Abstract
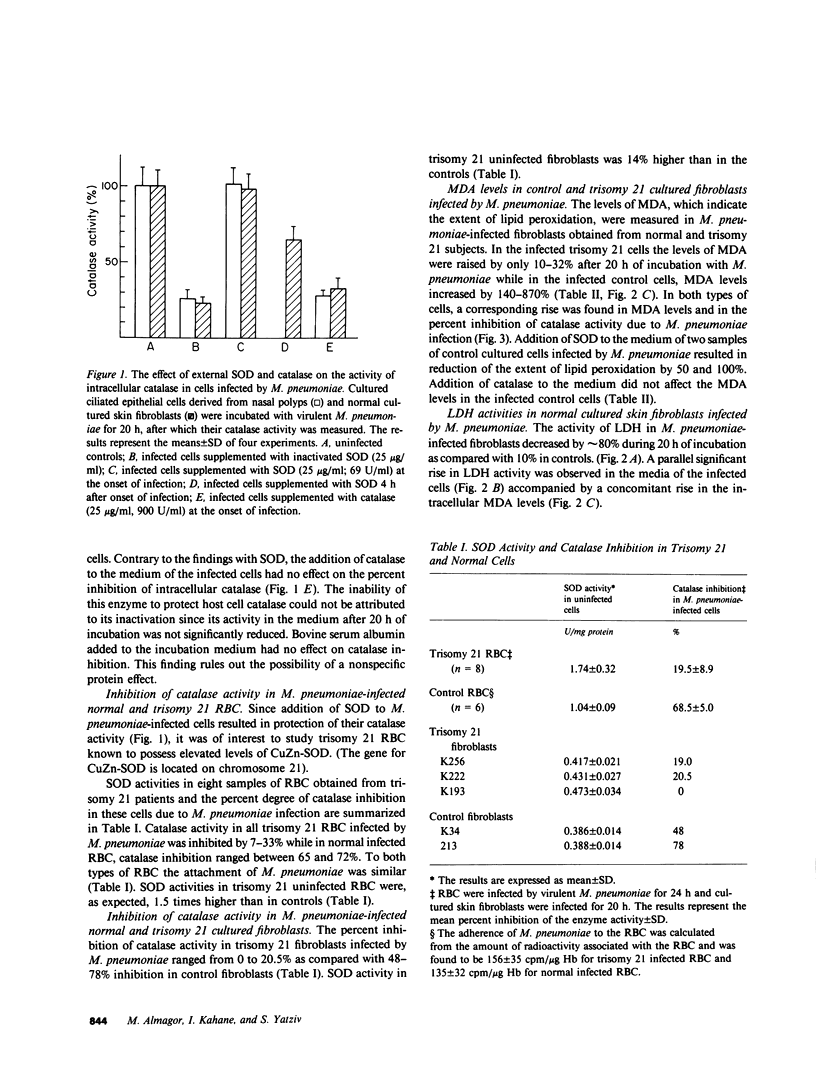
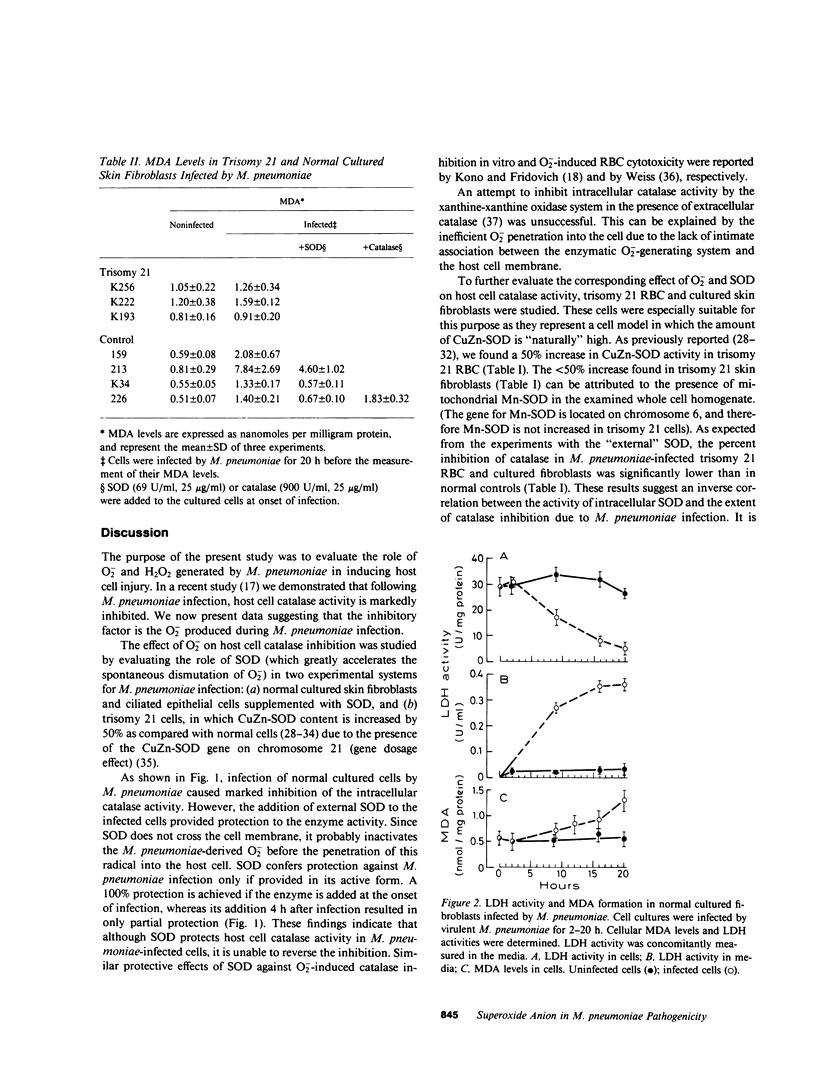
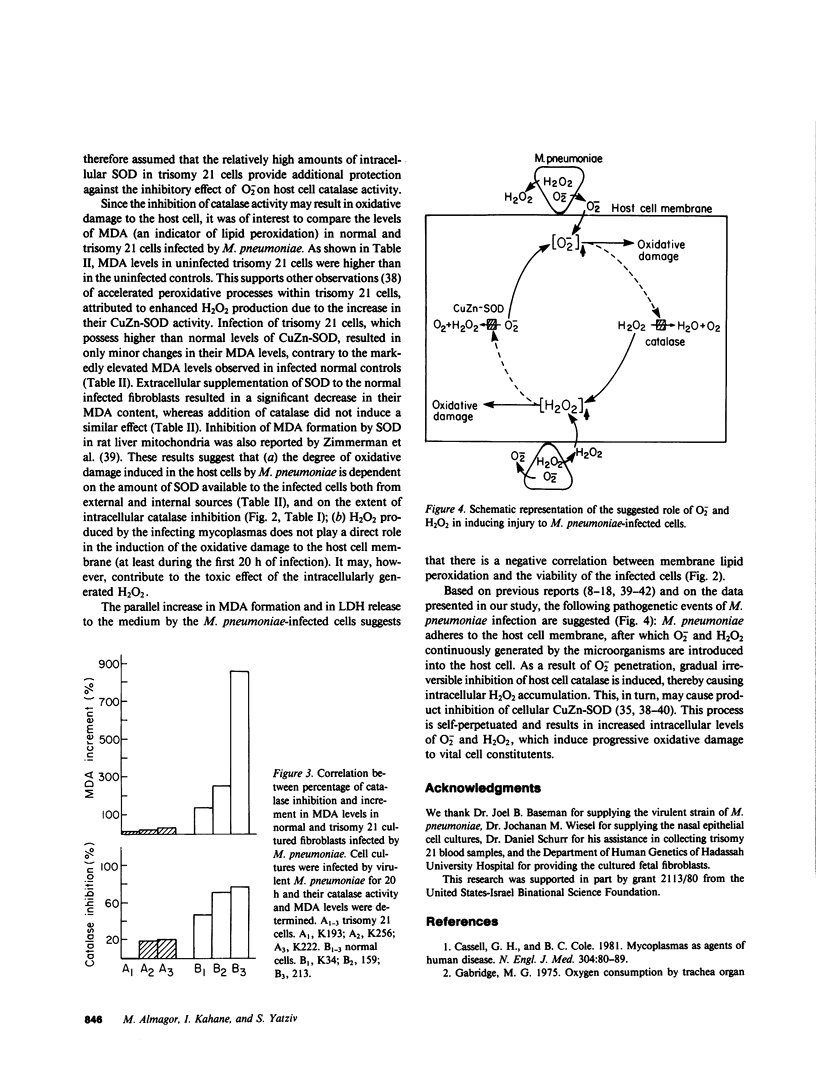
The role of Mycoplasma pneumoniae-generated superoxide and hydrogen peroxide in inducing host cell injury was studied in normal and trisomy 21 human cells. As a result of M. pneumoniae infection, catalase activity in infected normal skin fibroblasts and ciliated epithelial cells decreased by 74-77% as compared with uninfected controls. Addition of superoxide dismutase to the infected cultured cells totally prevented the inhibition whereas addition of catalase or catalytically inactivated superoxide dismutase had no protective effect. Trisomy 21 erythrocytes and cultured skin fibroblasts in which CuZn-superoxide dismutase content is 50% greater than in normal cells were infected by M. pneumoniae. The inhibition of catalase activity in these cells was 7-33% and 0-20.5%, respectively, as compared with 65-72% and 48-68% inhibition in normal infected controls. Following M. pneumoniae infection, the levels of malonyldialdehyde, an indicator for membrane lipid peroxidation were raised in trisomy 21 cultured fibroblasts by 10-32% while in normal cells malonyldialdehyde increased by 140-870%. Externally added superoxide dismutase, but not catalase, reduced the extent of lipid peroxidation in normal infected cells. Lactate dehydrogenase release from normal infected cells was time correlated with the increase in their malonyldialdehyde formation. It is suggested that superoxide generated during M. pneumoniae infection is involved in the inhibition of host cell catalase activity. The inactivation of this cellular antioxidative defense mechanism results in progressive oxidative damage to the M. pneumoniae-infected cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almagor M., Yatziv S., Kahane I. Inhibition of host cell catalase by Mycoplasma pneumoniae: a possible mechanism for cell injury. Infect Immun. 1983 Jul;41(1):251–256. doi: 10.1128/iai.41.1.251-256.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai M., Razin S., Bredt W., Kahane I. Isolation of binding sites to glycophorin from Mycoplasma pneumoniae membranes. Infect Immun. 1980 Dec;30(3):628–634. doi: 10.1128/iai.30.3.628-634.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baret A., Baeteman M. A., Mattei J. F., Michel P., Broussolle B., Giraud F. Immunoreactive Cu SOD and Mn SOD in the circulating blood cells from normal and trisomy 21 subjects. Biochem Biophys Res Commun. 1981 Feb 27;98(4):1035–1043. doi: 10.1016/0006-291x(81)91215-8. [DOI] [PubMed] [Google Scholar]
