Abstract
Ultrasound (US) is often the first imaging modality employed in patients with suspected focal liver lesions. The role of US in the characterisation of focal liver lesions has been transformed with the introduction of specific contrast media and the development of specialized imaging techniques. Ultrasound now can fully characterise the enhancement pattern of hepatic lesions, similar to that achieved with contrast enhanced multiphasic computed tomography (CT) and magnetic resonance imaging (MRI). US contrast agents are safe, well-tolerated and have very few contraindications. Furthermore, real-time evaluation of the vascularity of focal liver lesions has become possible with the use of the newer microbubble contrast agents. This article reviews the enhancement pattern of the most frequent liver lesions seen, using the second generation US contrast media. The common pitfalls for each type of lesion are discussed. The recent developments in US contrast media and specific imaging techniques have been a major advance and this technique, in view of the intrinsic advantages of US, will undoubtedly gain popularity in the years to come.
Keywords: Microbubble contrast agents, Ultrasound, Focal liver lesions
INTRODUCTION
The non-invasive detection and characterisation of focal liver lesions is an important component of cross-sectional imaging studies. Depending on their histological nature, different focal liver lesions vary in their blood supply, with the malignant ones generally having a preferential hepatic arterial supply[1]. The enhancement pattern of a lesion is based on the blood supply and constitutes the mainstay of its characterization with contrast-enhanced computed tomography or magnetic resonance imaging[2].
Ultrasound (US) is often the first imaging investigation for patients with liver disease. The sensitivity and specificity of gray-scale US for the characterization of focal lesions is inferior to that of CT or MRI[3,4]. One of the main reasons for this was the absence, until the 1990s, of US contrast media. The advent of microbubble contrast agents has led to improved characterisation and detection of focal liver lesions since the enhancement characteristics can be visualised in real time over a 5 min period. As a result, recent studies have reported sensitivities and specificities that rival that of CT and MRI[5].
US CONTRAST MEDIA AND SPECIFIC IMAGING TECHNIQUE
Ultrasound contrast agents consist of microbubbles of gas with a protein, lipid or polymer shell. The microbubbles are approximately 1 to 10 μm, which is the size of a red blood cell. These particles are too large to pass through the vascular endothelium and, as such, are considered pure blood pool agents[6]. After several minutes in the circulation, the microbubbles dissolve, the gas is exhaled and the shell is metabolized, mainly in the liver[7]. Furthermore, the microbubbles are well tolerated by patients after intravenous injection and there are very few contraindications to their use.
When subjected to an US wave, the microbubbles respond by changing their size: they expand during the rarefaction phase and contract during the pressure phase. These changes are much greater than the minor changes that occur in the soft tissues. The bubbles, like every oscillating system, have a natural frequency (the resonance frequency) at which their response is maximal. Fortunately, the bubbles resonate at frequencies used for diagnostic US imaging. This coincidence accounts for their high reflectivity, even when they are present in a small concentration. Furthermore, the expansion of these bubbles during the rarefaction phase exceeds their contraction during the pressure phase. This asymmetric oscillation produces a returning signal (echo) that contains harmonics, i.e. multiples of the driving frequency[8]. The first microbubble-specific imaging technique exploited this property by sending an US pulse into the tissue and selectively detecting echoes at twice that frequency, such that one could theoretically image only the bubbles. However, in practice, soft tissues also produce harmonic echoes. Consequently, the images produced with this technique were of poor quality, in part due to poor tissue suppression.
Subsequently, another mode of imaging with microbubbles was developed using high-power colour Doppler US. When submitted to a high-energy US beam, the microbubbles often break up into much smaller bubbles that dissolve rapidly. As they are disrupted, the bubbles emit a strong, brief echo which is easily detected, a phenomenon known as stimulated acoustic emission (SAE)[9]. The drawback of this technique, however, is that the microbubbles are destroyed by the US beam and therefore real-time imaging is not feasible.
At the present time, the most common imaging technique is based on the principle of phase-inversion, in which two US pulses, 180° out of phase, are sent sequentially. The returning echoes are added up by the US machine[10]. The linear echoes returned by the tissues nullify each other, while the non-linear echoes returned by the microbubbles produce a detectable signal. There are two main advantages of this technique. First, excellent tissue suppression is obtained. Second, a detectable signal is obtained even when a very low-power US beam is employed, consequently, the bubbles are not destroyed. The first generation contrast media, such as Levovist® (Schering, AG, Berlin, Germany), produced a very weak signal when submitted to low mechanical index US beam owing to its fragility and lent itself to use with SAE[11]. Since then, contrast media, such as SonoVue® (Bracco, Milan, Italy), Definity® (Bristol-Myers-Squibb, Billerica, Mass, USA) and Optison® (Nycomed/Amersham, Little Chalfont, UK), which have a strong, non-linear, harmonic response, even when insonated with low acoustic power, were able to provide real-time imaging using low mechanical index (MI) modes[12].
In the following sections we review the common enhancement patterns of the most frequent focal liver lesions seen with the second generation contrast agents.
