Abstract
AIM: To investigate the change in expression of p53, Bcl-2, and Bax genes in human colon cancer cells transplanted into nude mice after hyperthermia, chemotherapy, radiotherapy, thermochemotherapy, thermoradiotherapy and thermochemoradiotherapy.
METHODS: Human colon cancer cell line (HT29) was transplanted into the hind limbs of nude mice. Under laboratory simulated conditions of hyperthermia (43°C, 60 min), the actual radiation doses and doses of mitomycin C (MMC) were calculated in reference to the clinical radiotherapy for human rectal cancer and chemotherapy prescription for colon cancer. The mice were divided into 6 groups according to the treatment approaches: hyperthermia, chemotherapy, radiotherapy, thermochemotherapy, thermoradiotherapy, and thermochemoradiotherapy. The mice were sacrificed at different time points and the tumor tissue was taken for further procedures. The morphologic changes in membrane, cytoplasm and nuclei of tumor cells of p53, Bcl-2, and Bax after treatment, were observed by immunohistochemistry staining.
RESULTS: All of the six treatment modalities down-regulated the expression of p53, Bcl-2 and up-regulated the expression of Bax at different levels. The combined therapy of hyperthermia, with chemotherapy, and/or irradiation showed a greater effect on down-regulating the expression of p53 (0.208 ± 0.009 vs 0.155 ± 0.0115, P < 0.01) and Bcl-2 (0.086 ± 0.010 vs 0.026 ± 0.0170, P < 0.01) and up-regulating Bax expression (0.091 ± 0.0013 vs 0.207 ± 0.027, P < 0.01) compared with any single therapy.
CONCLUSION: Hyperthermia enhances the effect of radio- and chemotherapy on tumors by changing the expression of apoptosis genes, such as p53, Bcl-2 and Bax.
Keywords: Hyperthermia, Apoptosis, p53, Bcl-2, Bax, Nude mice, Colon cancer, Cell line
INTRODUCTION
Tumor regression after a high fever due to erysipels was first reported in 1866 by the German physician Busch. In 1910 Müller described the potential of hyperthermia as an adjuvant to radiotherapy[1]. In the past two decades there has been a great interest in application of hyperthermia in conjunction with irradiation or/and chemotherapy in cancer treatment. This is associated with a better understanding of several biological parameters such as radiosensitization, chemosensitization, direct cytoxicity, thermotolerance, and stepdown heating, as well as complete changes in micromilieu, especially involving the microvasculature[2-6]. Hyperthermia has become the fifth method of therapy after surgery, chemotherapy, radiation and biological therapy, and plays an important role in multidiscipline therapy for cancer. Hyperthermia is considered a very hopeful anticancer method in the near future. For the first time we observed that hyperthermia at 43°C for 60 min upregulated E-cadherin and γ-catenin expressions but downregulated β-catenin expression on colon carcinoma cells in vitro. α-catenin expression is not affected by hyperthermia[3]. However, the pathways between apoptosis and hyperthermia (which is one of the inducers) remain unclear. The relative amount of Bcl-2 and Bax proteins determines cell survival or death following an apoptotic stimulus. To clarify the molecular mechanism of hyperthermic cell destruction, radiosensitivity and interaction of heat with chemotherapeutic agents, we designed an experiment to identify the change in expression of p53, Bax and Bcl-2 proteins after hyperthermia, chemotherapy, radiotherapy and combined therapies in human colon cancer transplanted into nude mice.
MATERIALS AND METHODS
Reagents
Human colon adenocarcinoma cell line HT29 was kindly provided by Center Laboratory of Tianjin Medical University Cancer Hospital. One hundred and forty-four 4-5 wk-old BLAB/C nu/nu mice of both sexes, weighing 18-20 g, were purchased from the Animal Center of Chinese Academy of Medical Sciences. The mice were fed with standard maintenance diet and water throughout the experiment period. All animals received care treatment in compliance with the guidelines of China Ministry of Public Health.