- Bray R. C., Cockle S. A., Fielden E. M., Roberts P. B., Rotilio G., Calabrese L. Reduction and inactivation of superoxide dismutase by hydrogen peroxide. Biochem J. 1974 Apr;139(1):43–48. doi: 10.1042/bj1390043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Cassell G. H., Cole B. C. Mycoplasmas as agents of human disease. N Engl J Med. 1981 Jan 8;304(2):80–89. doi: 10.1056/NEJM198101083040204. [DOI] [PubMed] [Google Scholar]
- Cohen G., Somerson N. L. Mycoplasma pneumoniae: hydrogen peroxide secretion and its possible role in virulence. Ann N Y Acad Sci. 1967 Jul 28;143(1):85–87. doi: 10.1111/j.1749-6632.1967.tb27648.x. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R., Martin C. H. Hemolysin and peroxide activity of Mycoplasma species. J Bacteriol. 1968 Jun;95(6):2022–2030. doi: 10.1128/jb.95.6.2022-2030.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosti N., Serra A., Rigo A., Viglino P. Dosage effect of SOD-A gene in 21-trisomic cells. Hum Genet. 1976 Feb 29;31(2):197–202. doi: 10.1007/BF00296146. [DOI] [PubMed] [Google Scholar]
- Feaster W. W., Kwok L. W., Epstein C. J. Dosage effects for superoxide dismutase-1 in nucleated cells aneuploid for chromosome 21. Am J Hum Genet. 1977 Nov;29(6):563–570. [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G. Oxygen consumption by trachea organ cultures infected with Mycoplasma pneumoniae. Infect Immun. 1975 Sep;12(3):544–549. doi: 10.1128/iai.12.3.544-549.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Polisky R. B. Intracellular levels of adenosine triphosphate in hamster trachea organ cultures exposed to Mycoplasma pneumoniae cells or membranes. In Vitro. 1977 Aug;13(8):510–516. doi: 10.1007/BF02615144. [DOI] [PubMed] [Google Scholar]
- Gilles L., Ferradini C., Foos J., Pucheault J., Allard D., Sinet P. M., Jerome H. The estimation of red cell superoxide dismutase activity by pulse radiolysis in normal and trisomic 21 subjects. FEBS Lett. 1976 Oct 15;69(1):55–58. doi: 10.1016/0014-5793(76)80652-7. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Heikkila R. E., Cabbat F. S., Cohen G. In vivo inhibition of superoxide dismutase in mice by diethyldithiocarbamate. J Biol Chem. 1976 Apr 10;251(7):2182–2185. [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of the enzyme. Biochemistry. 