CONTRAST-ENHANCED US PATTERNS OF FOCAL LIVER LESIONS
The characterization of a hepatic lesion with microbubbles requires careful examination through all phases of contrast enhancement, i.e. arterial (10-20 to 25-35 s after injection), portal (30-45 to 120 s) and late parenchymal (> 120 s) phases[13]. Simply put, the late phase is useful to determine the benign or malignant nature of a lesion while the arterial phase helps in predicting its histology[14-19]. Between 86% to 93% of benign lesions retain the contrast in the late phase, while 78% to 98% of the malignant ones demonstrate wash out of the contrast[14,16,18]. The persistence of the second generation contrast agent in a healthy liver is thought to be the result of a very slow flow through the sinusoid[20]. Consequently, lesions devoid of normal sinusoids do not retain the contrast.
HAEMANGIOMAS
Haemangiomas are the most common solid benign lesion of the liver, with a prevalence ranging from 1% to 20% in the general population[21]. These lesions are more common in females and are frequently located peripherally or adjacent to a large hepatic vein branch. The most common sonographic appearance of haemangiomas is a homogeneously hyperechoic focal lesion, less than 3 cm in size[22]. These characteristic features, when present in a patient at a low risk for malignancy, are usually sufficient to allow a confident diagnosis. In a significant number of patients however, further imaging is required.
The characteristic early arterial nodular enhancement with delayed centripetal fill-in described on CT or MRI[23-25] is the most common appearance of haemangiomas during the arterial phase of contrast-enhanced US, seen in 52% to 88% of cases (Figure 1)[15,16,18,19]. Sustained enhancement has been reported in 83% to 100% during the late phase[16-19]. The real time nature of contrast-enhanced US is particularly useful in diagnosing small, rapidly perfusing (flash-filling) haemangiomas, where the typical enhancement pattern can be appreciated[6]. Complete enhancement does not always occur, especially in lesions larger than 3 cm, which often undergo central thrombosis or fibrosis[6,26,27].
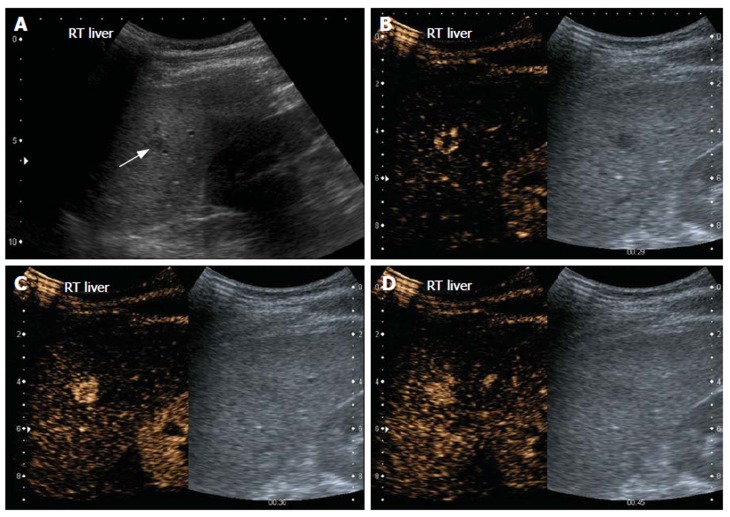
Figure 1.
Haemangioma. Gray-scale US image (A) and split-screen display images of contrast-enhanced US scan using a low MI technique (B-D). The left panel shows the contrast sensitive image while the corresponding gray-scale image is on the right. On gray-scale US, the liver is hyperechoic, consistent with fatty infiltration and an ill-defined hypoechoic lesion is seen (arrow, A). On contrast enhanced US, the lesion demonstrates peripheral nodular enhancement during the arterial phase (B). At 36 s, the lesion has almost completely filled in (C). At 45 s, the lesion is completely enhanced (D) and sustained enhancement was observed in the late phase scan (not shown).
FOCAL NODULAR HYPERPLASIA
Focal nodular hyperplasia (FNH) is the second most common solid benign hepatic lesion, with a prevalence between 0.9% and 3%[28-30]. This lesion occurs in all age groups and both sexes, but is found predominantly in women (80%-95% of the cases) during the 3rd to 5th decade of life. Oral contraceptive use has been incriminated, but a definite relationship has not been established[31,32]. FNH is not a dysplastic or neoplastic tumour, but is a hyperplastic lesion, probably occuring in response to a pre-existing arterial malformation[33]. FNH does not have a malignant potential, nor is it likely to bleed or rupture[34]. Consequently, differentiation from other lesions, particularly hepatocellular adenoma and carcinoma (especially the fibrolamellar form), is essential since FNH is managed conservatively, whereas the other lesions require surgery. FNH is often discovered incidentally.
The most common sonographic appearance of FNH is that of a homogeneous, near isoechoic lesion; some lesions are detected only because of their mass effect on adjacent blood vessels[32]. A central hypoechoic scar is detected in 20% to 45% of the cases[22,35,36]. In larger lesions, colour Doppler may show a central feeding artery with a spoke-wheel pattern of vessels radiating to the periphery.
FNH is a hypervascular tumour and consequently manifests as a strongly and homogeneously enhancing lesion during the arterial phase of contrast enhanced US in nearly 100% of the cases (Figure 2). The central spoke-wheel type of contrast enhancement can be demonstrated in 45% to 89% of FNH. These lesions become isoechoic or slightly hyperechoic, compared with the surrounding liver parenchyma during the portal and late phases of enhancement in 87% to 100% of the time[15-19,37]. The central scar is seen in 23% to 31% of cases[15,17]. However, in contradistinction to the pattern seen on CT or MRI, the central scar stands out as a defect instead of the late enhancement seen with the other imaging modalities. This finding can be explained by the fact that microbubbles are purely intravascular agents and therefore do not diffuse into the interstitium, unlike iodine and gadolinium-based contrast agents used with CT and MRI[6].
