Abstract
Desmoid tumor is a monoclonal, fibroblastic proliferation arising in musculoaponeurotic structures. This connective tissue hyperplasia infiltrates locally, recurs frequently after resection but does not metastasize. Abdominal desmoid occurs sporadically, in association with some familial syndromes and often represents a clinical dilemma for surgeons. The enigmatic biology and anatomical location of abdominal desmoids make treatment recommendations difficult. This distinct pathological entity is reviewed with a specific focus on aetiology and management.
Keywords: Desmoid, Abdomen, Fibromatosis, Familial adenomatous polyposis, Gardner’s syndrome
INTRODUCTION
Desmoid tumor, also known as aggressive fibromatosis or musculo-aponeurotic fibromatosis[1], is a monoclonal[2,3], fibroblastic proliferation arising in musculoaponeurotic structures.
Although Mueller in 1838 coined the term desmoid tumor[4] (derived from the Greek desmos that means tendon-like), the first description of the tumor is credited to McFarlane, who reported the disease occurring in the abdominal wall of a young woman after delivery in 1832[5].
Histologically, these tumors consist of spindle-shaped cells in a collagenous matrix without the pleomorphic, atypical, or hyperchromatic nuclei of malignancy[1]. The connective tissue hyperplasia infiltrates locally, recurs frequently after resection but does not metastasize[6].
Desmoid tumors have been recently subdivided according to their location into extra-abdominal, abdominal and intra-abdominal, and the latter have been subclassified further into mesenteric fibromatosis and pelvic fibromatosis[7]. This tumor may occur at the site of any fascia, but in particular in muscle, hence the descriptive term musculo-aponeurotic fibromatosis. The most frequent sites involved by these tumors are the torso and the extremities. Many studies have shown that between 37% and 50% of desmoids arise in the abdominal region[6,8,9]. Abdominal desmoid occurs sporadically[8], in association with some familial syndromes[9] and often represents a clinical dilemma for surgeons. Most surgical reports emphasize the difficulty in achieving adequate resection margins, while maintaining acceptable function and cosmesis[10,11]. These are major factors contributing to the high rates of relapse after surgery, especially after conservative resections[12,13].
The enigmatic biology and anatomical location of intra-abdominal desmoids make treatment recommendations difficult. A significant factor limiting the attempted generalization concerning management is the small number of cases available for analysis, reflecting the relative rarity of the disease.
EPIDEMIOLOGY AND ETIOLOGY
Desmoid tumor is a rare lesion representing < 3% of all soft tissue tumors with an estimated incidence of 2-4 new cases per million per year[14].
These tumors have been well characterized from a morphologic standpoint, but their nature and pathogenesis have remained obscure for many years, to the point that Stout[15] defined it as “the most incomprehensible group” of fibromatosis. They have been considered non-neoplastic processes by some authors and well-differentiated low-grade sarcomas by others[2].
An association with familial adenomatous polyposis of the colon (FAP) and Gardner’s syndrome has been well documented. Abdominal and extra-abdominal desmoids occur more frequently in FAP patients, with an incidence of 3.5%-32%. In the original Gardner kindred the incidence was 29%[9,16].
The etiology of desmoids has not been well defined. Numerous factors are acknowledged to be strongly associated with their development. An antecedent history of trauma to the site of the tumor, often surgical in nature, may be elicited in approximately 25% of cases[17,18].
Within the FAP population, there is a strong correlation between prophylactic procto-colectomy and the subsequent development of desmoid tumours[19-22].
Other forms of trauma, such as physiologic trauma associated with pregnancy, are also thought to contribute to the development of desmoid tumors and several papers report an increased association between pregnancy and desmoid tumors[10,14,16,17].
A predominance of the disease in the female population has been reported, with a female to male ratio ranging from 1.4 to 1.8. The peak of incidence is between the ages of 25 and 35 years, even though cases occurring in patients younger than 10 years have been described[10,17,23,24]. The preponderance of cases described afflicting women in reproductive age shows a clear association of this disease with the endogenous hormonal environment and exogenous sex hormones[10,16,17].
