or verifying a current or past exposure to a mitochondrial toxin and/or toxicant may be even more complex. Currently, biomarkers of mitochondrial function to facilitate population studies of environmental exposures and their consequences are deficient. Thus, there is a pressing need to develop a biomarker for mitochondrial toxin and/or toxicant exposures.
In order to effectively detect either populations at risk or those exposed to pesticides, biomarkers, generally in peripheral tissues, are utilized. However, issues such as tissue distribution and pharmacokinetics of the pesticide may be unknown. Low-level exposure may not produce a clinical phenotype, and depending on the assay chosen, functional mitochondrial impairment, or inhibition may not persist beyond the acute exposure (or metabolism/excretion of the toxin/toxicant). Alternatively, one can attempt to assess the functional status of intact mitochondria. Although such assays of mitochondrial function (i.e., respiration, membrane potential, production of reactive oxygen species, etc.) may be useful under some circumstances, these typically require fresh or cultured specimens, and may suffer from a relative lack of sensitivity or poor reproducibility (Greenamyre et al., 2001). Interestingly, indicators of DNA damage have been a focus of pesticide biomarker development. DNA has several characteristics that might be useful when developing a biomarker. It is stable and can be isolated easily from fresh or long-frozen specimens. However, while many studies to assess environmental exposures in humans are promising, the particular markers of DNA damage that have been used are plagued with confounding factors and inconsistencies (Kapka-Skrzypczak et al., 2011).
Rotenone, a mitochondrial toxicant, is a potent inhibitor of the mitochondrial electron transfer chain (ETC). It is an environmental toxicant that is widely used to kill nuisance fish in lakes and reservoirs and, until recently, was used as an insecticide in home vegetable gardens. Rotenone is lipophilic and crosses biological membranes rapidly, independent of transporters. Rotenone specifically inhibits complex I throughout the body and brain, thereby producing systemic mitochondrial impairment. As such, rotenone is a prototypical clinically relevant, environmental mitochondrial toxicant that may be used as an ideal initial platform to develop accessible biomarkers of exposure.
The over-arching goal of this work is to explore and validate peripheral DNA damage as a biomarker of mitochondrial toxicant exposure using the rotenone model. In this effort, we utilized a quantitative polymerase chain reaction (QPCR)-based assay that measures mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) damage. The QPCR-based assay has been used to quantify DNA damage in cells and tissues that have been exposed to various mutagens, as well as is particularly useful in detecting reactive oxygen species-mediated damage (Ayala-Torres et al., 2000; Furda et al., 2012b). The QPCR-based assay has recently been used to identify mtDNA damage in blood of patients with a “mitochondrial” disease, Friedreich’s ataxia (Haugen et al., 2010). Thus, this assay is ideally suited to provide readouts of exposures to environmental toxins/toxicants that affect mitochondria.
METHODS AND MATERIALS
In Vivo Rotenone Treatment and Sample Collection
The Institutional Animal Care and Use Committee of the University of Pittsburgh approved all the experiments utilizing animals. Male Lewis rats (7–9 months old, Hilltop Lab Animals, Inc., Scottsdale, PA, USA) were injected intraperitoneally with vehicle or 3.0 mg/kg/day rotenone (Sigma-Aldrich) either once or for 5 daily injections, which is a treatment paradigm in which there is no neurodegeneration (Cannon et al., 2009; Sanders et al., 2014b). Animals were also treated to Parkinsonian endpoint, which is characterized by behavioral features including bradykinesia, postural instability/gait disturbances, rigidity, and nigrostriatal neurodegeneration (Cannon et al., 2009). Animals were then euthanized as described (Cannon et al., 2009). Total whole blood was collected at the time of sacrifice into EDTA coated tubes (Fisher Scientific) and stored at 4°C (not for more than 5 days) until DNA isolation. Skeletal muscle (gracilis) was dissected and subsequently flash frozen in liquid nitrogen. The number of animals analyzed for each in vivo experiment is listed in figure legends.
DNA Isolation and Quantification
Blood and skeletal muscle nuclear and mitochondrial DNA was isolated according to a high molecular weight genomic DNA purification kit using the manufacturer’s protocol (QIAGEN Genomic tip). Muscle was first homogenized using the TissueRuptor with disposable probes (QIAGEN). One volume of buffer C1 (QIAGEN) and three volumes of distilled water were added to the blood sample, mixed, and then incubated on ice for 10 min. Both blood and muscle homogenates were centrifuged at 10 000× g for 20 min at 4°C. The pellet was either stored at −20°C or immediately processed further as described below. DNA was quantified using the Picogreen dsDNA quantification assay as suggested by the manufacturer (Molecular Probes). Fluorescence from the Picogreen was measured with a 485 nm excitation filter and a 530 nm emission filter using a microplate reader (SpectraMax Gemini EM). Lambda DNA was used to construct a standard curve in order to determine the concentration of unknown samples. Quality of the DNA prior to QPCR analysis was verified by running the DNA on a 0.6% ethidium bromide-stained agarose gel. Only DNA of intact high molecular weight which showed negligible evidence of degradation was used in the DNA damage assays. DNA samples were aliquoted and stored at −20°C.
