Abstract
AIM: To understand the role and significance of side population (SP) cells from hepatocellular carcinoma (HCC) in hepatocarcinogenesis, development, relapse and metastasis, we simulated the denutrition conditions that cancer cells experience in clinical therapy, observed the different anti-apoptosis ability of SP cells and non-SP cells under such conditions, and established the possible effects of P53, Bcl-2 and Bax on survival of SP cells.
METHODS: We used flow cytometry to analyze and sort the SP and non-SP cells in established HCC lines MHCC97 and hHCC. We evaluated cell proliferation by methyl thiazolyl tetrazolium (MTT) assay and investigated the expression of p53, bcl-2 and bax genes during denutrition, by RT-PCR and immunofluorescence staining.
RESULTS: The percentage of SP cells in the two established HCC lines was 0.25% and 0.5%, respectively. SP cells had greater anti-apoptosis and proliferation ability than non-SP cells. Expression of Bcl-2 and Bax in SP and non-SP cells differed during denutrition. The former was up-regulated in SP cells, and the latter was up-regulated in non-SP cells.
CONCLUSION: It may be that different upstream molecules acted and led to different expression levels of Bcl-2 and Bax in these two cell lines. There was a direct relationship between up-regulation of Bcl-2 and down-regulation of Bax and higher anti-apoptosis ability in SP cells. It may be that the existence and activity of SP cells are partly responsible for some of the clinical phenomena which are seen in HCC, such as relapse or metastasis. Further research on SP cells may have potential applications in the field of anticancer therapy.
Keywords: Side population, Hepatocellular carcinoma, Bax, Bcl-2, Apoptosis
INTRODUCTION
It is believed that cancer is unicellular in origin[1], although cancer cells from a lot of tumors generally exhibit functional heterogeneity in experimental and clinical settings[2]. There are two theories[3,4]. One is the stochastic model, which figures that cancer is composed of a comparatively homogeneous population; only a few cells undergo stochastic events, so that they have the potential to proliferate extensively and form new tumors. The other is the hierarchy model, which suggests that there is some kind of pyramid scale in cancer cells. In this model, the subpopulation cells, which are on acme in the pyramid scale, comprise cancer stem cells (CSCs) that self-renew, generate downstream descendants, and initiate new tumors.
Recently, the latter hypothesis has gained significant recognition. The possible existence of CSCs has been shown in leukemia and some solid tumors, including breast cancer and brain tumors[5-9]. These cells are detected by their own ability to efflux Hoechst 33 342 dye through an ATP-binding cassette (ABC) membrane transporter. They are also named side population (SP) cells for their location on flow cytometry charts.
Hepatocellular carcinoma (HCC) is a common malignancy and still has a high mortality rate[10]. Clinical operations and chemotherapy can lead most such cancer cells to death or proliferation inhibition through denutrition. However, there are always some cells that can survive and result in relapse or metastasis, which often leads to therapeutic failure and poor prognosis. The CSC hypothesis offers an explanation for these clinical phenomena. In some studies, it has been found that SP cells are easier to initiate tumors than non-SP cells are in non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice[11]. Under conditions of denutrition that are similar to those after clinical treatment, do SP cells survive more easily and have higher anti-apoptosis ability than non-SP cells?
p53, the most frequently mutated gene in human malignancies, is found inactivated in about 50% of tumors in any location and of any histological type. It has been named the “guardian of the genome”[12]. bcl-2 was the first example of an oncogene that inhibits cell death rather than promotes proliferation. According to differences in structure, the Bcl-2 family can be divided into two subgroups. Bax belongs to one subfamily that can oligomerize and integrate into the outer mitochondrial membrane to initiate events during apoptosis. Normally, Bcl-2 inhibits Bax activation. But, when cells are under stress (either oncogenic or genotoxic stress), Bcl-2 is inactivated by P53 as its downstream molecule. As a consequence, apoptotic cell death continues[13,14]. Under denutrition conditions, how are these three genes expressed in SP cells? What is their relationship with the anti-apoptosis ability of SP cells?
Taking clinical and experimental data together, we consider that the understanding of biologic characteristics of SP cells from HCC cells may serve to elucidate the mechanism of hepatocarcinogenesis and lead to novel therapeutic approaches.