Anecdotal reports of tumor regression during menopause[25,26], the development of desmoids in patients taking oral contraceptives[19,27], and reports of tumor regression with tamoxifen treatment[28], serve to underline an evident role of estrogen in the multifactorial pathogenesis of desmoid.
While most of the cases are sporadic, some are associated with familial syndromes (FAP, Gardner’s syndrome) and these are most often intra-abdominal[19,29]. There are also cases of familial desmoid tumors at multiple sites, in patients without FAP, often involving one extremity. In both FAP and familial non-FAP tumors, mutations of the adenomatous polyposis coli (APC) gene on the long arm of chromosome 5 have been incriminated. The resultant loss of ability to degrade beta-catenin and elevated beta-catenin levels promotes fibroblastic proliferation through a nuclear mechanism[30].
CLINICAL PRESENTATION AND DIAGNOSTIC EVALUATION
Desmoid tumors most frequently present as a slowly enlarging mass (Figure 1). Symptoms depend on the location of the tumor. Patients with intra-abdominal desmoid may have asymptomatic masses, symptoms of intestinal, vascular and urinary obstruction or neural involvement[6,31,32]. The diagnosis of desmoid is based on clinical suspicion. A history of FAP or of similarly affected family members is frequently elicited. A history of trauma or recent pregnancy is also common.
Figure 1.
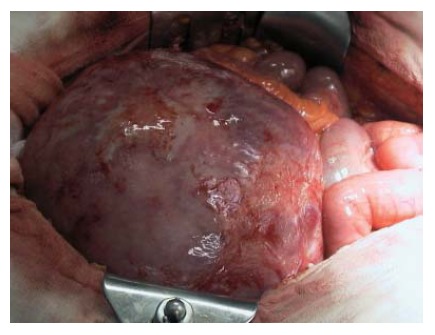
Intraoperative finding of giant retroperitoneal desmoid tumor.
The role of imaging (computed tomography and magnetic resonance) is to define the degree of extension to local structures and tumor relationship to neurovascular structure[33,34]. This tumor does not metastasize to regional nodes or distant sites so that search for metastatic disease is unnecessary. Biopsy is not usually necessary but does not seem to induce further growth, if performed. At the moment there is no accepted staging system for this disease[35].
MANAGEMENT
The management of desmoid tumors requires special attention. A strong family history of desmoid tumors and a high-risk location of the mutation on the APC gene increase the risk for the development of desmoid tumors[30,36]. Due to a reported 85% recurrence rate of desmoid tumors after surgical excision, surgery should be performed only when absolutely necessary[37,38].
Tamoxifen, toremifene, and sulindac have been used as treatments, but the results are controversial. Reports have suggested that therapy might be associated with an initial benefit but the long-term clinical improvement is minimal[39-41]. Cytotoxic chemotherapy (doxorubicin, dacarbazine, and carboplatin) may be effective in treating aggressive, rapidly growing and unresectable abdominal desmoids[42,43]. Radiation therapy might be effective in selected cases but it is frequently limited by the presence of the small bowel in the radiation field in mesenteric and pelvic desmoids[44,45].
Intra-abdominal desmoid tumors usually involve the mesentery and often involve the mesenteric vessels. They invade the mesentery diffusely, kink loops of bowel and can cause ureters obstruction (Figure 2). This feature requires a complex surgery and often radical resection is impossible to achieve[11]. Therefore the management of intra-abdominal desmoid tumors is complex and is dependant on their clinical behavior.
Figure 2.
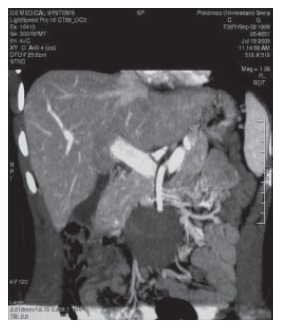
Coronal view of computed tomography scan shows a mesenteric desmoid tumor.