Reagents and instruments
Mitromycin-C (MMC) was produced by Kyowa Hacko Kogyo Co. Ltd. Human anti-p53 gene polycolonal antibodies were purchased from Beijing Zhongshan Biotechnology, Inc. Polycolonal human antibody against Bcl-2 and Bax gene, ultrasensitiveTMS-P box for immunohistochemical staining and DAB box were the products of Fujian Maixing Biotechnology Development Inc. Electroheat hemothermia water bath trunk (Shanghai No 7 Medical Equipment Co. Lit., HHW21. Cu 600), 30 self-made wooden shelves for fixing nude mice, WMY-01 digital thermometer were the products of Shanghai Medical Instrument Co. Lit. SL-7510 accelerator, medical adhesive tape, Leitz-1212 paraffin section machine, Olympus CH-2 optical microscope and Bei Hang CMIAS image analysis system were provided by Pathological Department of Tianjin Cancer Hospital.
Xenografts of HT29 cells in nude mice
HT29 cells were incubated in vitro to logarithmic growth period. The cells were separated and counted. A total of 1 × 107/mL HT29 cells were subcutaneously xenografted in bilateral groins of nude mice. After 22 d, the mice were sacrificed when tumors reached about 9 mm × 10 mm. The mass of tumors was cut into 1 mm × 1 mm × 1 mm pieces, which were then subcutaneously xenografted in bilateral hind limbs of all 144 nude mice. When the tumors grew about 0.8-1.0 cm in diameter, laboratory study was undertaken.
Treatment of nude mice with xenografts of HT29
The mice were randomly divided into 6 groups (24 mice in each group) and treated as follows.
The mice in group A (hyperthermia) were fixed on a special frame, their bilateral hind limbs were immersed in 43°C water for 60 min. The mice in group B (chemotherapy) were given MMC through local injection into the tumor at a dose of 1.4 mg/kg. Briefly, 5 mL NS was injected into an ampoule containing 2 mg MMC. MMC was dissolved and the dose for each mouse was calculated according to the formula: X = 5 mgY (1.4/1000) [X: mL of MMC solution for injection; Y: weigh of mice (g)]. The mice in group C (themochemotherapy) administrated MMC, hyperthermia was performed after chemotherapy. SL-7510 accelerator with 8 Mev electron beam was used in mice of group D (radiation) with a 1.0 cm-thick plexiglass placed on the surface. Radiation was performed once at 10GY. The mice in group E (themoradotherapy) underwent radiation and then hyperthermia. The mice in group F (themochemoradiotherapy) received radiation followed by chemotherapy and hyperthermia.
Three mice from each group were sacrificed at 8 different time points (before treatment, and 2, 4, 8, 12, 24, 48 and 72 h after treatment). The tumor of the hind limbs was resected, fixed with 10% formalin solution, embedded in paraffin wax blocks and cut into 4-μm thick sections for immunohistochemical analysis.
Immunohistochemical staining for expression of p53, Bcl-2 and Bax genes in tumor tissue
Expression of p53, Bcl-2 and Bax genes in harvested tumor was determined immunohistochemically using the S-P method. Unless otherwise stated, all steps were carried at room temperature. Briefly, after dewaxed and rehydrated, slides were washed for 5 min, put into distilled water for another 1 min and then dried in a microwaven (95°C-98°C) in citric acid buffer (pH 6.0) for 15-20 min to retrieve antigens. The sections were washed three times with phosphate-buffered saline (PBS) for 5 min. Normal goat serum was added to the slides for 20 min. Rabbit anti-human polyclonal antibody against Bcl-2, p53 and Bax was applied to sections and incubated overnight at 4°C. After incubated in PBS for 5 min, second antibody (biotin-conjugated) was added to the sections at 37°C for 30 min. The enzyme conjugated HRP-streptavidin (third antibody) at 37°C for 30 min. Between each step, the sections were washed three times with PBS for 5 min. Sites of immunoreaction were visualized with 3, 3’-diaminobenzidine (DAB) for 2-20 min under microscope. Then the sections were washed with tap water for 5 min, soaked in distilled water for 2 min, counterstained with Mayer’s haematoxylin, dehydrated in alcohol, and mounted on Canada balsam.
Evaluation criteria for immuohistochemical staining
Immunostaining specific for Bcl-2 and Bax gene was membranous and cytoplasmic, p53 gene was nucleolar. These genes were stained yellow or brown under optical microscope. Bei-Hang CMIAS pathological image analysis system was adopted. The brightest area of image was calibrated both for size and optical density; 5 high power fields of each section were selected for image collection. The target area of section was fixed and color division was carried out. The results of division were analyzed statistically and an average optical density was obtained.