In this study we therefore analyzed and sorted SP cells from established HCC cell lines. We observed the anti-apoptosis ability of two cell lines under denutrition conditions by methyl thiazolyl tetrazolium (MTT) assay. We further estimated the expression level of P53, Bcl-2 and Bax in SP cells and non-SP cells by RT-PCR and immunofluorescence during denutrition.
MATERIALS AND METHODS
Cell culture
The human liver cancer cell line MHCC97 was obtained from the Institute of Biochemistry and Cell Biology (Shanghai, China), the hHCC cell line was from the Department of Biochemistry and Molecular Biology of Fourth Military Medical University (Xi’an, China). These cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen Life Technologies, Carlsbad, CA, USA) containing 10% fetal calf serum (FCS) and 1% penicillin/streptomycin (Invitrogen), and incubated at 37°C in an atmosphere containing 5% CO2.
SP cell analysis and purification using flow cytometry
Cells were detached from the dishes with trypsin-EDTA (Invitrogen) and suspended at 1 × 106 cells/mL in Hank’s balanced salt solution (HBSS) supplemented with 3% fetal calf serum and 10 mmol/L HEPES. These cells were then incubated at 37°C for 90 min with 20 μg/mL Hoechst 33 342 (Sigma, St Louis, MO, USA), either alone or in the presence of 50 μmol/L verapamil (Sigma), which is an inhibitor of verapamil-sensitive ABC transporter. After 90 min incubation, the cells were centrifuged immediately for 5 min at 300 × g, 4°C and resuspended in ice-cold HBSS. The cells were kept on ice to inhibit efflux of Hoechst dye. Then, 1 μg/mL propidium iodide (PI; BD Pharmingen, San Diego, CA, USA) was added to discriminate dead cells. Finally, these cells were filtered through a 40-μm cell strainer (BD Falcon; BD Pharmingen, San Diego, CA, USA) to obtain single suspension cells. Cell dual-wavelength analysis and purification were performed using dual-laser cytometry (FACSVantage; BD Biosciences, Franklin Lakes, NJ. Hoechst 33 342 solution was excited at 355 nm UV light; blue fluorescence was collected with a 450/20 band-pass (BP) filter and red fluorescence with a 675-nm edge filter long-pass (EFLP). A 610-nm dichroic mirror short-pass was used to separate the emission wavelengths. PI-positive dead cells were excluded from the analysis.
MTT assay
All cells were maintained in PBS for 3, 6 and 9 h to induce denutrition. Then, cell proliferation was evaluated by MTT assay. MTT solution in PBS was added to a final concentration of 0.5 mg/mL, and cells incubated for 4 h at 37°C. Supernatant was removed and cells were resuspended in 150 μL DMSO for 10 min, and absorbance was measured at 490 nm using a microplate reader (Bio-rad, Japan).
Semi-quantitative RT-PCR
After all cells were induced in PBS for their respective number of hours, total RNA was extracted from SP and non-SP cells using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions, and was reverse-transcribed by using a First-Strand cDNA Synthesis Kit (Fermentas, Lithuania), as described in the instructions. RT-PCR was carried out in a 50 μL reaction mixture that contained 1 μL cDNA as template, 1 μmol/L specific oligonucleotide primer pair, and 25 μL Taq mixture that contained 0.5 U Taq DNA polymerase (Tangen, Beijing, China). Cycle parameters for p53, bcl-2, bax and glyceraldehyde-3-phosphate dehydrogenase (G3PDH) cDNAs were 15 s at 95°C, 30 s at 55°C, and 60 s at 72°C for 33, 32, 30 and 20 cycles, respectively. Primer sequences were as follows: p53, 5'-GTTTCCGTCTGGGCTTCTTG-3' and 5'-CCTGGGCATCCTTGAGTTCC-3'; bcl-2, 5'-ACACTGTTAAGCATGTGCCG-3' and 5'-CCAGCTCATCTCACCTCACA-3'; bax, 5'-GGATGCGTCCACCAAGAA-3' and 5'-ACTCCCGCCACAAAGATG-3'; and G3PDH, 5'-ACCACAGTCCATGCCATCAC-3' and 5'-TCCACCACCCTGTTGCTGTA-3'.