Given the problems related to the treatment of desmoids, there is a good case for simple observation of many tumors, particularly if asymptomatic. Following diagnosis, a small tumor which is not encroaching on any nearby structures may be followed up by regular clinical examination (every 6 mo) with or without imaging, usually by CT. Desmoids that are growing slowly or are mildly symptomatic can be treated with sulindac and tamoxifen or with vinblastine and methotrexate, since these are less toxic regimens. Aggressive desmoids are treated with anti-sarcomachemotherapy such as doxorubicin and dacarbazine.
It would thus seem that surgery is a reasonable first-line treatment for abdominal wall tumors, since they are easier to excise than intra-abdominal desmoid tumors, recurrence rates are lower, and morbidity rates associated with the procedure are lower. The excision should be completed with a one cm margin. A mesh can be used to cover the defect if required[46].
For intra-abdominal desmoids surgery should only be used in specific circumstances. These would include tumors which do not appear to involve vital organs and vessels on preoperative imaging, those resistant to drug treatment and in cases where a risky operation is the only possible option in the case of a rapidly growing, life-threatening tumor. High rates of recurrence should be expected and patients must be counseled pre-operatively about the risks of death. Such cases should only be attempted in specialist centers with sufficiently experienced staff.
At the moment one center has also reported a technique where the tumor and small bowel are removed en bloc, perfused and cooled, and the tumor resected on the bench in a bloodless field with subsequent autotransplantation of the small bowel back into the patient[47]. Recently a report has been published where two desmoids (one intra-abdominal) were treated with percutaneous chemical ablation with acetic acid under-radiological guidance[48]. In both cases there was substantial regression of the tumours within a few months.
Unfortunately, despite any treatment, some patients deteriorate, become dependent on TPN, and have life-threatening complications develop. Intestinal transplantation is the only remaining option for these patients[49].
In conclusion the optimal treatment protocol has not yet been established and, in many cases, a multidisciplinary approach including surgery, chemotherapy, and radiation therapy has been employed. The rarity of cases in even major tumor centers has traditionally limited the ability to study this disease. The notion that a specific genotype can predict the development of an aggressive desmoid tumor in a given patient could prove to be valuable in allowing appropriate patient selection for early therapy or even a chemopreventive strategy. Several novel pharmacologic and biologic treatment approaches are actively being developed, although long-term follow-up is needed for their substantiation[50].
Footnotes
S- Editor Liu Y L- Editor Alpini GD E- Editor Liu Y
References
- 1.Enzinger FM, Weiss SW. Soft tissue tumors. 4th ed. St. Louis: CV Mosby; 2001. pp. 1472–1475. [Google Scholar]
- 2.Li M, Cordon-Cardo C, Gerald WL, Rosai J. Desmoid fibromatosis is a clonal process. Hum Pathol. 1996;27:939–943. doi: 10.1016/s0046-8177(96)90221-x. [DOI] [PubMed] [Google Scholar]