Statistical analysis
Statistical analysis was performed by SPSS11.5 for Windows (SPSS, Chicago IL). Comparison of average optical density for Bcl-2, Bax and p53 genes in different groups was made by mono-factor variance analysis. SNQ test was used in comparison of the two groups.
RESULTS
Change in p53 gene expression
Down regulation of p53 expression was found in all the six groups for treatment. The reduction of p53 expression in group F was the highest and occurred at first. In contrast, the reduction of p53 expression in group D was the lowest and occurred at last. There was no significant difference in the reduction of p53 expression in groups A, C and E. The extent of reduction in the above mentioned groups was significant in comparison to that in groups B and D. The extent of reduction of p53 expression was greater in group B than in group D.
Dynamic analysis showing reduction of p53 expression was begun immediately in group F after the treatment. The lowest level of p53 expression was found 48 h after the treatment. The optical density was reduced 50% in comparison to that before treatment, and then elevated again 48 h after treatment. p53 expression was down regulated gradually in groups A, C and E 2 h after treatment, and elevated again in group C 48 h after treatment. p53 expression was down regulated gradually in groups B and D, 4 and 12 h following treatment (Figures 1 and 2).
Figure 1.
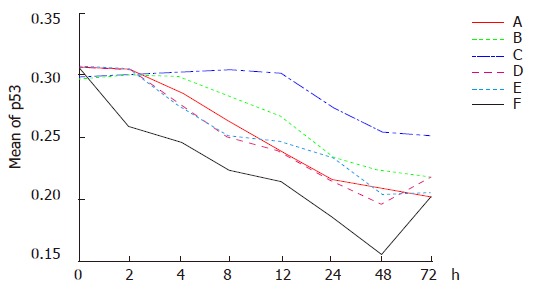
Expressions of p53 in different groups at different time points after hyperthermia (A), chemotherapy (B), themochemotherapy (C), radiation (D), themoradiotherapy (E), and themochemoradiotherapy (F).
Figure 2.
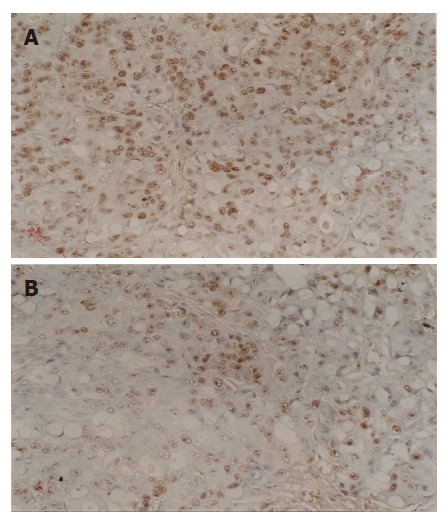
Expressions of p53 before (A) and after thermochemoradiotherapy (B) (SP, × 200).
The average optical densities of p53 in different groups 48 h after treatment were analyzed by SNQ test (Table 1). There was no statistical difference between groups A and B, groups A and C, groups A and E (P = 0.425, P = 0.129, P = 0.279), though statistical differences were found between groups D and E (P = 0.014, P = 0.000). There was no statistical difference between groups B and A, groups B and E (P = 0.129, P = 0.279), but between groups B and D, groups B and F (P = 0.014, P = 0.000). There were statistical differences between groups D and C, groups D and E, groups D and F (P = 0.001, P = 0.000, P = 0.000) while no statistical difference occurred between groups C and E (P = 0.97). On the other hand, statistical difference was identified between groups C and F (P = 0.001). These results indicated that p53 expression was significantly down regulated in group F in comparison to other groups and the combined therapy had a greater ability to down regulate p53 expression than any single therapy.
Table 1.