Immunofluorescence staining
Cells were cultured on coverslips and received the same treatment of induced denutrition in PBS as described above. Cells were fixed in methanol at -20°C for 20 min and washed in PBS containing 0.1% Tween 20 (Sanland Chemicals, Xiamen, China). After blocking with 10% goat normal serum for 1 h, fixed cells were incubated with primary antibodies, rabbit anti-Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse anti-Bax (Santa Cruz Biotechnology), in a moist chamber at 4°C overnight. Cells were washed in PBS containing 0.1% Tween 20, blocked again for 30 min, and treated with TRITC (Tetramethyl Rhodamine Isothiocyanate)-conjugated goat anti-rabbit IgG (Santa Cruz) and FITC (Fluorescein Isothiocyanate)-conjugated goat anti-mouse IgG (Santa Cruz) at 37°C for 1 h. After washing in PBS, the coverslips were covered on slips with 30% glycerol phosphate buffer and examined under an Olympus IX70 microscope (Olympus, Japan).
Statistical analysis
Bands from RT-PCR were quantified by Smart View Bio-electrophoresis Image Analysis System software (Furi Science & Technology, Shanghai, China). Relative mRNA levels were calculated by referring them to the amount of G3PDH. Numerical data from the MTT assay were presented as the mean ± SEM. The difference between means was measured with Student's t test. All statistical analyses were performed using SPSS11.0 software (Chicago, IL, USA). P < 0.05 was considered as statistically significant.
RESULTS
Detection of SPs in HCC cells
Flow cytometry analysis with Hoechst 33 342 staining demonstrated that MHCC97 and hHCC cells included 0.25% and 0.5% SP cells, respectively. The number of these SP cells was diminished in the presence of Hoechst 33 342 and verapamil, a calcium channel blocker. The SP and non-SP cells in MHCC97 and hHCC cells were sorted separately and used for further experiments (Figure 1).
Figure 1.
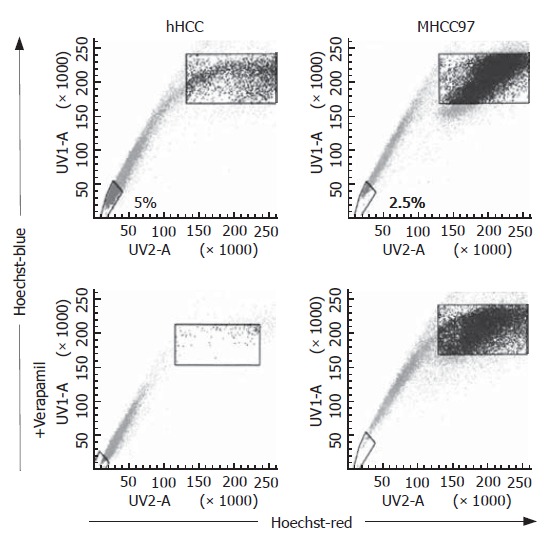
SP cells were detected in 0.25 and 0.5% of MHCC97 and hHCC cells, respectively. The SP cells disappeared with Hoechst 33 342 and verapamil co-treatment.
MTT assay
MTT assay was used to determine the proliferation of SP and non-SP cells under denutrition conditions. The results illustrated that denutrition inhibited proliferation of SP and non-SP cells in both cell lines in a time-dependent manner. The data showed that there was a difference between SP and non-SP cells at 6 and 9 h, and it seemed that SP cells had a greater proliferation ability than non-SP cells under denutrition (Figure 2).
Figure 2.
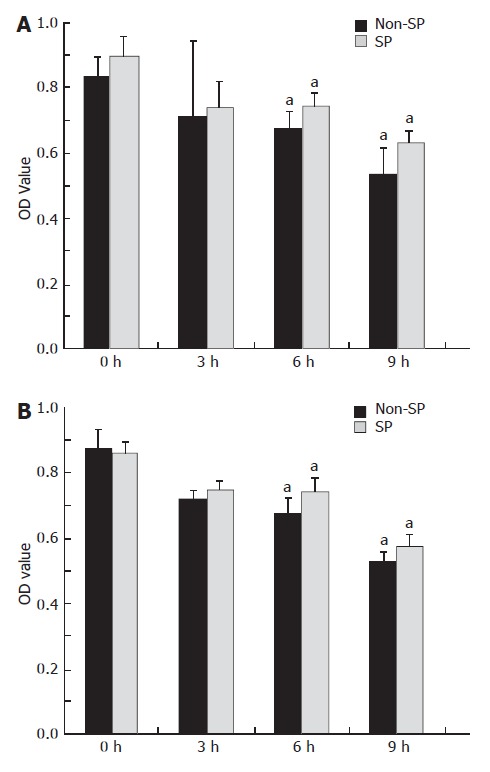
Proliferation of MHCC97 and hHCC cells at 3, 6 and 9 h during denutrition, as observed by MTT assay. SP cells purified from MHCC97 (A) and hHCC (B) cell lines demonstrated greater viability than the corresponding non-SP cells. aP < 0.05.