Figure 2.
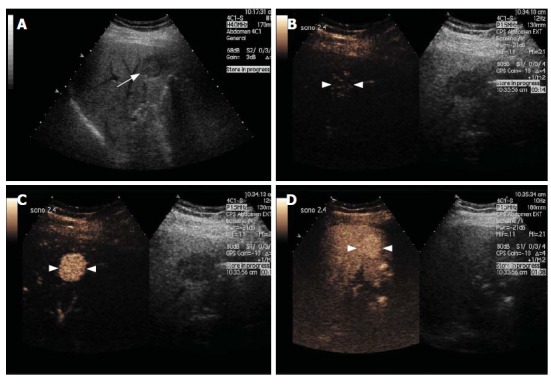
Focal Nodular Hyperplasia. Gray-scale US image (A) and split-screen display images of a contrast-enhanced US scan using a low MI technique (B-D). The gray-scale US image shows a focal hypoechoic lesion (arrow, A) in a diffusely hyperechoic liver in keeping with fatty infiltration. After contrast injection, the lesion enhances avidly in the arterial phase with filling seen from a central feeding vessel, demonstrating the classical spoke-wheel appearance (arrowheads, B and C). The lesion remains slightly hyperechoic during the portal and late phases (arrowheads, D).
In patients with chronic liver disease, caution should be used since well-differentiated hepatocellular carcinoma (HCC) may mimic the enhancement pattern of FNH (see below). In these patients, all hypervascular lesions should be regarded with suspicion.
HEPATOCELLULAR ADENOMA
Hepatocellular adenoma (HA) is a rare, benign neoplasm of hepatocellular origin. Approximately 90% of HAs occur in young women[38]. Up to 90% of females with HA have reported the use of oral contraceptives[39]. HA is also associated with the use of anabolic steroids in men and in some storage diseases[40]. In contrast to FNH, HA is a true neoplasm.
Management of HA is by surgical resection, in contrast to FNH, because of the risk of malignant degeneration and haemorrhage[41]. Patients with HA may present with pain secondary to its mass effect (40%) or intratumoral/intraperitoneal haemorrhage (40%). Alternatively, the tumour may be discovered incidentally (20%)[22].
The most common sonographic appearance of HA is a well-defined, large, solitary, hyperechoic mass owing to its high lipid content. Since the lesions have a propensity to bleed, HAs are usually heterogeneous in appearance. Colour Doppler findings are non-specific. The typical spoke-wheel pattern of FNH is absent.
The contrast-enhanced US characteristics of HA are relatively non-specific, but these lesions usually enhance during the arterial phase. Smaller lesions are likely to show homogeneous enhancement whereas the larger ones will be heterogeneous owing to previous intratumoral haemorrhage or necrosis. In one study with Sonovue, HAs were iso- or more often hypoechoic in comparison with the surrounding liver parenchyma in the portal venous and late phases of enhancement[37]. Unfortunately, this pattern of enhancement is not unique to HA, as it is also a common appearance of HCC on contrast-enhanced US (see below). In some cases, even the histopathological differentiation of HA from well-differentiated HCC is difficult[42]. The differential diagnosis should include hypervascular metastases which can exhibit similar enhancement characteristics.
HEPATOCELLULAR CARCINOMA
HCC is the most common primary malignancy of the liver. It usually occurs in patients with chronic liver disease, particularly in those with chronic hepatitis B and C infection where the risk is approximately 100 times that of patients with cirrhosis of other aetiologies. Men are affected three times more frequently than women[43]. Early detection is crucial for curative treatment, since patients with small HCC (< 2 cm) who are treated with liver transplantation have a survival rate of about 80%[44], whereas the 5-year survival of untreated HCC is less than 5%.
The gray scale US appearance of HCC is variable and non-specific. Hyperechoic foci related to the presence of fibrosis, haemorrhage and necrosis are found in approximately 50% of large HCCs[22]. On colour Doppler, approximately 75% of HCCs have a fine peripheral network of vessels, surrounding and penetrating the lesion (the so-called basket pattern)[45]. Non-invasive imaging diagnosis of HCC is often based on CT or MRI detection of a hypervascular mass in a patient with chronic liver disease because of the lack of specificity of conventional US.
With the use of contrast-enhanced US, more than 90% of HCCs behave like other hypervascular lesions and enhance avidly during the arterial phase (Figure 3)[15,17-19]. The basket pattern is reportedly seen in about one-third of the cases[19]. Also, like other malignant lesions, the majority (83% to 97%) of HCCs washout the contrast and appear as a defect during the late phase. However, caution should be used since well-differentiated HCCs may not show this washout very reliably. Moreover, it has been observed that the more differentiated a lesion, the more slowly it is likely to washout[46]. Consequently, in a patient with known chronic liver disease, a hypervascular lesion in the arterial phase should not be considered as benign on the sole finding of persistent enhancement during the portal and late phases.
Figure 3.