Average optical density of p53, Bcl-2 and Bax in different groups 48 h after treatment (mean ± SD)
| Group | n | p53 | Bcl-2 | Bax |
| A | 6 | 0.208 ± 0.009 | 0.086 ± 0.010 | 0.091 ± 0.0013 |
| B | 6 | 0.223 ± 0.0133 | 0.056 ± 0.0064 | 0.135 ± 0.0095 |
| C | 6 | 0.255 ± 0.0111 | 0.064 ± 0.0048 | 0.128 ± 0.0144 |
| D | 6 | 0.197 ± 0.0157 | 0.058 ± 0.0095 | 0.151 ± 0.011 |
| E | 6 | 0.205 ± 0.100 | 0.054 ± 0.0090 | 0.150 ± 0.028 |
| F | 6 | 0.155 ± 0.0115 | 0.026 ± 0.0170 | 0.207 ± 0.027 |
| F | 40.810b | 21.466b | 23.912b |
Group A: hyperthermia; group B: chemotherapy; group C: themo-chermotherapy; group D: radiation; group E: themoradiotherapy; group F: thempchempradiotherapy.
P < 0.01 in groups A-F. Comparison of average optical density of Bcl-2, Bax and p53 genes in different groups was made by mono-factor variance analysis. SNQ test was used to compare the two groups.
Change in Bcl-2 gene expression
Down regulation of Bcl-2 expression was demonstrated in six groups. The lowest level of Bcl-2 expression was first observed in group F. Hyperthermia by itself (in group A) could not greatly down regulate Bcl-2 expression. Down regulation of Bcl-2 could be enhanced when hyperthermia was combined with chemotherapy (in group C) or radiation (in group E), but obvious effect of down regulation was found in group E compared to group C.
Dynamic analysis indicated that down regulation of Bcl-2 expression was observed in group F 2 h after treatment and the greatest reduction occurred 48 h after treatment and then elevated. In group A, Bcl-2 expression was down regulated temporarily 4 h after hyperthermia, then began to increase at 8 h and returned to the original level at 12 h. Down regulation of Bcl-2 gene was observed again at 24 h following hyperthermia. There was no significant difference at various time points. Down regulation of Bcl-2 gene was identified 8 h after chemotherapy (in group B) and Bcl-2 expression was down regulated in group D after an additional 4 h. Bcl-2 expression increased at 48 h after chemotherapy and kept stable at 48 h following radiation (in group D). Down regulation of Bcl-2 gene was demonstrated 4 h after themoradiation (in group E) and Bcl-2 decreased in group C after an additional 4 h. Bcl-2 expression increased at 48 h following treatment in groups C and E (Figures 3 and 4).
Figure 3.
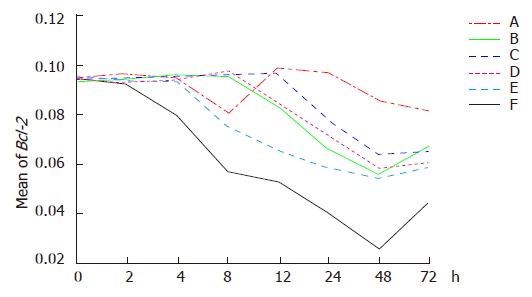
Expression of Bcl-2 in different groups at the different time points after hyperthermia (A), chemotherapy (B), themochemotherapy (C), radiation (D), themoradiotherapy (E), and themochemoradiotherapy (F).
Figure 4.
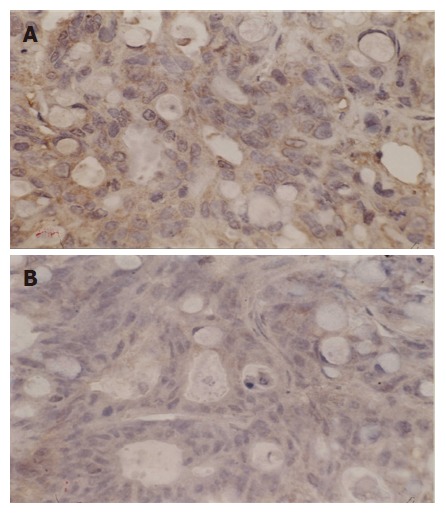
Expression of Bcl-2 before (A) and after thermochemoradiotherapy (B) (SP, × 400).