QPCR-based Assay to Measure mtDNA Damage
To measure levels of mtDNA damage, we used a QPCR-based assay (Ayala-Torres et al., 2000; Furda et al., 2012a; Santos et al., 2006). This method is based on the principle that various forms of DNA damage have the propensity to slow down or block DNA polymerase progression. Thus, if equal amounts of mtDNA from experimental and control specimens are amplified under identical conditions, the mtDNA sample with the least mtDNA damage will produce the greatest amount of PCR product. The PCR product used here amplifies almost the entire mitochondrial genome, excluding the D-loop to avoid bias from this mutation hotspot (Sanchez-Cespedes et al., 2001). In addition to mtDNA damage, the QPCR-based assay measures certain mtDNA mutations, such as the common 4.9 kb and other large deletions (based on a size change of the product), as well as smaller deletions or rearrangements that compromise primer complementarity. In contrast, typical sequencing strategies only detect mutations and not mtDNA damage.
To confirm that QPCR-based assays were performed in the linear range, optimal number of cycles for each template was conducted to show that 50% reduction in the amount of template resulted in about 50% reduction in amplification. QPCR products that demonstrated 40%–60% in the amplification of the target sequence when using 50% of the original template were considered acceptable. The PCR amplification profile for a 13.4 kb rat mitochondrial fragment was as follows: hot start for 10 min at 75°C when the DNA polymerase was added, an initial denaturation step for 1 min at 94°C, followed by 20–24 cycles (this range was dependent on the length of rotenone exposure or tissue source) of denaturation for 15 s at 94°C, and then annealing/extension at 66°C for 14 min. To complete the profile, a final extension for 10 min at 72°C was performed. Primers 10 633 and 13 559 were used for the amplification of the rat mitochondrial fragment and the primer nucleotide sequences were described previously (Ayala-Torres et al., 2000). The final concentration of magnesium in the PCR was 1.1 mM for skeletal muscle and 1.3 mM for blood.
To ensure quality and specificity, all PCR products were resolved on a 0.6% agarose gel and UV light used to visualize ethidium bromide-stained gels. Relative fluorescence of PCR products was quantified using Picogreen. All QPCR experiments were conducted in triplicate. Assuming a random distribution, the Poisson equation was used to calculate the number of DNA lesions. Based on this equation, the amplification is directly proportional to the fraction of undamaged DNA templates. As such, the average lesion frequency per strand is calculated as –ln(AD/AO), where AD is the amplification of the damaged or experimental template, while AO is the amplification of the undamaged or control template. Therefore, the results are shown as the number of lesions per strand normalized to 10 kb. DNA damage can then be expressed mathematically as the number of DNA lesions per kilobase (Furda et al., 2012a). By normalizing this way, direct comparisons of different tissues or different chemical exposures are facilitated.
QPCR of a Small mtDNA Fragment
To ensure that the amplification of the large mtDNA fragment was not due to possible changes in mtDNA steady state levels, we amplified a rat (235-bp) small mtDNA fragment, since the amplification of this small fragment should be independent of damage. The profile for this PCR amplification was as follows: hot start for 10 min at 75°C when the DNA polymerase was added, an initial denaturation step for 1 min at 94°C, followed by 18–21 cycles (this range was dependent on the length of rotenone exposure or tissue source) of denaturation for 1 min at 94°C and then annealing at 60°C rat for 45 s and extension at 72°C for 45 s. To complete the profile, a final extension for 10 min at 72°C was performed. The primer nucleotide sequences were 14 678 and 14 885 for rat (Ayala-Torres et al., 2000). The PCR product was resolved on a 1.5% agarose gel and UV light used to visualize ethidium bromide-stained gels. Relative fluorescence of PCR products were quantified using Picogreen. The final concentration of magnesium in the PCR was 1.1 mM for skeletal muscle and 1.3 mM for blood.
QPCR-based Assay to Measure Nuclear DNA Damage
The PCR amplification profile for the 12.5-kb rat fragment from the nuclear clusterin (TRPM-2) gene was as follows: hot start for 10 min at 75°C when the DNA polymerase was added, an initial denaturation step for 1 min at 94°C, followed by 23–28 cycles (this range was dependent on the length of rotenone exposure or tissue source) of denaturation for 15 s at 94°C, and then annealing/extension at 66°C for 13 min (Ayala-Torres et al., 2000). Primers 5781 and 18 314 were used for the amplification of the nuclear fragment and the primer nucleotide sequences were described previously (Ayala-Torres et al., 2000). The final concentration of magnesium in the PCR was 1.1 mM for skeletal muscle and 1.3 mM for blood.
Statistical Analysis
The statistical software package GraphPad Prism was used for statistical computation. Data were analyzed by ANOVA followed by Bonferroni post hoc test and P < .0001 was deemed significant. For all graphs, the bars represent mean ± standard error of the mean (SEM).
RESULTS
Blood mtDNA Damage Detects Subclinical Exposures and Outlasts Acute Effects of Rotenone
In an effort to develop a peripheral biomarker for mitochondrial toxin exposure, we measured mtDNA and nDNA damage in the rat rotenone model utilizing a QPCR-based assay (Furda et al., 2012a). A single bolus injection of rotenone (3 mg/kg) produces no detectable pathology, loss of striatal dopamine terminals or of nigral neurons (Cannon et al., 2009). After injection, rotenone maximally inhibits complex I by about 45% at 8 h, with full recovery of activity within 24 h (Cannon et al., 2009). Our initial goal was to define the useful time window for DNA damage as a potential biomarker (i.e., how soon and for how long) in 2 accessible tissues (blood and skeletal muscle) after a single rotenone exposure.