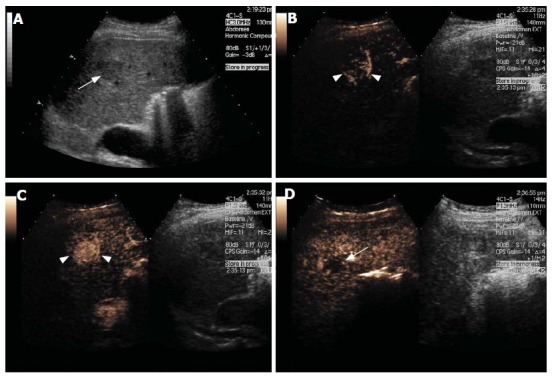
Hepatocellular Carcinoma. Gray-scale US image (A) and split-screen display images of a contrast-enhanced US scan using a low MI technique (B-D). The gray-scale US image shows a slightly hypoechoic lesion in segment 2 of the liver (arrow, A). After contrast injection, the lesion shows marked hypervascularity in the arterial phase with a basket-pattern peripheral network of vessels (arrowheads, B and C). The lesion becomes isoechoic and finally washes out to leave a defect in the late phase (arrow, D).
METASTASES
The liver is a frequent site of metastases of extrahepatic tumours, and metastatic disease is one of the most common indications for imaging the liver. The gray-scale sonographic appearances of metastases are varied depending on several factors such as the histology of the primary tumour and the treatment received by the patient. In a patient with a known malignancy with interval development of hepatic masses, the diagnosis is straightforward and characterisation is not an issue. However, when there is no history of malignancy or no previous imaging for comparison, characterisation becomes essential.
The accuracy of US for the assessment of liver metastases is lower than that of CT or MRI[47]. However, the use of first generation US contrast media, improved significantly the sensitivity for the detection of liver metastases[48]. It has been proposed by the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) that any US staging study of the liver should be contrast enhanced, although the cost and feasibility of such a recommendation remains a big hurdle[13].
On contrast-enhanced US, metastases show different patterns of enhancement during the arterial phase, depending upon the vascularity pattern of the primary tumour. Regardless of their behaviour during the early phase, metastasis consistently show rapid and complete contrast washout and appear as enhancement defects on late phase scans (Figure 4)[15-17,19]. Recent publications have shown that, with the second generation US contrast media, the vast majority (> 85%) of metastases show some arterial enhancement, often more pronounced in the periphery[15,16,19,49]. This phase of hypervascularity is often not recognized on multiphasic CT or MRI because it is very brief and the lesion starts to washout within 20 s of the injection in most cases, before the arterial phase of CT and MRI, which is around 40 s from the beginning of the injection. The arterial enhancement may be rim-like, diffuse or mosaic-like. Rim-like enhancement and early and complete washout of the lesion are typical of metastasis, while complete enhancement with a later washout is most suggestive of a HCC. However, when a hypervascular metastasis shows complete enhancement (Figure 5), differentiation from HCC is difficult and correlation with the clinical history and alpha-fetoprotein is often helpful.
Figure 4.
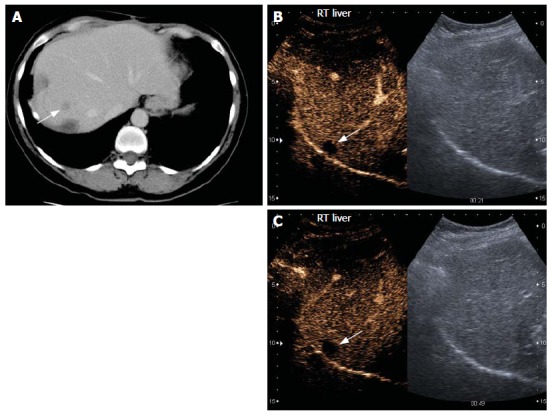
Liver metastasis in a patient with known metastatic colon carcinoma. The patient previously had liver resection and radiofrequency ablation. Contrast-enhanced CT scan image (A) and split-screen display images of a contrast-enhanced US scan using a low MI technique (B and C). On CT, a 1cm lesion is seen in segment 8 (arrow, A). The lesion was not visualized on gray-scale US. After injection of microbubbles, a 1 cm hypoechoic rounded lesion is seen as a defect in all the phases of enhancement (arrow, B and C). These findings are suspicious for a metastatic deposit.
Figure 5.
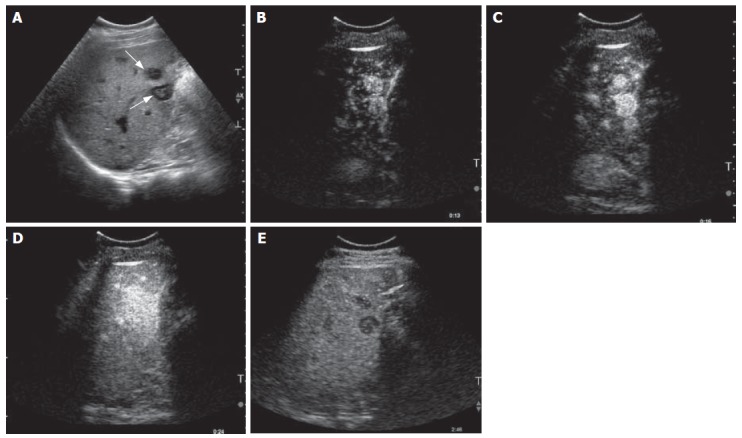
Hypervascular metastases in a patient with a known carcinoid tumour. Gray-scale US image (A) and contrast-enhanced US scan images (B-E) using a microbubble sensitive technique. On the gray-scale image, two hypoechoic target-like lesions are seen (arrows, A). After microbubble enhancement, an avid uptake of the contrast medium was seen in the early phase (B-D). The contrast washed out in the later phases leaving the metastases as defects (E).