The average optical densities of Bcl-2 in different groups 48 h after treatment were analyzed by SNQ test (Table 1). Statistical differences were found between groups A and B, groups A and D, groups A and C, groups A and E, group A and F (P = 0.03, P = 0.021, P = 0.008, P = 0.003, P = 0.001). However, there were no statistical differences between groups B and D, groups B and C, groups B and E, groups B and F (P = 0.339, P = 1.000, P = 1.000, P = 0.054). No statistical differences existed between groups D and C, groups D and E (P = 0.905, P = 0.356). On the other hand, statistical differences were shown between groups D and F (P = 0.040). There were no statistical differences between groups C and E (P = 0.999) though statistical differences were indicated between group C and F (P = 0.040). Finally, there were no statistical difference between groups E and F (P = 0.078). The results indicated that Bcl-2 expression was significantly down regulated in groups E and F in comparison to other groups and the combined therapy demonstrated its greater ability to down regulate Bcl-2 expression than any single therapy.
Change in Bax gene expression
Up regulation of Bax expression was observed in six groups. The highest Bax expression was first found in group F. Once again hyperthermia by itself could not obviously up regulate Bax expression which occurred at last. Chemotherapy increased Bax expression (in group B) followed by radiation (in group D). Bax expression increased more significantly in group E than in group C.
Dynamic analysis indicated that Bax expression was up-regulated gradually in group F 2 h after treatment. The highest level was observed 48 h after treatment and then decreased. Bax expression was up regulated 24 h after hyperthermia treatment (in group A), 4 h after themoradiation (in group E), 8 h after themochemotherapy (in group C), 8 h after chemotherapy (in group B), and 12 h after radiation (in group D), respectively (Figures 5 and 6).
Figure 5.
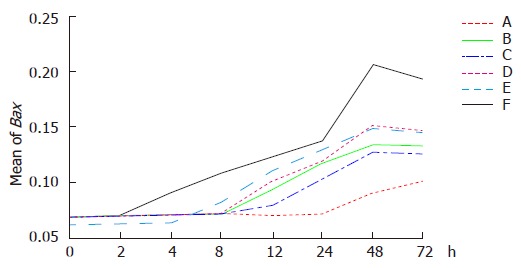
Expression of Bax in different groups at the different time points after hyperthermia (A), chemotherapy (B), themochemotherapy (C), radiation (D), themoradiotherapy (E), and themochemoradiotherapy (F).
Figure 6.
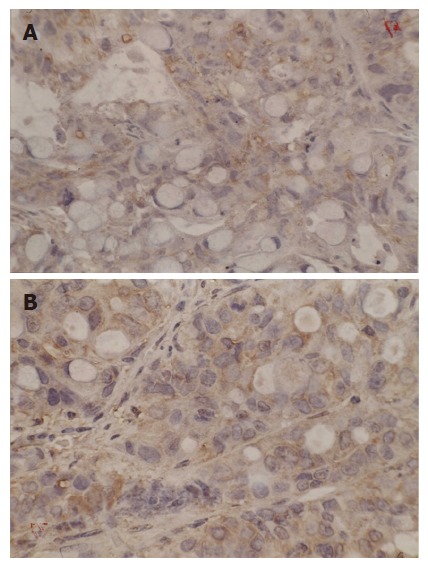
Expression of Bax before (A) and after (B) thermochemoradiotherapy (SP, × 400).
The average optical densities of Bax in different groups 48 h after treatment were analyzed by SNQ test (Table 1). Statistical differences were shown between groups A and B, groups A and D, groups A and C, groups A and E, groups A and F (P = 0.01, P = 0.012, P = 0.000, P = 0.029, P = 0.000). However, there were no statistical differences between groups B and D, groups B and C, groups B and E (P = 0.988, P = 0.242, P = 0.952). On the other hand, statistical differences existed between groups B and F (P = 0.008). No statistical differences were observed between groups D and C, groups D and E (P = 0.134, P = 0.767), though statistical difference occurred between groups D and F (P = 0.004). There were no statistical differences between groups C and E (P = 1.000), but there was between groups C and F (P = 0.028). Finally, there was no statistical difference between groups E and F (P = 0.059). The results indicated that Bax expression was significantly up regulated in groups E and F compared to other groups and the combined therapy had a greater ability to up regulate Bax expression than any single therapy.