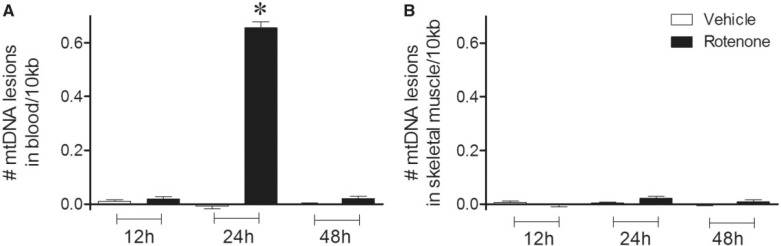
To do this, DNA was purified from blood and skeletal muscle 12, 24, and 48 h after rats were treated with a single dose of rotenone or vehicle. In blood, mtDNA damage (or lesions) was significantly increased 24 h after exposure to rotenone relative to vehicle-treated rats, but mtDNA damage was not detected at 12 or 48 h (Fig. 1A). Thus, the time course of detectable mtDNA damage in blood lags slightly behind the time course of complex I inhibition (Cannon et al., 2009) and lasts longer. In contrast, mtDNA damage was not detected in skeletal muscle at any time point after rats were exposed to rotenone or vehicle (Fig. 1b). Because rotenone might alter levels of mtDNA, we used a small mitochondrial QPCR product to control for mtDNA copy number. Levels of mtDNA damage in blood and skeletal muscle in response to rotenone treatment (12 h, 24 h, or 48 h) were not related to changes in mtDNA steady-state levels (Table 1). Notably, nDNA damage was not seen in either tissue at any time point after a single dose of rotenone (Table 2). Overall, these data suggest that blood mtDNA damage serves as a relatively sensitive marker of sub-clinical exposure to rotenone that persists even after complex I activity has returned to normal.
FIG. 1.
mtDNA damage in blood 24 h after a single bolus injection of rotenone (3 mg/kg). A, Blood collected 24 h after rats received a single dose of rotenone, exhibited greater levels of mtDNA damage than vehicle-treated animals (black bar, 0.66 lesions ± 0.02/10 kb; *p < .0001). Blood collected 12 h or 48 h after rats received a single dose of rotenone did not display differences in mtDNA damage when vehicle (white bars) or rotenone (black bars) treated rats were compared (black bars, 0.02 lesions ± 0.01/10 kb). B, No differences in mtDNA damage were found in skeletal muscle in vehicle compared to rotenone (single dose) treated animals at either 12, 24 or 48 h (black bars, −0.01 lesions ± 0.01/10 kb; 0.02 lesions ± 0.01/10 kb; 0.01 lesions ± 0.01/10 kb; 12, 24 and 48 h, respectively). n = 6 (vehicle) and n = 7 (rotenone) treated rats were used for 12 h blood and muscle DNA damage analysis. n = 5 (vehicle) and n = 5 (rotenone) treated rats were used for 24 h blood and muscle DNA damage analysis. n = 7 (vehicle), n = 7 (rotenone) for 48 h blood and muscle DNA damage analysis. Data are presented as mean ± SEM.
TABLE 1.
Mitochondrial DNA (mtDNA) copy number in blood and skeletal muscle following rotenone treatment. Data are presented as mean ± SEM.
| Tissue | Treatment | mtDNA Copy Number Relative to Control |
|---|---|---|
| Blood | 12 h post single injection | 1.01 ± 0.01 |
| Muscle | 12 h post single injection | 1.02 ± 0.02 |
| Blood | 24 h post single injection | 1.03 ± 0.08 |
| Muscle | 24 h post single injection | 1.05 ± 0.02 |
| Blood | 48 h post single injection | 0.98 ± 0.02 |
| Muscle | 48 h post single injection | 1.01 ± 0.01 |
| Blood | 6 h post 5 daily injections | 1.04 ± 0.07 |
| Muscle | 6 h post 5 daily injections | 0.95 ± 0.02 |
| Blood | 48 h post 5 daily injections | 0.99 ± 0.02 |
| Muscle | 48 h post 5 daily injections | 1.00 ± 0.02 |
| Blood | Endpoint | 1.01 ± 0.02 |
| Muscle | Endpoint | 0.99 ± 0.02 |
TABLE 2.