RT-PCR
RT-PCR was used to detect mRNA expression levels in SP and non-SP cells in two cancer cell lines. The p53 gene was expressed weakly but steadily during the whole experiment. The bcl-2 gene was up-regulated, while the bax gene was down-regulated in SP cells under denutrition. Interestingly, the regulation of the two genes was reversed in non-SP cells. It may be that the upstream molecule which adjusted these two genes’ expression was not P53 (Figure 3).
Figure 3.
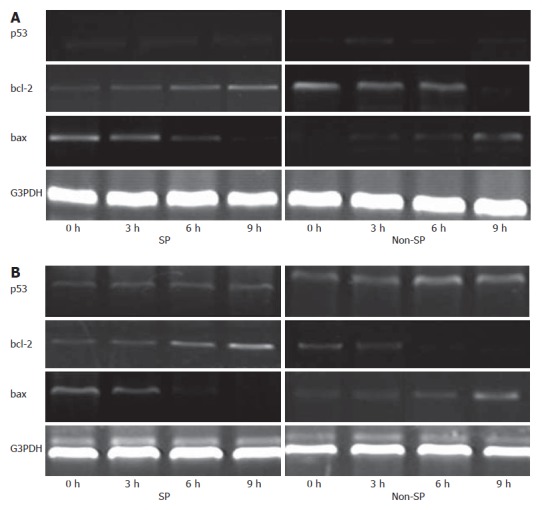
p53, bcl-2 and bax mRNA levels were evaluated by RT-PCR. bcl-2 levels were up-regulated in SP cells from MHCC97 (A) and hHCC (B) cell lines, and bax levels were down-regulated in non-SP cells during denutrition.
Immunofluorescence staining
Expression levels of Bcl-2 and Bax proteins were examined by immunofluorescence staining in hHCC and MHCC97 cell lines (Figure 4). It was clear that the expression of Bcl-2 increased in a time-dependent manner in SP cells, in contrast with non-SP cells. Conversely, the expression of Bax increased in non-SP cells in the same manner and decreased in SP cells.
Figure 4.
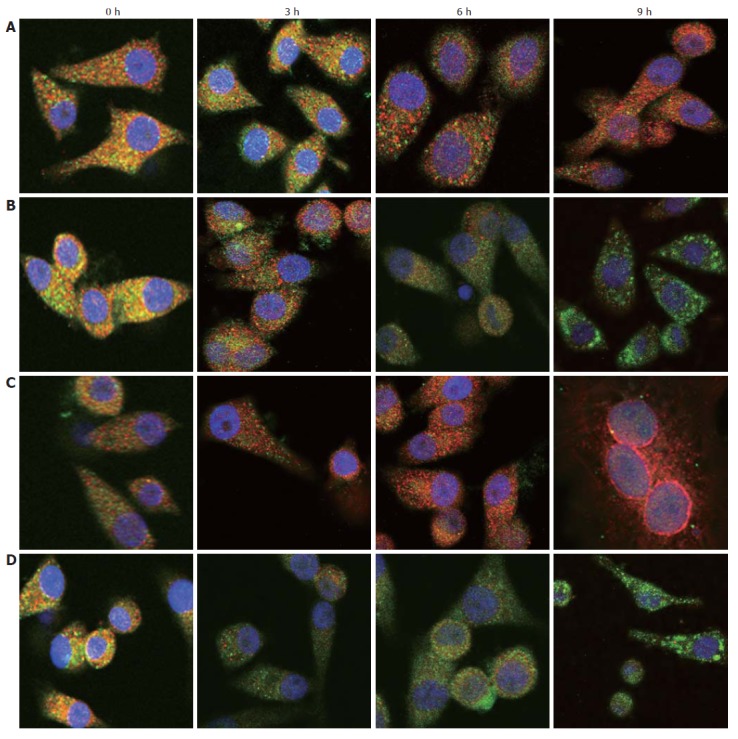
Bcl-2 (red) and Bax (green) expression was examined in SP (A) and non-SP (B) cells of MHCC97, and SP (C) and non-SP (D) cells of hHCC cells. The expression of Bcl-2 in SP cells was up-regulated, while Bax was down-regulated.