DISCUSSION
p53 seems to play an important role in mediating DNA damage induced by various drugs because mutations in p53 are associated with decreased susceptibility to chemotherapeutic agents[8], and there is evidence that p53 expression is also related to multidrug resistance (MDR)[9]. It was reported that human non-small-cell lung cancer cell lines with mutant p53 have become more sensitive to cisplatin following transduction of wild-type p53[10]. Finally, p53 molecule has been fused to chemotherapeutic prodrugs to enhance its chemosensitizing properties. It was originally shown that thymocytes readily undergo apoptosis following exposure to γ-irradiation, but loss of wild-type p53 dramatically reduces the number of apoptosis cells[11]. Therefore, efforts to combine p53 gene therapy with radiotherapy have focused on enhancing the radiosensitivity of cancer cells lacking functional p53 to render them[12]. In our experiment, mutant p53 was detected by immunohistochemical staining. We found that themochemotherapy and themoradiation could significantly down regulate mutant p53 expression. Down regulation of mutant p53 enhances radio- and chemosensitivity indirectly with the similar therapeutic effect produced by p53 gene therapy combined with chemotherapy and radiation. Furthermore, hyperthermia has a greater ability to down regulate mutant p53 expression than radiotherapy or chemotherapy. Combined hyperthermia with chemotherapy and radiation can achieve the best result. A recent report also indicates that heat-induced growth inhibition of transplanted neck squamous cell carcinoma (HNSCC) may be correlated with the induction of p53-dependent Bax-mediated apoptosis. Thus, p53 status appears to be one of the useful parameters for predictive assays in hyperthermic cancer therapy[13].
Bcl-2 family is a huge family comprising various members, which are key regulators of apoptosis, tissue homeostasis, and protection against pathogens[14]. High levels and aberrant patterns of Bcl-2 expression have been reported in colorectal, lung, gastric, renal and other cancers[15]. Elevation of Bcl-2 protein expression contributes not only to the development of cancer, but also to resistance against a wide variety of anti-cancer agents[16]. The protection afforded by Bcl-2 against chemotherapeutic agents defines a novel type of drug resistance in which Bcl-2 over-expression does not prevent drug entry and accumulation in tumor cells or the interaction of drugs with their primary molecular targets. Bcl-2 appears to block the transmission of signals originating from cellular damage to molecular effectors of apoptosiss[15]. This allows cells to survive in the presence of other lethal damage, repairing drug-induced damage after withdrawal of the drugs. Via this mechanism, Bcl-2 essentially converts anti-cancer drugs from cytotoxic to cytostatic, thus increasing the chances of acquiring genetic alterations and favoring the development of a more malignant phenotype. Bcl-2 protein expression is negatively correlated with the radiosensitivity of nasopharyhgeal carcinoma[17]. Our study suggested that hyperthermia combined with chemotherapy could enhance the ability of Bcl-2 to down regulate its expression, although hyperthermia itself could not change the expression of Bcl-2 gene significantly. This may be one of the mechanisms of hyperthermia underlying the enhancement of radiosensitization and cytotoxicity of many chemotherapeutic agents.
In contradiction to the Bcl-2 gene, Bax enhances cell death. It was reported that when expression or mutation of Bax gene is low in tumor tissue, cells resist apoptosis and have no response to radiation and chemotherap[18]. On the other hand, over-expression of Bax in human cancer cell lines has been reported to induce apoptosis independently of endogeneous p53 status and to enhance the chemosensitivity or radiosensitivity of selected tumor lines[18]. Research in cell line of childhood acute lymphoblastic leukemia indicates that down regulation of Bcl-2 expression and up regulation of Bax expression by radiation tend to be correlated with increased radiosensitivity[19].
In the present study, expression of Bcl-2 gene was relatively high and expression of Bax gene was low, the ratio of Bcl-2 and Bax was high. However, the expression of Bax was up-regulated significantly after treatment. Bcl-2 gene expression was down regulated in groups B-F. Down regulation of Bcl-2 expression in group F was more obvious than that in groups E and C as well as in groups D and B. Although hyperthermia itself did not change Bcl-2 gene expression, hyperthermia in combination with radiation and chemotherapy increased down regulation of Bcl-2 gene expression significantly, suggesting that hyperthermia can enhance radio- and chemosensitivity.
Anti-cancer therapy itself can influence the expression of apoptosis-associated genes. Radiation and chemotherapy can change the level of apoptosis[5]. A recent report indicates that apoptosis induced by abating HSP70 expression may enhance the sensitivity of human bladder cancer cells to MMC[20]. In vitro study with malignant histiofibrocytoma cell line has illustrated that the expression of Bax is up-regulated in protein 30 min after 43°C hyperthermia, accompanying the change in mRNA[6]. In the present study, the extent of up regulation of Bax expression in themoradiation group 24 h after treatment was greater than that in the hyperthermia and radiation treatment groups. This phenomenon was not found in the thermochemotherapy group.