Nuclear DNA (nDNA) damage in blood and skeletal muscle following rotenone treatment. Data are presented as mean ± SEM.
| Tissue | Treatment | No. of nDNA lesions/10 kb |
|---|---|---|
| Blood | 12 h post single vehicle injection | 0.02 ± 0.01 |
| Blood | 12 h post single rotenone injection | 0.03 ± 0.02 |
| Muscle | 12 h post single vehicle injection | 0.01 ± 0.01 |
| Muscle | 12 h post single rotenone injection | 0.01 ± 0.01 |
| Blood | 48 h post single vehicle injection | 0.01 ± 0.01 |
| Blood | 48 h post single rotenone injection | 0.02 ± 0.01 |
| Muscle | 48 h post single vehicle injection | 0.01 ± 0.003 |
| Muscle | 48 h post single rotenone injection | 0.0001 ± 0.003 |
| Blood | 48 h post 5 daily vehicle injections | −0.01 ± 0.01 |
| Blood | 48 h post 5 daily rotenone injections | −0.01 ± 0.002 |
| Muscle | 48 h post 5 daily vehicle injections | −0.003 ± 0.002 |
| Muscle | 48 h post 5 daily rotenone injections | −0.01 ± 0.01 |
| Blood | 24 h post single vehicle injection | 0.01 ± 0.01 |
| Blood | 24 h post single rotenone injection | 0.05 ± 0.02 |
| Muscle | 24 h post single vehicle injection | 0.02 ± 0.02 |
| Muscle | 24 h post single rotenone injection | 0.05 ± 0.01 |
| Blood | 6 h post 5 daily vehicle injections | 0.0001 ± 0.0001 |
| Blood | 6 h post 5 daily rotenone injections | 0.0001 ± 0.00003 |
| Muscle | 6 h post 5 daily vehicle injections | 0.0003 ± 0.0002 |
| Muscle | 6 h post 5 daily rotenone injections | 0.0003 ± 0.0002 |
| Blood | Endpoint vehicle injections | 0.02 ± 0.01 |
| Blood | Endpoint rotenone injections | 0.02 ± 0.01 |
| Muscle | Endpoint vehicle injections | 0.01 ± 0.01 |
| Muscle | Endpoint rotenone injections | 0.01 ± 0.01 |
mtDNA Damage Persists in Both Blood and Skeletal Muscle with Chronic Rotenone Exposure
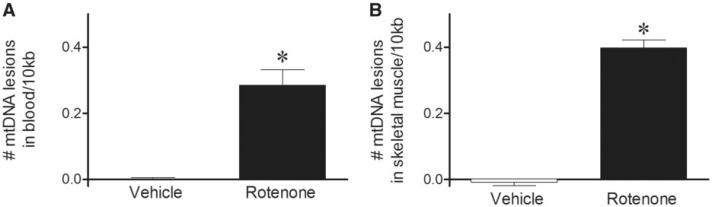
We next investigated whether a more prolonged exposure to rotenone would further increase mtDNA damage in blood and/or become detectable in skeletal muscle. Five days of rotenone dosing produces no overt clinical signs and no striatal dopamine terminal or nigral neuron loss in rats (Cannon et al., 2009; Sanders et al., 2014b). Similar to a single rotenone injection (Fig. 1A), 6 h after the 5th daily dose, mtDNA damage was significantly increased in blood (Fig. 2A); mtDNA damage was also found in skeletal muscle at this time point (Fig. 2A). No differences in mtDNA copy number could be detected in blood or muscle with rotenone treatment relative to vehicle (Table 1). DNA damage was specific to the mitochondria, as no nDNA damage was observed in either tissue with rotenone or vehicle treatment (Table 2).
FIG. 2.
mtDNA damage increased in both blood and skeletal muscle samples obtained 6 h after the 5th daily dose of rotenone. A, Blood collected 6 h after rats received 5 daily doses of rotenone, exhibited greater levels of mtDNA damage than vehicle-treated animals (black bar, 0.29 lesions ± 0.05/10 kb; *P < .0001). B, Skeletal muscle also showed increased mtDNA damage in rotenone-treated animals relative to vehicle (black bar, 0.40 lesions ± 0.02/10 kb; *P < .0001). Levels of blood and skeletal muscle mtDNA damage are not statistically different from each other. n = 8 (vehicle) and n = 8 (rotenone) treated rats were used for blood DNA damage analysis. n = 7 (vehicle) and n = 7 (rotenone) treated rats were used for muscle DNA damage analysis. Data are presented as mean ± SEM.
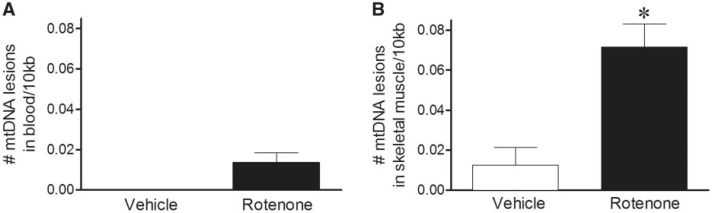
To investigate whether extended rotenone treatment caused mtDNA damage to persist even longer, rats were treated with 5 daily doses and then 48 h later the blood and skeletal muscle assayed for DNA damage. Although mtDNA damage was detected 6 h after the 5th daily dose, the mtDNA damage was apparently repaired in the blood by 48 h (Fig. 3A). However, mtDNA damage was still detectable in skeletal muscle 48 h after the 5th daily rotenone exposure (Fig. 3B), albeit to a lesser magnitude than at 6 h. Differences in mtDNA copy number (Table 1) or nDNA damage (Table 2) were not observed with vehicle or rotenone treatment in either blood or skeletal muscle.