DISCUSSION
HCC ranks among the most common cancers in many countries. A recent estimate indicates that HCC represents the fifth most common cancer in males, and the eighth most common in females, with a total of 560 000 new cases each year, 83% of which occur in developing countries, and more than one-half in China alone. Moreover, because of its very poor prognosis, HCC represents the third leading cause of cancer death worldwide[10].
From the clinical standpoint, there are some conspicuous biological characteristics in HCC, such as anti-apoptosis, chemotherapy resistance, extensive proliferation, and even early metastasis. These characteristics are particularly prevalent in cases of relapse and metastasis.
Recently, it has been reported that CSCs are seen in many kinds of tumors and established cancer cell lines[15-18]. Using the previously described methods, we analyzed and sorted some of these CSCs, namely SP cells, in established HCC cell lines. The proportion of SP cells in the two cell lines was 0.25% and 0.5%, respectively. The MHCC97 (Metastatic Human Hepatocellular cancer 97) cell line was established from a highly metastatic case of HCC in 1997, and the hHCC (human Hepatocellular cancer) cell line was cultured from a case with a high level of chemotherapy resistance, with the proportion of SP cells being a little higher than those reported previously.
In clinic, whatever surgery or chemotherapy, it will bring denutrition directly or indirectly so as to inhibit the proliferation of cancer cells, beside destroy the structure of tumors or kill them. In clinics, surgery, radiotherapy and chemotherapy are used to destroy the structure of tumors, induce denutrition, kill cancer cells directly, and inhibit cancer cell proliferation. However, there are still some cells that can survive in denutrition conditions, and these may lead to relapse and metastasis. What difference is there between these and other cells? What mechanism is behind these phenomena? In our experiment, we simulated the denutrition conditions and observed the anti-apoptosis or proliferation ability of SP cells.
By MTT assay, we found that SP cells had better resistance to denutrition than non-SP cells. Using RT-PCR and immunofluorescence staining, we found that P53 may not be the key molecule that is responsible for the anti-apoptosis ability of SP cells. p53 was one of the most important genes in stabilizing the cell genome. It regulated the expression of numerous pro-apoptotic genes, such as bcl-2 and bax. Our results showed that the normal activity of P53 in the two cell lines may have been inhibited. The expression levels of two members of the Bcl-2 family were clearly altered between SP and non-SP cells; specifically, the expression of Bax was inhibited in SP and activated in non-SP cells, but the expression of Bcl-2 was reversed. Bax was a cytosolic monomer in viable cells but during apoptosis, it changed its conformation, integrated into the outer mitochondrial membrane, and was oligomerized. It provoked the permeabilization of the outer mitochondrial membrane (PT) and contributed to the release of pro-apoptotic factors into the cytosol, such as cytochrome C, which led to formation of the apoptosome and activation of the caspase cascade. However, the anti-apoptotic guardian Bcl-2 could bind Bax strongly, and this interaction inhibited Bcl-2 activation, which sustained cell survival. In our study, the up-regulation of Bcl-2 and down-regulation of Bax were effective during the anti-apoptosis in SP cells. In other words, in MHCC97 and hHCC cell lines, SP cells had greater anti-apoptosis or proliferation ability than non-SP cells had. Expression of Bcl-2 and Bax had a pivotal role in the anti-apoptosis procedure during denutrition.