In conclusion, hyperthermia in combination with radio-and chemotherapy can achieve a better effect and enhance changes in apoptosis-related genes though its definite mechanism is still unknown. Further research is required to determine the precise effect of hyperthermia in combination with other therapies.
ACKNOWLEDGMENTS
The authors thank Professor Riu-Fang Niu, Professor Yun Niu and Ms Yu-Rong Shi at Center Laboratory of Tianjin Cancer Hospital, Tianjin Medical University for their technical supports. The authors also thank Dr. Wienert, Andreas for his kindly review of the English manuscript.
COMMENTS
Background
In the past two decades there has been a great interest in application of hyperthermia in conjunction with radiotherapy or/and chemotherapy in cancer treatment. This is associated with a better understanding of several biological parameters such as radiosensitization, chemoxicity, thermotolerance, as well as complete changes in micromilieu, especially involving the microvasculature. However, the pathways between apoptosis and hyperthermia remain unclear.
Research frontiers
We observed that hyperthermia at 43°C for 60 min up regulated E-cadherin and γ-catenin expression but down regulated β-catenin expression. Anti-cancer therapy itself can influence the expression of apoptosis-associated genes. Radiation and chemotherapy can change the expression of apoptosis.
Innovations and breakthroughs
The results of this study show that hyperthermia combined with chemotherapy or/and radiation can change the expression of apoptosis genes such as p53, Bcl-2 and Bax significantly when combined with chemotherapy or/and radiation compared to hyperthermia itself. To our knowledge, no similar studies are available at present.
Applications
According to the results of this study, the molecular mechanism of hyperthermic radiosensitivity and chemosensitivity can be understood. In clinical practice, hyperthermia in combination with radiation and chemotherapy can achieve a better effect.
Terminology
(1) Hyperthermia: A therapeutic method by increasing tissue temperature (range 40-43°C), potentially induces tumor cell death by a spectrum of molecular, metabolic, cellular, and tumor tissue changes. (2) p53 gene: It is a tumor suppressor gene, which is mutated or deleted in over half of all human malignancies. Therefore, one strategy in cancer therapy focuses on the replacement or over-expression of tumor suppressor genes. (3) Bcl-2: It is a huge family composed of various members, which are key regulators of apoptosis. High levels and aberrant patterns of Bcl-2 expression have been reported in a wide variety of human caners. (4) Bax: It is a pro-apoptosis member of the Bcl-2 family, thought to induce apoptosis. Loss of Bax in genetically engineered mice results in increased tumor incidence, suggesting that Bax may play a role in suppressing tumor growth in vivo.
Peer review
The study investigated the expression of three apoptosis-related genes (Bcl-2, Bax, p53) in human colon cancer transplanted into nude mice after hyperthermia, chemotherapy and radiotherapy. The authors tried to set up an in vivo tumor model, in which the effect of various treatment modalities and their combination could be investigated. The authors used a histology based model to study the protein expression related to the above mentioned three genes.