FIG. 3.
mtDNA damage persists longer in skeletal muscle with chronic rotenone exposure. A, Blood and B, skeletal muscle were collected 48 h after rats received 5 daily doses of rotenone. Only skeletal muscle exhibited increased mtDNA damage in rotenone-treated animals relative to vehicle (black bar, 0.01 lesions ± 0.01/10 kb; 0.07 lesions ± 0.01/10 kb; *P < .0001, blood and skeletal muscle, respectively). n = 6 (vehicle and rotenone) treated rats were used for blood and muscle DNA damage analysis. Data are presented as mean ± SEM.
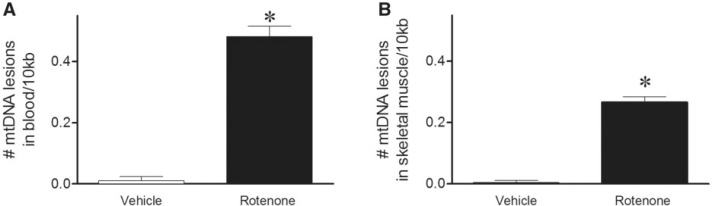
Biomarkers for chronic exposures are particularly difficult to identify. To produce an in vivo Parkinsonian phenotype with loss of nigrostriatal dopamine neurons, rats are typically treated to endpoint with rotenone (3 mg/kg/day) for about 2 weeks (Cannon et al., 2009). DNA damage levels were determined using this chronic rotenone paradigm. Mitochondrial DNA damage was significantly increased in both blood and skeletal muscle in rats treated with rotenone to Parkinson’s disease (PD) endpoint relative to vehicle (Fig. 4A and B). No differences in mtDNA copy number or nDNA damage could be detected in blood or muscle with chronic rotenone treatment relative to vehicle-treated animals (Tables 1 and 2). In summary, depending on the length of exposure, skeletal muscle, and/or blood mtDNA damage may provide a useful marker of mitochondrial toxin exposure.
FIG. 4.
mtDNA damage detectable in rotenone animals treated to PD endpoint. A, Blood collected after rats were treated with rotenone to endpoint, exhibited greater levels of mtDNA damage than vehicle-treated animals (black bar, 0.48 lesions ± 0.03/10 kb; *P < .0001). B, Skeletal muscle also showed increased mtDNA damage in rotenone-treated animals relative to vehicle (black bar, 0.27 lesions ± 0.02/10 kb; *P < .0001). Levels of blood mtDNA damage are statistically higher than muscle mtDNA damage (P < .0001). n = 7 (vehicle) and n = 10 (rotenone) treated rats were used for blood DNA damage analysis. n = 5 (vehicle) and n = 5 (rotenone) treated rats were used for muscle DNA damage analysis. Data are presented as mean ± SEM.
DISCUSSION
Demonstrating or verifying a current or past exposure to an environmental mitochondrial toxin is extraordinarily difficult. Thus, there is a pressing need to develop a biomarker for exposure to environmental mitochondrial inhibitors that is (i) sensitive, (ii) at least semi-quantitative, (iii) enduring after toxin exposure has ceased, (iv) stable after specimen collection, and (v) highly reproducible. Overall, the data presented here support the idea that mtDNA damage in both blood and skeletal muscle meet these criteria. By using a sensitive QPCR-based assay of DNA damage that simultaneously allows the assessment of multiple forms of damage, we found that mtDNA damage in accessible, peripheral tissues may provide a biomarker of recent or ongoing mitochondrial toxin exposure.
In our efforts to develop a biomarker for mitochondrial toxin exposure we chose the rotenone model, as it is ideally suited for these studies in that this model reproduces both the environmental (pesticide) contribution to PD and the systemic mitochondrial impairment of the human disease. A single bolus of rotenone in rats (3 mg/kg) does not produce behavioral phenotypes or neuronal loss (i.e., it is subclinical), yet repeated boluses produce a profound Parkinsonian syndrome, including gastrointestinal dysfunction/pathology (Cannon et al., 2009; Drolet et al., 2009). As might be expected, 24 h after a single rotenone exposure, detectable mtDNA damage in the blood lags behind the time course of complex I inhibition (Cannon et al., 2009). Therefore, mtDNA damage in blood may be useful as a biomarker with acute doses in two regards: (i) mtDNA damage is detected shortly after the exposure; and (ii) mtDNA damage as a “footprint” outlives the acute toxin exposure. Despite the lipophilicity of rotenone, which enables this molecule to cross all biological membranes and inhibit complex I in all tissues, mtDNA damage in muscle is not detected 24 h after a single bolus of rotenone. Further investigation is required to understand the mechanism(s) by which skeletal muscle is resistant to mtDNA damage with an acute dose of rotenone treatment. However, the fact that we detect mtDNA damage after 5 days of dosing demonstrates that the skeletal muscle response simply trails behind the blood response. Overall, these results indicate that blood mtDNA damage may be a useful biomarker early after a mitochondrial toxin exposure.