1975 Dec 2;14(24):5294–5299. doi: 10.1021/bi00695a010. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Alterations in the metabolism of hamster tracheas in organ culture after infection by virulent Mycoplasma pneumoniae. Infect Immun. 1975 Apr;11(4):704–710. doi: 10.1128/iai.11.4.704-710.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T., Blum J., Kahane I., Fridovich I. Distinguishing between Mn-containing and Fe-containing superoxide dismutases in crude extracts of cells. Arch Biochem Biophys. 1980 May;201(2):551–555. doi: 10.1016/0003-9861(80)90544-5. [DOI] [PubMed] [Google Scholar]
- Kono Y., Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982 May 25;257(10):5751–5754. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leith D. K., Hansen E. J., Wilson R. M., Krause D. C., Baseman J. B. Hemadsorption and virulence are separable properties of Mycoplasma pneumoniae. Infect Immun. 1983 Feb;39(2):844–850. doi: 10.1128/iai.39.2.844-850.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman R. P., Clyde W. A., Jr The interrelationship of virulence, cytadsorption, and peroxide formation in Mycoplasma pneumoniae. Proc Soc Exp Biol Med. 1969 Sep;131(4):1163–1167. doi: 10.3181/00379727-131-34061. [DOI] [PubMed] [Google Scholar]
- Lott I. T. Down's syndrome, aging, and Alzheimer's disease: a clinical review. Ann N Y Acad Sci. 1982;396:15–27. doi: 10.1111/j.1749-6632.1982.tb26840.x. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Miura T., Ogiso T. Lipid peroxidation of erythrocyte membrane induced by xanthine oxidase system: modification of superoxide dismutase effect by hemoglobin. Chem Pharm Bull (Tokyo) 1982 Oct;30(10):3662–3668. doi: 10.1248/cpb.30.3662. [DOI] [PubMed] [Google Scholar]
- Sichitiu S., Sinet P. M., Lejeune J., Frézal J. Surdosage de la forme dimérique de l'indophénoloxydase dans la trisomie 21, secondaire au surdosage génique. Humangenetik. 1974 Jun 26;23(1):65–72. doi: 10.1007/BF00295684. [DOI] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Sinet P. M., Allard D., Lejeune J., Jérôme H. Augmentation d'activité de la superoxyde dismutase érythrocytaire dans la trisomie pour le chromosome 21. C R Acad Sci Hebd Seances Acad Sci D. 1974 Jun 17;278(25):3267–3270. [PubMed] [Google Scholar]