OTHER MALIGNANT LESIONS
Other primary malignant lesions of the liver, such as intra-hepatic cholangiocarcinoma and lymphoma, show the typical behaviour of malignant lesions and washout contrast rapidly and appear as defects on the portal and late phases.
Cholangiocarcinoma frequently enhances during the arterial phase[40,49]. The rapid washout of this lesion observed on contrast-enhanced US is discordant with the typical behaviour of the lesion seen on CT and MRI (hypoenhancement with mild peripheral centripetal progression of enhancement over time)[50]. Again, this may be explained by the fact that microbubbles are purely intravascular agents. Some gray-scale features may help in differentiating cholangiocarcinoma from other abnormalities such as biliary duct dilatation.
ABSCESS
Liver abscesses result from bacterial, amoebic or fungal infections. Pyogenic abscess are by far the most frequent (88%)[22]. The gray-scale US findings of pyogenic abscesses vary with the stage of the disease. During early disease, the shape of the lesion is usually irregular and the echogenicity is variable. As the abscess matures the lesion becomes more rounded and hypoechoic, with debris in the middle and thick walls on the outside. Since an abscess is a fluid-filled lesion, there is usually associated posterior enhancement. Internal septations are seen commonly. Bright punctate echoes with “dirty” shadowing are present if there is gas within the cavity.
On contrast-enhanced US, pyogenic hepatic abscesses show areas of increased enhancement relative to the surrounding parenchyma[51]. Mature lesions with fluid show an enhancing rim. The internal septations also show enhancement giving the lesion a honeycomb appearance. Early (solid-appearing) lesions usually enhance diffusely, but heterogeneously. The enhancement appears early and usually persists during the portal and late phases (Figure 6),
Figure 6.
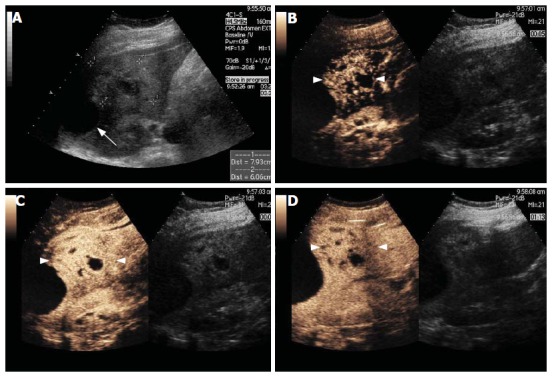
Hepatic abscess. Gray-scale US image (A) and split-screen display images of a contrast-enhanced US scan using a low MI technique (B-D).The gray-scale image shows an ill-defined heterogeneous mass in the left lobe of the liver (between callipers, A). A subcapsular anechoic fluid collection is also seen (arrow, A). After microbubble injection, regional hypervascularity during the arterial phase is shown (arrowheads, B-D). The abscess appears as a cluster of non-enhancing collections separated from each other by enhancing septations (B and C). In the late phase scan (D), there is no enhancement of the fluid collections and no wash out of the enhancing portions.
with no contrast enhancement seen in the liquefied portions. In the arterial phase, a transient peri-lesional enhancement has been reported. In a minority of cases, this is followed by portal venous phase hypovascularity[51]. The main differential diagnosis for a hepatic abscess is a necrotic metastasis. The latter would appear as a punched-out enhancement defects in the late phase, while the former appears as an ill-defined area of decreased enhancement, although, as stated above, this is not a common finding.
LIVER CYSTS
Liver cysts are common incidental findings on liver US. The diagnosis is straightforward when their pathognomonic features (anechoic, thin-walled and posterior enhancement) are present on gray-scale US.
Liver cysts are mentioned in this review because, on contrast-enhanced US, these lesions represent a potential pitfall if they have not first been recognized on gray scale US, since their gray-scale appearances are essential for characterisation. Liver cysts present as enhancement defects on all phases of contrast-enhanced US scan (Figure 7) and can be erroneously mistaken as malignant lesions.
Figure 7.
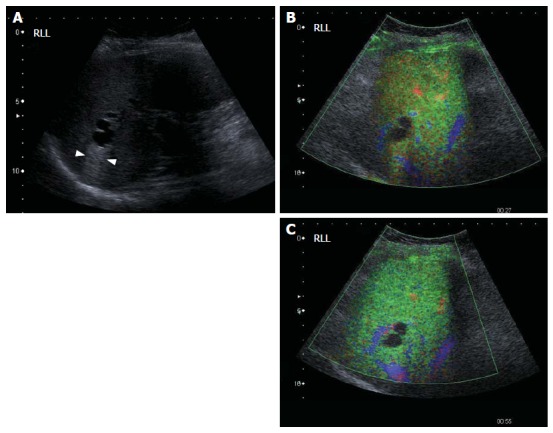
Potential pitfall: simple cysts. Gray-scale US image (A) and contrast-enhanced US scan images using a low MI technique (B and C). The gray-scale image (A) shows a typical simple hepatic cyst which is completely anechoic, has a thin wall and posterior acoustic enhancement (between arrowheads, A). After microbubble injection, no enhancement of the cyst is seen throughout all phases of enhancement (B and C). The diagnosis is straightforward if the lesion was recognised prior to contrast injection. If not, it may be misinterpreted as an enhancement defect and be categorized as a malignant lesion.