We found mtDNA damage in both blood and skeletal muscle with 5 daily doses of rotenone and with chronic rotenone treatment to PD endpoint. Importantly, the fact that mtDNA damage is still detectable indicates that repair mechanisms have not adequately compensated despite ongoing damage. Interestingly, even though mtDNA damage resolved in blood within 48 h after a single or 5 daily rotenone injections, mtDNA damage persisted in skeletal muscle 48 h after 5 daily rotenone injections. With a more chronic rotenone exposure, perhaps more so than with a single rotenone exposure, the measured mtDNA damage is an integrated function of ongoing damage, repair mechanisms and mitochondrial biogenesis (Van Laar and Berman, 2013 ). Although the mechanism(s) is unclear, skeletal muscle may be slower to repair the mtDNA damage after more sustained rotenone treatment, suggesting this peripheral tissue may provide a useful biomarker depending on the length of exposure.
Rotenone specifically binds complex I of the ETC and, under our experimental conditions, appears to exert its toxicity through oxidative stress rather than ATP depletion (Sanders and Greenamyre, 2013 ). The generation of reactive oxygen species can damage not only the ETC complexes, but also other macromolecules in the mitochondria, including DNA. Interestingly, the peak of mtDNA damage in blood was 24 h after rotenone exposure relative to 5 doses or endpoint treatment; levels of mtDNA damage were highest in skeletal muscle from rats treated with 5 doses of rotenone. Therefore, chronic rotenone exposure did not result in increased levels of mtDNA damage in either blood or skeletal muscle. Together these results suggest that complex I inhibition beyond a certain threshold does not result in increased levels of mtDNA damage in these tissues. Potential compensatory mechanism(s) such as the removal of damaged mitochondria by autophagy, require further investigation (Akbari et al., 2014).
While we were able to detect mtDNA damage using the QPCR-based assay shortly after the onset of complex I inhibition and after more prolonged mitochondrial impairment, thus far, we have no evidence for nDNA damage in peripheral tissues after rotenone exposure. Since nDNA damage has been observed in other systems after rotenone treatment (Jia et al., 2010; Seo et al., 2006; Sherer et al., 2002; Swarnkar et al., 2012), it is possible that nDNA damage occurs in blood and skeletal muscle but is repaired prior to DNA damage analysis. Alternatively, certain types of nDNA damage may accumulate that cannot be detected using the QPCR-based assay. Nonetheless, this emphasizes the specificity of mtDNA damage as a biomarker of mitochondrial toxin exposure.
It should be emphasized that the QPCR-based assay simultaneously assesses a wide variety of DNA damage, including apurinic/apyrimidinic sites, and single and double-strand breaks; it may also detect DNA repair intermediates (Ayala-Torres et al., 2000; Furda et al., 2012a; Santos et al., 2006). On the other hand, the assay does not detect all forms of DNA damage, only those that impede or stop DNA polymerase progression. Moreover, it does not provide information of the specific type(s) of DNA damage present in a sample. However, for biomarker development, we propose that the ability to simultaneously detect and measure a broad range of types of DNA damage—even if the specific biochemical alterations are not identified—is a strength, rather than a weakness. And, if one considers mtDNA damage in the context of exposure to mitochondrial toxins, mtDNA detected by the QPCR-assay could provide a convergent readout across a divergent range of toxicants. Future studies will evaluate whether peripheral mtDNA damage is a common feature of systemically active complex I inhibitors and/or ETC inhibitors in general.
Further validation of mtDNA damage as a peripheral biomarker of rotenone exposure might include the presence of mtDNA damage in the brain. Despite systemic inhibition of complex I, chronic rotenone administration causes selective nigrostriatal neurodegeneration (Betarbet et al., 2000). We recently found that mtDNA damage selectively accumulates in midbrain neurons and this occurred well before there is any sign of degeneration of the nigrostriatal system (Sanders et al., 2014b). Taken together, these results suggest that after rotenone exposure mtDNA damage in peripheral tissues may accurately predict mtDNA damage in brain regions susceptible to neurodegeneration.
To perform the QPCR-based analysis tissue homogenates are required to isolate genomic DNA (which includes both mtDNA and nDNA). One advantage of this method is that since a separate mitochondrial isolation is not necessary, artificial oxidation to the DNA is not a confounding factor (Maynard et al., 2010). However, one limitation of the homogenate preparation is that the observed DNA damage cannot be ascribed to a particular cell type. For instance, there are numerous components in a blood homogenate (e.g., leukocytes, platelets, and red blood cells). A more detailed analysis is required to identify the specific cell type(s) in blood (or skeletal muscle) with increased mtDNA damage.
In the current study in rats, we have validated mtDNA damage as a biomarker of mitochondrial toxin exposure in 2 peripheral tissues. The QPCR-based assay can easily be translated into studies of human exposures. It is worth noting that the amount of starting material required for this assay (<10 mg tissue or <1 ml blood) is easily obtained in a routine clinical needle biopsy of muscle or from blood draws. In addition, the QPCR-based assay can be performed on long-frozen DNA samples. In considering the assay as a biomarker for at-risk human populations, it would be of interest to test blood or skeletal muscle samples from individuals who apply rotenone in lakes or reservoirs, or from pesticide applicators who use insecticides that act on mitochondria.