We have previously found that the expression level of alpha-fetoprotein (AFP) in SP cells is significantly higher than in non-SP cells in established HCC cell lines, e.g. MHCC97[19]. AFP is one of the most useful markers, and has been used in clinical diagnosis of HCC. AFP is synthesized in large quantities by the fetal yolk sac and the liver during embryonic development[20,21]. According to clinical experience, if a high level of AFP is found in the serum, the first thought is that the patient has HCC. If this appears after surgery or chemotherapy, it indicates a poor prognosis, such as recurrence or metastasis[22-27]. The ABC transporter for discharging Hoechst 33 342, which is called the breast cancer resistance protein, has a high efflux capacity with a wide substrate range, including mitoxantrone and methotrexate[28]. Further, the higher expression level of ABC transporter on SP cells indicates a possible relationship between them and clinical chemotherapy resistance.
Taking the experimental results and clinical experiences together, we found that the characteristics displayed in SP cells, such as high expression level of AFP and Bcl-2 and drug efflux capacity, are consistent with the characteristics displayed in tumors, such as high expression level of AFP, high anti-apoptosis ability and chemotherapy resistance. We conclude that perhaps the existence and activity of SP cells are responsible for these clinical phenomena shown in HCC; moreover, it is reasonable to recognize SP cells as CSCs.
ACKNOWLEDGMENTS
We thank Mr. Shi-Liang Ma from Peking University Health Science Center for his patience and dedication during our flow cytometry analysis. We also thank Professor Qing-Chuan Zhao, Professor Zhen-Shun Song and Professor Yong Chen from the Department of Hepatobiliary Surgery for their helpful discussion during the preparation of this paper.
COMMENTS
Background
The theory of CSCs is one of the most significant theory in tumor research. According to the theory, there is some kind of pyramid cell structure in tumor cells, and CSCs are at its apex. They have self-renewal and differentiation abilities, and other cells are their descendents. CSCs have many biological activities, such as anti-drug and greater ability to proliferate. CSC theory also explains some clinic phenomena such as chemotherapy resistance and relapse. Therefore, research on the characteristics of CSCs may be the foundation of future clinical therapy.
Research frontiers
In 1996, Goodell sorted a certain kind of cells from mouse whole bone cells by cytometry, and he found that those cells expressed different biological characteristics, such as higher proliferation and multi-differentiation. Many workers have reported the existence of such cells in different tissues, and they have been collectively named SP cells. SP cells from tumors or tumor cell lines have greater proliferation and tumor formation ability, they resist drugs, and they maintain themselves in whole cells at a rate of a few percent.
Innovations and breakthroughs
The observation of SP cells backs up the theory of CSCs, which was introduced many years ago. Using Hoechst 33 342 dye, it is possible to sort the cells by cytometry. SP cells have been found in HCC, brain tumor, prostate cancer and leukemia. By analyzing their biological abilities, it seemed that side population cells existed between the tip and bottom of the pyramid cell structure, and that they had intersection with real CSCs. Thus, though we can not be certain that SP cells are CSCs, it seems that that SP cells have a typical CSC phenotype.
Applications
In clinical cases, there have heretofore been many failures caused by tumor chemotherapy resistance or metastisis. CSC theory and the discovery of SP cells bring new hope for tumor therapy in the future. How to locate these cells in tumors, how to remove them completely by surgery, and how to inhibit or kill them by chemotherapy, are just a few of the clinical questions that need to be answered. Even a small advancement in this research field may result in a new breakthrough in tumor therapy.
Terminology
SP cells were first discovered by Goodell when he analyzed mouse bone cells by cytometry using Hoechst 33 342. They were named after their location in 2-D cytometry charts. They display low fluorescence, and are located at the edge of the chart, away from other cells.
Peer review
SP cells from tumors or tumor cell lines are one of the hottest topics in tumor research. This study confirmed their existence and numbers in two human HCC cell lines.