CONCLUSION
The introduction of second generation microbubble US contrast media has allowed real-time imaging of a liver lesion in every phase of enhancement. The ability to observe the complete pattern of enhancement of a lesion has improved significantly the specificity of US for focal liver lesions, and rivals that of CT and MRI, thus reducing the need for further investigations. As a screening tool, US is ideal owing to its relative accessibility and portability. Microbubble agents have extended the utility of US further and are applicable to most imaging departments worldwide.
Footnotes
Supported by Research Grant from the British Medical Research Council, Pfizer Global Research (Sandwich, UK) and the United Kingdom Department of Health Research and Development Fund. SM is funded by a clinical and research fellowship from the Société des Radiologistes de l’Hôpital St-François d’Assise, Québec, Canada
S- Editor Liu Y L- Editor Anand BS E- Editor Lu W
References
- 1.Libbrecht L, Bielen D, Verslype C, Vanbeckevoort D, Pirenne J, Nevens F, Desmet V, Roskams T. Focal lesions in cirrhotic explant livers: pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transpl. 2002;8:749–761. doi: 10.1053/jlts.2002.34922. [DOI] [PubMed] [Google Scholar]
- 2.Matsui O, Kadoya M, Kameyama T, Yoshikawa J, Takashima T, Nakanuma Y, Unoura M, Kobayashi K, Izumi R, Ida M. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology. 1991;178:493–497. doi: 10.1148/radiology.178.2.1846240. [DOI] [PubMed] [Google Scholar]
- 3.Wernecke K, Rummeny E, Bongartz G, Vassallo P, Kivelitz D, Wiesmann W, Peters PE, Reers B, Reiser M, Pircher W. Detection of hepatic masses in patients with carcinoma: comparative sensitivities of sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;157:731–739. doi: 10.2214/ajr.157.4.1892027. [DOI] [PubMed] [Google Scholar]
- 4.Noone TC, Semelka RC, Chaney DM, Reinhold C. Abdominal imaging studies: comparison of diagnostic accuracies resulting from ultrasound, computed tomography, and magnetic resonance imaging in the same individual. Magn Reson Imaging. 2004;22:19–24. doi: 10.1016/j.mri.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Bryant TH, Blomley MJ, Albrecht T, Sidhu PS, Leen EL, Basilico R, Pilcher JM, Bushby LH, Hoffmann CW, Harvey CJ, et al. Improved characterization of liver lesions with liver-phase uptake of liver-specific microbubbles: prospective multicenter study. Radiology. 2004;232:799–809. doi: 10.1148/radiol.2323030596. [DOI] [PubMed] [Google Scholar]
- 6.Brannigan M, Burns PN, Wilson SR. Blood flow patterns in focal liver lesions at microbubble-enhanced US. Radiographics. 2004;24:921–935. doi: 10.1148/rg.244035158. [DOI] [PubMed] [Google Scholar]
- 7.Cosgrove D, Blomley M. Liver tumors: evaluation with contrast-enhanced ultrasound. Abdom Imaging. 2004;29:446–454. doi: 10.1007/s00261-003-0126-7. [DOI] [PubMed] [Google Scholar]
- 8.Uhlendorf V, Scholle FD, Reinhardt M. Acoustic behaviour of current ultrasound contrast agents. Ultrasonics. 2000;38:81–86. doi: 10.1016/s0041-624x(99)00128-6. [DOI] [PubMed] [Google Scholar]
- 9.Blomley M, Albrecht T, Cosgrove D, Jayaram V, Butler-Barnes J, Eckersley R. Stimulated acoustic emission in liver parenchyma with Levovist. Lancet. 1998;351:568. doi: 10.1016/S0140-6736(05)78554-8. [DOI] [PubMed] [Google Scholar]
- 10.Burns PN, Wilson SR, Simpson DH. Pulse inversion imaging of liver blood flow: improved method for characterizing focal masses with microbubble contrast. Invest Radiol. 2000;35:58–71. doi: 10.1097/00004424-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Youk JH, Kim CS, Lee JM. Contrast-enhanced agent detection imaging: value in the characterization of focal hepatic lesions. J Ultrasound Med. 2003;22:897–910. doi: 10.7863/jum.2003.22.9.897. [DOI] [PubMed] [Google Scholar]
- 12.Correas JM, Bridal L, Lesavre A, Méjean A, Claudon M, Hélénon O. Ultrasound contrast agents: properties, principles of action, tolerance, and artifacts. Eur Radiol. 2001;11:1316–1328. doi: 10.1007/s003300100940. [DOI] [PubMed] [Google Scholar]
- 13.Albrecht T, Blomley M, Bolondi L, Claudon M, Correas JM, Cosgrove D, Greiner L, Jäger K, Jong ND, Leen E, et al. Guidelines for the use of contrast agents in ultrasound. January 2004. Ultraschall Med. 2004;25:249–256. doi: 10.1055/s-2004-813245. [DOI] [PubMed] [Google Scholar]