PD is a neurodegenerative movement disorder that has been associated with both environmental exposures (Bronstein et al., 2009; Dick et al., 2007; Elbaz and Tranchant, 2007 ; Vance et al., 2010) and systemic mitochondrial defects (Abeliovich, 2010; Murphy, 2009; Orsucci et al., 2011; Zheng et al., 2010). The first proof-of-concept that systemic mitochondrial dysfunction could result in selective neurodegeneration similar to PD came from the rotenone model (Betarbet et al., 2000). Repeated daily bolus injection of rotenone (3 mg/kg; ip) over a period of about 2 weeks results in consistent, selective degeneration of the nigrostriatal dopamine system, as occurs in PD (Cannon et al., 2009). Moreover, recent epidemiological studies have confirmed rotenone exposure as a bona fide risk factor for development of PD (Dhillon et al., 2008; Tanner et al., 2011). Given (i) the current results, (ii) the fact that rotenone and other mitochondrial toxicants increase the risk of PD, (iii) our recent finding that PD-associated pathogenic LRRK2 mutations cause mtDNA damage, and (iv) midbrain neurons selectively accumulate mtDNA damage in idiopathic PD, future studies will include measurement of mtDNA damage in peripheral tissues from PD patients (Sanders et al., 2014a, 2014b; Tanner et al., 2011). In conclusion, detection of mtDNA damage may provide the basis for an accessible, sensitive, stable biomarker of environmental mitochondrial toxin exposure, and could possibly have utility for additional human diseases.
FUNDING
This work was supported by grants from the National Institutes of Health T32MH18273 (L.H.S.), 1F32ES019009-01 (L.H.S.), 1R01ES020718 (J.T.G.), and the JPB Foundation (J.T.G.).
ACKNOWLEDGMENTS
We would like to thank members of the Greenamyre laboratory.
REFERENCES
- Abeliovich A. (2010). Parkinson’s disease: Mitochondrial damage control. Nature 463, 744–745. [DOI] [PubMed] [Google Scholar]
- Akbari M., Keijzers G., Maynard S., Scheibye-Knudsen M., Desler C., Hickson I. D., Bohr V. A. (2014). Overexpression of DNA ligase III in mitochondria protects cells against oxidative stress and improves mitochondrial DNA base excision repair. DNA Repair 16, 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala-Torres S., Chen Y., Svoboda T., Rosenblatt J., Van Houten B. (2000). Analysis of gene-specific DNA damage and repair using quantitative polymerase chain reaction. Methods 22, 135–147. [DOI] [PubMed] [Google Scholar]
- Betarbet R., Sherer T. B., MacKenzie G., Garcia-Osuna M., Panov A. V., Greenamyre J. T. (2000). Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 3, 1301–1306. [DOI] [PubMed] [Google Scholar]
- Bronstein J., Carvey P., Chen H., Cory-Slechta D., DiMonte D., Duda J., English P., Goldman S., Grate S., Hansen J., et al. (2009). Meeting report: Consensus statement-Parkinson’s disease and the environment: Collaborative on health and the environment and Parkinson's Action Network (CHE PAN) conference 26–28 June 2007. Environ. Health Perspect. 117, 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. R., Tapias V., Na H. M., Honick A. S., Drolet R. E., Greenamyre J. T. (2009). A highly reproducible rotenone model of Parkinson’s disease. Neurobiol. Disease 34, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon A. S., Tarbutton G. L., Levin J. L., Plotkin G. M., Lowry L. K., Nalbone J. T., Shepherd S. (2008). Pesticide/environmental exposures and Parkinson’s disease in East Texas. J. Agromed. 13, 37–48. [DOI] [PubMed] [Google Scholar]
- Dick F. D., De Palma G., Ahmadi A., Osborne A., Scott N. W., Prescott G. J., Bennett J., Semple S., Dick S., Mozzoni P., et al. (2007). Gene-environment interactions in parkinsonism and Parkinson’s disease: The Geoparkinson study. Occupat. Environ. Med. 64, 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet R. E., Cannon J. R., Montero L., Greenamyre J. T. (2009). Chronic rotenone exposure reproduces Parkinson's disease gastrointestinal neuropathology. Neurobiol. Disease 36, 96–102. [DOI] [PubMed] [Google Scholar]
- Elbaz A., Tranchant C. (2007). Epidemiologic studies of environmental exposures in Parkinson’s disease. J. Neurol. Sci. 262, 37–44. [DOI] [PubMed] [Google Scholar]
- Furda A. M., Bess A. S., Meyer J. N., Van Houten B. (2012a). Analysis of DNA damage and repair in nuclear and mitochondrial DNA of animal cells using quantitative PCR. Methods Mol. Biol. 920, 111–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furda A. M., Marrangoni A. M., Lokshin A., Van Houten B. (2012b). Oxidants and not alkylating agents induce rapid mtDNA loss and mitochondrial dysfunction. DNA Repair 11, 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenamyre J. T., Sherer T. B., Betarbet R., Panov A. V. (2001). Complex I and Parkinson’s disease. IUBMB Life 52, 135–141. [DOI] [PubMed] [Google Scholar]