Footnotes
Supported by the Clinical Research Foundation of Tianjin Medical University, No. 2002KY18
S- Editor Zhu LH L- Editor Wang XL E- Editor Liu Y
References
- 1.Lin SY, Li RY, Mao HS, Zhang SW. New developmental therapies, Hyperthermia. In: Oncology (in Chinese), editor. Zhang TZ, Xu GW, editors. Tianjin and Shenyang: Tianjin Science & Technology Press and Liao Ning Science & Technology Press; 2005. pp. 849–880. [Google Scholar]
- 2.Song CW, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia. 2005;21:761–767. doi: 10.1080/02656730500204487. [DOI] [PubMed] [Google Scholar]
- 3.Xia T, Sun Q, Shi X, Fan N, Hiraoka M. Relationship between thermal parameters and tumor response in hyperthermia combined with radiation therapy. Int J Clin Oncol. 2001;6:138–142. doi: 10.1007/pl00012096. [DOI] [PubMed] [Google Scholar]
- 4.Engin K, Leeper DB, Tupchong L, Waterman FM. Thermoradiotherapy in the management of superficial malignant tumors. Clin Cancer Res. 1995;1:139–145. [PubMed] [Google Scholar]
- 5.Liang H, Hao XS. The biological mechanisms of hyperthermia. Guowai Yixue (Oncological Section) 2001;28:438–441. [Google Scholar]
- 6.Zhan HJ, Liang H. The status of research for hyperthermia. Guowai Yixue (Oncological Section) 2005;32:35–38. [Google Scholar]
- 7.Liang H, Li JW, Shi YR, Niu RF, Wang P, Hao XS. Change in E-cadherin, alpha-, beta- and gamma-catenin expression after hyperthermia of a human colon carcinoma cell line in vitro. Zhonghua YiXue ZaZhi. 2004;84:1299–1303. [PubMed] [Google Scholar]
- 8.Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 9.Matsuhashi N, Saio M, Matsuo A, Sugiyama Y, Saji S. The evaluation of gastric cancer sensitivity to 5-FU/CDDP in terms of induction of apoptosis: time- and p53 expression-dependency of anti-cancer drugs. Oncol Rep. 2005;14:609–615. [PubMed] [Google Scholar]
- 10.Fujiwara T, Cai DW, Georges RN, Mukhopadhyay T, Grimm EA, Roth JA. Therapeutic effect of a retroviral wild-type p53 expression vector in an orthotopic lung cancer model. J Natl Cancer Inst. 1994;86:1458–1462. doi: 10.1093/jnci/86.19.1458. [DOI] [PubMed] [Google Scholar]
- 11.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 12.Badie B, Goh CS, Klaver J, Herweijer H, Boothman DA. Combined radiation and p53 gene therapy of malignant glioma cells. Cancer Gene Ther. 1999;6:155–162. doi: 10.1038/sj.cgt.7700009. [DOI] [PubMed] [Google Scholar]
- 13.Tamamoto T, Yoshimura H, Takahashi A, Asakawa I, Ota I, Nakagawa H, Ohnishi K, Ohishi H, Ohnishi T. Heat-induced growth inhibition and apoptosis in transplanted human head and neck squamous cell carcinomas with different status of p53. Int J Hyperthermia. 2003;19:590–597. doi: 10.1080/0265673031000150858. [DOI] [PubMed] [Google Scholar]
- 14.Lanave C, Santamaria M, Saccone C. Comparative genomics: the evolutionary history of the Bcl-2 family. Gene. 2004;333:71–79. doi: 10.1016/j.gene.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Reed JC. Regulation of apoptosis by bcl-2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol. 1995;7:541–546. doi: 10.1097/00001622-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Fisher TC, Milner AE, Gregory CD, Jackman AL, Aherne GW, Hartley JA, Dive C, Hickman JA. bcl-2 modulation of apoptosis induced by anticancer drugs: resistance to thymidylate stress is independent of classical resistance pathways. Cancer Res. 1993;53:3321–3326. [PubMed] [Google Scholar]
- 17.Liu MC, Gelmann EP. P53 gene mutations: case study of a clinical marker for solid tumors. Semin Oncol. 2002;29:246–257. doi: 10.1053/sonc.2002.32900. [DOI] [PubMed] [Google Scholar]
- 18.Komatsu K, Suzuki S, Shimosegawa T, Miyazaki JI, Toyota T. Cre-loxP-mediated bax gene activation reduces growth rate and increases sensitivity to chemotherapeutic agents in human gastric cancer cells. Cancer Gene Ther. 2000;7:885–892. doi: 10.1038/sj.cgt.7700181. [DOI] [PubMed] [Google Scholar]
- 19.Findley HW, Gu L, Yeager AM, Zhou M. Expression and regulation of Bcl-2, Bcl-xl, and Bax correlate with p53 status and sensitivity to apoptosis in childhood acute lymphoblastic leukemia. Blood. 1997;89:2986–2993. [PubMed] [Google Scholar]
- 20.He LF, Guan KP, Ye HY, Ren L, Yan Z, Wang SW, Hou SK. Heat shock protein 70 expression in relation to apoptosis in primary bladder transitional cell carcinoma. Chin Med J (Engl) 2005;118:2093–2096. [PubMed] [Google Scholar]