- 14.Nicolau C, Vilana R, Catalá V, Bianchi L, Gilabert R, García A, Brú C. Importance of evaluating all vascular phases on contrast-enhanced sonography in the differentiation of benign from malignant focal liver lesions. AJR Am J Roentgenol. 2006;186:158–167. doi: 10.2214/AJR.04.1009. [DOI] [PubMed] [Google Scholar]
- 15.Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, Pozzi-Mucelli R. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420–430. doi: 10.1148/radiol.2322031401. [DOI] [PubMed] [Google Scholar]
- 16.von Herbay A, Vogt C, Willers R, Häussinger D. Real-time imaging with the sonographic contrast agent SonoVue: differentiation between benign and malignant hepatic lesions. J Ultrasound Med. 2004;23:1557–1568. doi: 10.7863/jum.2004.23.12.1557. [DOI] [PubMed] [Google Scholar]
- 17.Ding H, Wang WP, Huang BJ, Wei RX, He NA, Qi Q, Li CL. Imaging of focal liver lesions: low-mechanical-index real-time ultrasonography with SonoVue. J Ultrasound Med. 2005;24:285–297. doi: 10.7863/jum.2005.24.3.285. [DOI] [PubMed] [Google Scholar]
- 18.Wilson SR, Burns PN. An algorithm for the diagnosis of focal liver masses using microbubble contrast-enhanced pulse-inversion sonography. AJR Am J Roentgenol. 2006;186:1401–1412. doi: 10.2214/AJR.04.1920. [DOI] [PubMed] [Google Scholar]
- 19.Leen E, Ceccotti P, Kalogeropoulou C, Angerson WJ, Moug SJ, Horgan PG. Prospective multicenter trial evaluating a novel method of characterizing focal liver lesions using contrast-enhanced sonography. AJR Am J Roentgenol. 2006;186:1551–1559. doi: 10.2214/AJR.05.0138. [DOI] [PubMed] [Google Scholar]
- 20.Kono Y, Steinbach GC, Peterson T, Schmid-Schönbein GW, Mattrey RF. Mechanism of parenchymal enhancement of the liver with a microbubble-based US contrast medium: an intravital microscopy study in rats. Radiology. 2002;224:253–257. doi: 10.1148/radiol.2241011352. [DOI] [PubMed] [Google Scholar]
- 21.Mergo PJ, Ros PR. Benign lesions of the liver. Radiol Clin North Am. 1998;36:319–331. doi: 10.1016/s0033-8389(05)70025-7. [DOI] [PubMed] [Google Scholar]
- 22.Harvey CJ, Albrecht T. Ultrasound of focal liver lesions. Eur Radiol. 2001;11:1578–1593. doi: 10.1007/s003300101002. [DOI] [PubMed] [Google Scholar]
- 23.Leslie DF, Johnson CD, Johnson CM, Ilstrup DM, Harmsen WS. Distinction between cavernous hemangiomas of the liver and hepatic metastases on CT: value of contrast enhancement patterns. AJR Am J Roentgenol. 1995;164:625–629. doi: 10.2214/ajr.164.3.7863883. [DOI] [PubMed] [Google Scholar]
- 24.Nino-Murcia M, Olcott EW, Jeffrey RB Jr, Lamm RL, Beaulieu CF, Jain KA. Focal liver lesions: pattern-based classification scheme for enhancement at arterial phase CT. Radiology. 2000;215:746–751. doi: 10.1148/radiology.215.3.r00jn03746. [DOI] [PubMed] [Google Scholar]
- 25.Semelka RC, Brown ED, Ascher SM, Patt RH, Bagley AS, Li W, Edelman RR, Shoenut JP, Brown JJ. Hepatic hemangiomas: a multi-institutional study of appearance on T2-weighted and serial gadolinium-enhanced gradient-echo MR images. Radiology. 1994;192:401–406. doi: 10.1148/radiology.192.2.8029404. [DOI] [PubMed] [Google Scholar]
- 26.Quaia E, Bertolotto M, Dalla Palma L. Characterization of liver hemangiomas with pulse inversion harmonic imaging. Eur Radiol. 2002;12:537–544. doi: 10.1007/s003300101132. [DOI] [PubMed] [Google Scholar]
- 27.Bartolotta TV, Midiri M, Quaia E, Bertolotto M, Galia M, Cademartiri F, Lagalla R. Liver haemangiomas undetermined at grey-scale ultrasound: contrast-enhancement patterns with SonoVue and pulse-inversion US. Eur Radiol. 2005;15:685–693. doi: 10.1007/s00330-004-2569-9. [DOI] [PubMed] [Google Scholar]
- 28.Karhunen PJ. Benign hepatic tumours and tumour like conditions in men. J Clin Pathol. 1986;39:183–188. doi: 10.1136/jcp.39.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wanless IR, Albrecht S, Bilbao J, Frei JV, Heathcote EJ, Roberts EA, Chiasson D. Multiple focal nodular hyperplasia of the liver associated with vascular malformations of various organs and neoplasia of the brain: a new syndrome. Mod Pathol. 1989;2:456–462. [PubMed] [Google Scholar]
- 30.Nguyen BN, Fléjou JF, Terris B, Belghiti J, Degott C. Focal nodular hyperplasia of the liver: a comprehensive pathologic study of 305 lesions and recognition of new histologic forms. Am J Surg Pathol. 1999;23:1441–1454. doi: 10.1097/00000478-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Shortell CK, Schwartz SI. Hepatic adenoma and focal nodular hyperplasia. Surg Gynecol Obstet. 1991;173:426–431. [PubMed] [Google Scholar]