- Haugen A. C., Di Prospero N. A., Parker J. S., Fannin R. D., Chou J., Meyer J. N., Halweg C., Collins J. B., Durr A., Fischbeck K., et al. (2010). Altered gene expression and DNA damage in peripheral blood cells from Friedreich’s ataxia patients: Cellular model of pathology. PLoS Genet. 6, e1000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H., Liu Z., Li X., Feng Z., Hao J., Shen W., Zhang H., Liu J. (2010). Synergistic anti-Parkinsonism activity of high doses of B vitamins in a chronic cellular model. Neurobiol. Aging 31, 636–646. [DOI] [PubMed] [Google Scholar]
- Kapka-Skrzypczak L., Cyranka M., Skrzypczak M., Kruszewski M. (2011). Biomonitoring and biomarkers of organophosphate pesticides exposure - state of the art. Ann. Agricultural Environ. Med. 18, 294–303. [PubMed] [Google Scholar]
- Liang C., Ahmad K., Sue C. M. (2014). The broadening spectrum of mitochondrial disease: Shifts in the diagnostic paradigm. Biochim. Biophys. Acta. 1840, 1360–1367 [DOI] [PubMed] [Google Scholar]
- Maynard S., de Souza-Pinto N. C., Scheibye-Knudsen M., Bohr V. A. (2010). Mitochondrial base excision repair assays. Methods 51, 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. N., Leung M. C., Rooney J. P., Sendoel A., Hengartner M. O., Kisby G. E., Bess A. S. (2013). Mitochondria as a target of environmental toxicants. Toxicol. Sci. 134, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A. N. (2009). In a flurry of PINK, mitochondrial bioenergetics takes a leading role in Parkinson’s disease. EMBO Mol. Med. 1, 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsucci D., Caldarazzo Ienco E., Mancuso M., Siciliano G. (2011). POLG1-related and other “mitochondrial Parkinsonisms”: An overview. J. Mol. Neurosci. 44, 17–24. [DOI] [PubMed] [Google Scholar]
- Pogribny I. P., Rusyn I. (2013). Environmental toxicants, epigenetics, and cancer. Adv. Exp. Med. Biol. 754, 215–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenburg R. J. (2011). Biochemical diagnosis of mitochondrial disorders. J. Inherited Metabol. Disease 34, 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Cespedes M., Parrella P., Nomoto S., Cohen D., Xiao Y., Esteller M., Jeronimo C., Jordan R. C., Nicol T., Koch W. M., et al. (2001). Identification of a mononucleotide repeat as a major target for mitochondrial DNA alterations in human tumors. Cancer Res. 61, 7015–7019. [PubMed] [Google Scholar]
- Sanders L. H., Greenamyre J. T. (2013). Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radic. Biol. Med. 62, 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L. H., Laganiere J., Cooper O., Mak S. K., Vu B. J., Huang Y. A., Paschon D. E., Vangipuram M., Sundararajan R., Urnov F. D., et al. (2014a). LRRK2 mutations cause mitochondrial DNA damage in iPSC-derived neural cells from Parkinson’s disease patients: Reversal by gene correction. Neurobiol. Dis. 62, 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L. H., McCoy J., Hu X., Mastroberardino P. G., Dickinson B. C., Chang C. J., Chu C. T., Van Houten B., Greenamyre J. T. (2014b). Mitochondrial DNA damage: Molecular marker of vulnerable nigral neurons in Parkinson’s disease. Neurobiol. Disease 70, 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J. H., Meyer J. N., Mandavilli B. S., Van Houten B. (2006). Quantitative PCR-based measurement of nuclear and mitochondrial DNA damage and repair in mammalian cells. Methods Mol. Biol. 314, 183–199. [DOI] [PubMed] [Google Scholar]
- Seo B. B., Marella M., Yagi T., Matsuno-Yagi A. (2006). The single subunit NADH dehydrogenase reduces generation of reactive oxygen species from complex I. FEBS Lett. 580, 6105–6108. [DOI] [PubMed] [Google Scholar]
- Sherer T. B., Betarbet R., Stout A. K., Lund S., Baptista M., Panov A. V., Cookson M. R., Greenamyre J. T. (2002). An in vitro model of Parkinson's disease: Linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J. Neurosci. 22, 7006–7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarnkar S., Goswami P., Kamat P. K., Gupta S., Patro I. K., Singh S., Nath C. (2012). Rotenone-induced apoptosis and role of calcium: A study on Neuro-2a cells. Arch. Toxicol. [DOI] [PubMed] [Google Scholar]
- Tanner C. M., Kamel F., Ross G. W., Hoppin J. A., Goldman S. M., Korell M., Marras C., Bhudhikanok G. S., Kasten M., Chade A. R., et al. (2011). Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 119, 866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laar V. S., Berman S. B. (2013). The interplay of neuronal mitochondrial dynamics and bioenergetics: Implications for Parkinson's disease. Neurobiol. Disease 51, 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance J. M., Ali S., Bradley W. G., Singer C., Di Monte D. A. (2010). Gene-environment interactions in Parkinson’s disease and other forms of parkinsonism. Neurotoxicol. 31, 598–602. [DOI] [PubMed] [Google Scholar]
- Zheng B., Liao Z., Locascio J. J., Lesniak K. A., Roderick S. S., Watt M. L., Eklund A. C., Zhang-James Y., Kim P. D., Hauser M. A., et al. (2010). PGC-1alpha, a potential therapeutic target for early intervention in Parkinson's disease. Sci. Trans. Med. 2, 52ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]