- Sinet P. M., Garber P. Inactivation of the human CuZn superoxide dismutase during exposure to O-2 and H2O2. Arch Biochem Biophys. 1981 Dec;212(2):411–416. doi: 10.1016/0003-9861(81)90382-9. [DOI] [PubMed] [Google Scholar]
- Sobeslavsky O., Chanock R. M. Peroxide formation by mycoplasmas which infect man. Proc Soc Exp Biol Med. 1968 Nov;129(2):531–535. doi: 10.3181/00379727-129-33362. [DOI] [PubMed] [Google Scholar]
- Somerson N. L., Walls B. E., Chanock R. M. Hemolysin of Mycoplasma pneumoniae: tentative identification as a peroxide. Science. 1965 Oct 8;150(3693):226–228. doi: 10.1126/science.150.3693.226. [DOI] [PubMed] [Google Scholar]
- Symonyan M. A., Nalbandyan R. M. Interaction of hydrogen peroxide with superoxide dismutase from erythrocytes. FEBS Lett. 1972 Nov 15;28(1):22–24. doi: 10.1016/0014-5793(72)80667-7. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Tischfield J., Ruddle F. H. The linkage of genes for the human interferon-induced antiviral protein and indophenol oxidase-B traits to chromosome G-21. J Exp Med. 1973 Feb 1;137(2):317–330. doi: 10.1084/jem.137.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keuren M. L., Goldman D., Merril C. R. Protein variations associated with Down's syndrome, chromosome 21, and Alzheimer's disease. Ann N Y Acad Sci. 1982;396:55–67. doi: 10.1111/j.1749-6632.1982.tb26843.x. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. The role of superoxide in the destruction of erythrocyte targets by human neutrophils. J Biol Chem. 1980 Oct 25;255(20):9912–9917. [PubMed] [Google Scholar]
- Wiesel J. M., Gamiel H., Vlodavsky I., Gay I., Ben-Bassat H. Cell attachment, growth characteristics and surface morphology of human upper-respiratory tract epithelium cultured on extracellular matrix. Eur J Clin Invest. 1983 Feb;13(1):57–63. doi: 10.1111/j.1365-2362.1983.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Yatziv S., Flowers H. M. Action of -galactosidase on glycoprotein from human B-erythrocytes. Biochem Biophys Res Commun. 1971 Oct 15;45(2):514–518. doi: 10.1016/0006-291x(71)90849-7. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Flohé L., Weser U., Hartmann H. J. Inhibition of lipid peroxidation in isolated inner membrane of rat liver mitochondria by superoxide dismutase. FEBS Lett. 1973 Jan 15;29(2):117–120. doi: 10.1016/0014-5793(73)80539-3. [DOI] [PubMed] [Google Scholar]