- 32.Vilgrain V. Focal nodular hyperplasia. Eur J Radiol. 2006;58:236–245. doi: 10.1016/j.ejrad.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 33.Wanless IR, Mawdsley C, Adams R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology. 1985;5:1194–1200. doi: 10.1002/hep.1840050622. [DOI] [PubMed] [Google Scholar]
- 34.Cherqui D, Rahmouni A, Charlotte F, Boulahdour H, Métreau JM, Meignan M, Fagniez PL, Zafrani ES, Mathieu D, Dhumeaux D. Management of focal nodular hyperplasia and hepatocellular adenoma in young women: a series of 41 patients with clinical, radiological, and pathological correlations. Hepatology. 1995;22:1674–1681. [PubMed] [Google Scholar]
- 35.Shamsi K, De Schepper A, Degryse H, Deckers F. Focal nodular hyperplasia of the liver: radiologic findings. Abdom Imaging. 1993;18:32–38. doi: 10.1007/BF00201698. [DOI] [PubMed] [Google Scholar]
- 36.Kim TK, Jang HJ, Wilson SR. Benign liver masses: imaging with microbubble contrast agents. Ultrasound Q. 2006;22:31–39. [PubMed] [Google Scholar]
- 37.Dietrich CF, Schuessler G, Trojan J, Fellbaum C, Ignee A. Differentiation of focal nodular hyperplasia and hepatocellular adenoma by contrast-enhanced ultrasound. Br J Radiol. 2005;78:704–707. doi: 10.1259/bjr/88181612. [DOI] [PubMed] [Google Scholar]
- 38.González A, Canga F, Cárdenas F, Castellano G, García H, Cuenca B, Sánchez F, Solís-Herruzo JA. An unusual case of hepatic adenoma in a male. J Clin Gastroenterol. 1994;19:179–181. doi: 10.1097/00004836-199409000-00026. [DOI] [PubMed] [Google Scholar]
- 39.Alobaidi M, Shirkhoda A. Benign focal liver lesions: discrimination from malignant mimickers. Curr Probl Diagn Radiol. 2004;33:239–253. doi: 10.1067/j.cpradiol.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Dietrich CF. Characterisation of focal liver lesions with contrast enhanced ultrasonography. Eur J Radiol. 2004;51 Suppl:S9–S17. doi: 10.1016/j.ejrad.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 41.Grazioli L, Federle MP, Brancatelli G, Ichikawa T, Olivetti L, Blachar A. Hepatic adenomas: imaging and pathologic findings. Radiographics. 2001;21:877–892; discussion 892-894. doi: 10.1148/radiographics.21.4.g01jl04877. [DOI] [PubMed] [Google Scholar]
- 42.Gordon SC, Reddy KR, Livingstone AS, Jeffers LJ, Schiff ER. Resolution of a contraceptive-steroid-induced hepatic adenoma with subsequent evolution into hepatocellular carcinoma. Ann Intern Med. 1986;105:547–549. doi: 10.7326/0003-4819-105-4-547. [DOI] [PubMed] [Google Scholar]
- 43.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 44.Taouli B, Losada M, Holland A, Krinsky G. Magnetic resonance imaging of hepatocellular carcinoma. Gastroenterology. 2004;127:S144–S152. doi: 10.1053/j.gastro.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka S, Kitamura T, Fujita M, Nakanishi K, Okuda S. Color Doppler flow imaging of liver tumors. AJR Am J Roentgenol. 1990;154:509–514. doi: 10.2214/ajr.154.3.2154912. [DOI] [PubMed] [Google Scholar]
- 46.Fan ZH, Chen MH, Dai Y, Wang YB, Yan K, Wu W, Yang W, Yin SS. Evaluation of primary malignancies of the liver using contrast-enhanced sonography: correlation with pathology. AJR Am J Roentgenol. 2006;186:1512–1519. doi: 10.2214/AJR.05.0943. [DOI] [PubMed] [Google Scholar]
- 47.Hagspiel KD, Neidl KF, Eichenberger AC, Weder W, Marincek B. Detection of liver metastases: comparison of superparamagnetic iron oxide-enhanced and unenhanced MR imaging at 1.5 T with dynamic CT, intraoperative US, and percutaneous US. Radiology. 1995;196:471–478. doi: 10.1148/radiology.196.2.7617863. [DOI] [PubMed] [Google Scholar]
- 48.Albrecht T, Blomley MJ, Burns PN, Wilson S, Harvey CJ, Leen E, Claudon M, Calliada F, Correas JM, LaFortune M, et al. Improved detection of hepatic metastases with pulse-inversion US during the liver-specific phase of SHU 508A: multicenter study. Radiology. 2003;227:361–370. doi: 10.1148/radiol.2272011833. [DOI] [PubMed] [Google Scholar]
- 49.Jang HJ, Kim TK, Wilson SR. Imaging of malignant liver masses: characterization and detection. Ultrasound Q. 2006;22:19–29. [PubMed] [Google Scholar]
- 50.Worawattanakul S, Semelka RC, Noone TC, Calvo BF, Kelekis NL, Woosley JT. Cholangiocarcinoma: spectrum of appearances on MR images using current techniques. Magn Reson Imaging. 1998;16:993–1003. doi: 10.1016/s0730-725x(98)00135-0. [DOI] [PubMed] [Google Scholar]
- 51.Catalano O, Sandomenico F, Raso MM, Siani A. Low mechanical index contrast-enhanced sonographic findings of pyogenic hepatic abscesses. AJR Am J Roentgenol. 2004;182:447–450. doi: 10.2214/ajr.182.2.1820447. [DOI] [PubMed] [Google Scholar]