- 3.Alman BA, Pajerski ME, Diaz-Cano S, Corboy K, Wolfe HJ. Aggressive fibromatosis (desmoid tumor) is a monoclonal disorder. Diagn Mol Pathol. 1997;6:98–101. doi: 10.1097/00019606-199704000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Mueller J Ueber den feinrn Bau und die Formen der Krankhaften geschwulste. Berlin: G Reimer and Erste Lieferung, 1838: 60 [Google Scholar]
- 5.MacFarlane J Clinical report on the surgical practice of Glasgow Royal Infirmary. Glasgow: D. Robertson, 1832: 63 [Google Scholar]
- 6.Lewis JJ, Boland PJ, Leung DH, Woodruff JM, Brennan MF. The enigma of desmoid tumors. Ann Surg. 1999;229:866–872; discussion 872-873. doi: 10.1097/00000658-199906000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss S, Goldblum JR, editors . Enzinger and Weiss's Soft Tissue tumors. 4th ed. St Louis: Mobis; 2001. pp. 641–693. [Google Scholar]
- 8.Bruce JM, Bradley EL 3rd, Satchidanand SK. A desmoid tumor of the pancreas. Sporadic intra-abdominal desmoids revisited. Int J Pancreatol. 1996;19:197–203. doi: 10.1007/BF02787368. [DOI] [PubMed] [Google Scholar]
- 9.Clark SK, Phillips RK. Desmoids in familial adenomatous polyposis. Br J Surg. 1996;83:1494–1504. doi: 10.1002/bjs.1800831105. [DOI] [PubMed] [Google Scholar]
- 10.Posner MC, Shiu MH, Newsome JL, Hajdu SI, Gaynor JJ, Brennan MF. The desmoid tumor. Not a benign disease. Arch Surg. 1989;124:191–196. doi: 10.1001/archsurg.1989.01410020061010. [DOI] [PubMed] [Google Scholar]
- 11.Smith AJ, Lewis JJ, Merchant NB, Leung DH, Woodruff JM, Brennan MF. Surgical management of intra-abdominal desmoid tumours. Br J Surg. 2000;87:608–613. doi: 10.1046/j.1365-2168.2000.01400.x. [DOI] [PubMed] [Google Scholar]
- 12.Burke AP, Sobin LH, Shekitka KM, Federspiel BH, Helwig EB. Intra-abdominal fibromatosis. A pathologic analysis of 130 tumors with comparison of clinical subgroups. Am J Surg Pathol. 1990;14:335–341. [PubMed] [Google Scholar]
- 13.Acker JC, Bossen EH, Halperin EC. The management of desmoid tumors. Int J Radiat Oncol Biol Phys. 1993;26:851–858. doi: 10.1016/0360-3016(93)90501-l. [DOI] [PubMed] [Google Scholar]
- 14.Reitamo JJ, Scheinin TM, Häyry P. The desmoid syndrome. New aspects in the cause, pathogenesis and treatment of the desmoid tumor. Am J Surg. 1986;151:230–237. doi: 10.1016/0002-9610(86)90076-0. [DOI] [PubMed] [Google Scholar]
- 15.STOUT AP. Juvenile fibromatoses. Cancer. 1954;7:953–978. doi: 10.1002/1097-0142(195409)7:5<953::aid-cncr2820070520>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 16.Reitamo JJ, Häyry P, Nykyri E, Saxén E. The desmoid tumor. I. Incidence, sex-, age- and anatomical distribution in the Finnish population. Am J Clin Pathol. 1982;77:665–673. doi: 10.1093/ajcp/77.6.665. [DOI] [PubMed] [Google Scholar]
- 17.Lopez R, Kemalyan N, Moseley HS, Dennis D, Vetto RM. Problems in diagnosis and management of desmoid tumors. Am J Surg. 1990;159:450–453. doi: 10.1016/s0002-9610(05)81243-7. [DOI] [PubMed] [Google Scholar]
- 18.McKinnon JG, Neifeld JP, Kay S, Parker GA, Foster WC, Lawrence W Jr. Management of desmoid tumors. Surg Gynecol Obstet. 1989;169:104–106. [PubMed] [Google Scholar]