Footnotes
S- Editor Liu Y L- Editor Kerr C E- Editor Li HY
References
- 1.Fialkow PJ, Gartler SM, Yoshida A. Clonal origin of chronic myelocytic leukemia in man. Proc Natl Acad Sci USA. 1967;58:1468–1471. doi: 10.1073/pnas.58.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heppner GH. Tumor heterogeneity. Cancer Res. 1984;44:2259–2265. [PubMed] [Google Scholar]
- 3.Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Warner JK, Wang JC, Hope KJ, Jin L, Dick JE. Concepts of human leukemic development. Oncogene. 2004;23:7164–7177. doi: 10.1038/sj.onc.1207933. [DOI] [PubMed] [Google Scholar]
- 5.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 10.Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 11.Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, Nakauchi H, Taniguchi H. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240–251. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 12.Royds JA, Iacopetta B. p53 and disease: when the guardian angel fails. Cell Death Differ. 2006;13:1017–1026. doi: 10.1038/sj.cdd.4401913. [DOI] [PubMed] [Google Scholar]
- 13.Zinkel S, Gross A, Yang E. BCL2 family in DNA damage and cell cycle control. Cell Death Differ. 2006;13:1351–1359. doi: 10.1038/sj.cdd.4401987. [DOI] [PubMed] [Google Scholar]
- 14.Walensky LD. BCL-2 in the crosshairs: tipping the balance of life and death. Cell Death Differ. 2006;13:1339–1350. doi: 10.1038/sj.cdd.4401992. [DOI] [PubMed] [Google Scholar]
- 15.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, Maclaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci USA. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 17.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 18.Hirschmann-Jax C, Foster AE, Wulf GG, Goodell MA, Brenner MK. A distinct "side population" of cells in human tumor cells: implications for tumor biology and therapy. Cell Cycle. 2005;4:203–205. [PubMed] [Google Scholar]
- 19.Zhang N, Dou KF, Li R, Fan J, Zhang FQ. Differential expression of AFP in subpopulations of MHCC97 cell lines. Disi Junyi daxue Xuebao. 2006;27:1799–1801. [Google Scholar]
- 20.Li D, Mallory T, Satomura S. AFP-L3: a new generation of tumor marker for hepatocellular carcinoma. Clin Chim Acta. 2001;313:15–19. doi: 10.1016/s0009-8981(01)00644-1. [DOI] [PubMed] [Google Scholar]
- 21.Tilghman SM, Belayew A. Transcriptional control of the murine albumin/alpha-fetoprotein locus during development. Proc Natl Acad Sci USA. 1982;79:5254–5257. doi: 10.1073/pnas.79.17.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oyunsuren T, Sanduijav R, Davaadorj D, Nansalmaa D. Hepatocellular carcinoma and its early detection by AFP testing in Mongolia. Asian Pac J Cancer Prev. 2006;7:460–462. [PubMed] [Google Scholar]
- 23.Kamiyama T, Takahashi M, Nakagawa T, Nakanishi K, Kamachi H, Suzuki T, Shimamura T, Taniguchi M, Ozaki M, Matsushita M, et al. AFP mRNA detected in bone marrow by real-time quantitative RT-PCR analysis predicts survival and recurrence after curative hepatectomy for hepatocellular carcinoma. Ann Surg. 2006;244:451–463. doi: 10.1097/01.sla.0000234840.74526.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao F, Guo JM, Xu CF, Lou YL, Xiao BX, Zhou WH, Chen J, Hu YR, Liu Z, Hong GF. Detecting AFP mRNA in peripheral blood of the patients with hepatocellular carcinoma, liver cirrhosis and hepatitis. Clin Chim Acta. 2005;361:119–127. doi: 10.1016/j.cccn.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Liu X, Zhou S, Li P, Li G. Effects of alpha fetoprotein on escape of Bel 7402 cells from attack of lymphocytes. BMC Cancer. 2005;5:96. doi: 10.1186/1471-2407-5-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding X, Yang LY, Huang GW, Yang JQ, Liu HL, Wang W, Peng JX, Yang JQ, Tao YM, Chang ZG, et al. Role of AFP mRNA expression in peripheral blood as a predictor for postsurgical recurrence of hepatocellular carcinoma: a systematic review and meta-analysis. World J Gastroenterol. 2005;11:2656–2661. doi: 10.3748/wjg.v11.i17.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross-Goupil M, Saffroy R, Azoulay D, Precetti S, Emile JF, Delvart V, Tindilière F, Laurent A, Bellin MF, Bismuth H, et al. Real-time quantification of AFP mRNA to assess hematogenous dissemination after transarterial chemoembolization of hepatocellular carcinoma. Ann Surg. 2003;238:241–248. doi: 10.1097/01.sla.0000080959.95226.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staud F, Pavek P. Breast cancer resistance protein (BCRP/ABCG2) Int J Biochem Cell Biol. 2005;37:720–725. doi: 10.1016/j.biocel.2004.11.004. [DOI] [PubMed] [Google Scholar]