- 19.Jones IT, Jagelman DG, Fazio VW, Lavery IC, Weakley FL, McGannon E. Desmoid tumors in familial polyposis coli. Ann Surg. 1986;204:94–97. doi: 10.1097/00000658-198607000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurbuz AK, Giardiello FM, Petersen GM, Krush AJ, Offerhaus GJ, Booker SV, Kerr MC, Hamilton SR. Desmoid tumours in familial adenomatous polyposis. Gut. 1994;35:377–381. doi: 10.1136/gut.35.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAdam WA, Goligher JC. The occurrence of desmoids in patients with familial polyposis coli. Br J Surg. 1970;57:618–631. doi: 10.1002/bjs.1800570816. [DOI] [PubMed] [Google Scholar]
- 22.Lynch HT, Fitzgibbons R Jr. Surgery, desmoid tumors, and familial adenomatous polyposis: case report and literature review. Am J Gastroenterol. 1996;91:2598–2601. [PubMed] [Google Scholar]
- 23.Järvinen HJ. Desmoid disease as a part of familial adenomatous polyposis coli. Acta Chir Scand. 1987;153:379–383. [PubMed] [Google Scholar]
- 24.Klemmer S, Pascoe L, DeCosse J. Occurrence of desmoids in patients with familial adenomatous polyposis of the colon. Am J Med Genet. 1987;28:385–392. doi: 10.1002/ajmg.1320280217. [DOI] [PubMed] [Google Scholar]
- 25.DAHN I, JONSSON N, LUNDH G. DESMOID TUMOURS. A SERIES OF 33 CASES. Acta Chir Scand. 1963;126:305–314. [PubMed] [Google Scholar]
- 26.Lotfi AM, Dozois RR, Gordon H, Hruska LS, Weiland LH, Carryer PW, Hurt RD. Mesenteric fibromatosis complicating familial adenomatous polyposis: predisposing factors and results of treatment. Int J Colorectal Dis. 1989;4:30–36. doi: 10.1007/BF01648547. [DOI] [PubMed] [Google Scholar]
- 27.Waddell WR. Treatment of intra-abdominal and abdominal wall desmoid tumors with drugs that affect the metabolism of cyclic 3',5'-adenosine monophosphate. Ann Surg. 1975;181:299–302. doi: 10.1097/00000658-197503000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilcken N, Tattersall MH. Endocrine therapy for desmoid tumors. Cancer. 1991;68:1384–1388. doi: 10.1002/1097-0142(19910915)68:6<1384::aid-cncr2820680634>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 29.GARDNER EJ, RICHARDS RC. Multiple cutaneous and subcutaneous lesions occurring simultaneously with hereditary polyposis and osteomatosis. Am J Hum Genet. 1953;5:139–147. [PMC free article] [PubMed] [Google Scholar]
- 30.Cheon SS, Cheah AY, Turley S, Nadesan P, Poon R, Clevers H, Alman BA. beta-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci USA. 2002;99:6973–6978. doi: 10.1073/pnas.102657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corbel L, Souissi M, Chrétien Y, Dufour B. [Desmoid tumor of the mesentery. An uncommon cause of ureteral obstruction] J Radiol. 1992;73:669–672. [PubMed] [Google Scholar]
- 32.Anthony T, Rodriguez-Bigas MA, Weber TK, Petrelli NJ. Desmoid tumors. J Am Coll Surg. 1996;182:369–377. [PubMed] [Google Scholar]
- 33.Einstein DM, Tagliabue JR, Desai RK. Abdominal desmoids: CT findings in 25 patients. AJR Am J Roentgenol. 1991;157:275–279. doi: 10.2214/ajr.157.2.1853806. [DOI] [PubMed] [Google Scholar]
- 34.Quinn SF, Erickson SJ, Dee PM, Walling A, Hackbarth DA, Knudson GJ, Moseley HS. MR imaging in fibromatosis: results in 26 patients with pathologic correlation. AJR Am J Roentgenol. 1991;156:539–542. doi: 10.2214/ajr.156.3.1899752. [DOI] [PubMed] [Google Scholar]
- 35.American Joint Committee on Cancer. ALCC Cancer Staging Manual, 6th ed, vol 22. New York: Springer; 2002. pp. 193–200. [Google Scholar]
- 36.Zayid I, Dihmis C. Familial multicentric fibromatosis--desmoids. A report of three cases in a Jordanian family. Cancer. 1969;24:786–795. doi: 10.1002/1097-0142(196910)24:4<786::aid-cncr2820240420>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 37.Ballo MT, Zagars GK, Pollack A, Pisters PW, Pollack RA. Desmoid tumor: prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol. 1999;17:158–167. doi: 10.1200/JCO.1999.17.1.158. [DOI] [PubMed] [Google Scholar]
- 38.Kulaylat MN, Karakousis CP, Keaney CM, McCorvey D, Bem J, Ambrus Sr JL. Desmoid tumour: a pleomorphic lesion. Eur J Surg Oncol. 1999;25:487–497. doi: 10.1053/ejso.1999.0684. [DOI] [PubMed] [Google Scholar]
- 39.Waddell WR, Gerner RE, Reich MP. Nonsteroid antiinflammatory drugs and tamoxifen for desmoid tumors and carcinoma of the stomach. J Surg Oncol. 1983;22:197–211. doi: 10.1002/jso.2930220314. [DOI] [PubMed] [Google Scholar]
- 40.Kinzbrunner B, Ritter S, Domingo J, Rosenthal CJ. Remission of rapidly growing desmoid tumors after tamoxifen therapy. Cancer. 1983;52:2201–2204. doi: 10.1002/1097-0142(19831215)52:12<2201::aid-cncr2820521204>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Hansmann A, Adolph C, Vogel T, Unger A, Moeslein G. High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer. 2004;100:612–620. doi: 10.1002/cncr.11937. [DOI] [PubMed] [Google Scholar]
- 42.Okuno SH, Edmonson JH. Combination chemotherapy for desmoid tumors. Cancer. 2003;97:1134–1135. doi: 10.1002/cncr.11189. [DOI] [PubMed] [Google Scholar]
- 43.Gega M, Yanagi H, Yoshikawa R, Noda M, Ikeuchi H, Tsukamoto K, Oshima T, Fujiwara Y, Gondo N, Tamura K, et al. Successful chemotherapeutic modality of doxorubicin plus dacarbazine for the treatment of desmoid tumors in association with familial adenomatous polyposis. J Clin Oncol. 2006;24:102–105. doi: 10.1200/JCO.2005.02.1923. [DOI] [PubMed] [Google Scholar]
- 44.Keus R, Bartelink H. The role of radiotherapy in the treatment of desmoid tumours. Radiother Oncol. 1986;7:1–5. doi: 10.1016/s0167-8140(86)80118-9. [DOI] [PubMed] [Google Scholar]
- 45.Leibel SA, Wara WM, Hill DR, Bovill EG Jr, de Lorimier AA, Beckstead JH, Phillips TL. Desmoid tumors: local control and patterns of relapse following radiation therapy. Int J Radiat Oncol Biol Phys. 1983;9:1167–1171. doi: 10.1016/0360-3016(83)90175-x. [DOI] [PubMed] [Google Scholar]
- 46.Sutton RJ, Thomas JM. Desmoid tumours of the anterior abdominal wall. Eur J Surg Oncol. 1999;25:398–400. doi: 10.1053/ejso.1999.0664. [DOI] [PubMed] [Google Scholar]
- 47.Tzakis AG, Tryphonopoulos P, De Faria W, Kato T, Nishida S, Levi DM, Madariaga J, Weppler D, Mittal N, Ruiz P, et al. Partial abdominal evisceration, ex vivo resection, and intestinal autotransplantation for the treatment of pathologic lesions of the root of the mesentery. J Am Coll Surg. 2003;197:770–776. doi: 10.1016/s1072-7515(03)00756-7. [DOI] [PubMed] [Google Scholar]
- 48.Clark TW. Percutaneous chemical ablation of desmoid tumors. J Vasc Interv Radiol. 2003;14:629–634. doi: 10.1097/01.rvi.0000064861.87207.3e. [DOI] [PubMed] [Google Scholar]
- 49.Tryphonopoulos P, Weppler D, Levi DM, Nishida S, Madariaga JR, Kato T, Mittal N, Moon J, Selvaggi G, Esquenazi V, et al. Transplantation for the treatment of intra-abdominal fibromatosis. Transplant Proc. 2005;37:1379–1380. doi: 10.1016/j.transproceed.2004.12.218. [DOI] [PubMed] [Google Scholar]
- 50.Janinis J, Patriki M, Vini L, Aravantinos G, Whelan JS. The pharmacological treatment of aggressive fibromatosis: a systematic review. Ann Oncol. 2003;14:181–190. doi: 10.1093/annonc/mdg064. [DOI] [PubMed] [Google Scholar]


