Abstract
AIM: To investigate the correlation between C/T single nucleotide polymorphism (SNP) in the promoter of the DNA methyltransferase 3B (DNMT3B) gene and risk for development and progression of primary hepatocellular carcinoma (HCC).
METHODS: One hundred case subjects were selected consecutively from Tongji Hospital (Wuhan, China). from March to November 2006. They did not receive radiotherapy or chemotherapy for newly diagnosed and histopathologically confirmed HCC. One hundred and forty control subjects having no history of cancerous or genetic diseases were healthy volunteers to Wuhan Blood Center in the same period. Frequency was matched for sex, age, alcohol consumption and cigarette smoking status of the case subjects. C/T polymorphism of the DNMT3B promoter was analyzed using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) and sequencing analysis. The association between genotypes of DNMT3B and clinicopathological parameters among cases was also studied.
RESULTS: The CC genotype was not detected in both HCC patients and controls. In control subjects, the frequency of TT and CT genotypes was 99.3% and 0.7% respectively, and that of T and C alleles was 99.6% and 0.4% respectively. The frequency of CT genotype was higher in HCC (3.0%). The frequency of T and C alleles was 98.5% and 1.5% respectively. However, the genotype and allelotype distribution in HCC patients was not significantly different from that in controls.
CONCLUSION: C/T polymorphism is not associated with the increased risk of HCC. DNMT3B genetic polymorphism is variable in different races, ethnic groups or geographic areas. Further study is needed to clarify the role of DNMT3B SNP in the development of HCC among other populations.
Keywords: DNA methyltransferase, Single nucleotide polymorphism, Susceptibility, Primary hepatocellular carcinoma
INTRODUCTION
Primary hepatocellular carcinoma (HCC) occurs frequently in Southeast Asia, especially in China. It ranks second and approximately accounts for 42.5% of all malignancies worldwide[1].
Although hepatitis B virus (HBV) is the major cause of HCC, only a fraction of patients with chronic HBV infection develop HCC during their lifetime, suggesting that genetic and epigenetic factors are important in determining individuals’ susceptibility to HCC.
DNA methylation plays an important role in chromatin structure stability, genome integrity, modulation of tissue-specific gene expression, embryonic development, genomic imprinting, X-chromosome inactivation and is essential for the development of mammals[2,3]. DNA methylation is mediated by DNA methyltransferases (DNMTs), of which three active forms have been identified: DNMT1, DNMT3A and DNMT3B. DNMT1 is thought to be primarily responsible for maintaining pre-existing methylation patterns after DNA replication because of its preference to hemimethylated DNA substrates and targeting replication foci. DNMT3A and DNMT3B have an equal preference to hemimethylated and unmethylated DNA substrates, and therefore they are believed to be principally required for de novo methylation[4-6]. Recent studies have shown that three different mechanisms play a role in the effect of methylation: global hypomethylation, hypermethylation of individual gene segments and deregulated expression of DNA methyltransferases. Changes in the methylation pattern correlate with the development of cancer. De novo hypermethylation in promoter CpG islands has been identified as a possible mechanism for tumor suppressor gene and DNA repair gene inactivation of in human cancer cells[7-12]. DNMT3B, regarded as a de novo DNA methyltransferase, is thought to play an important role in the generation of aberrant methylation in carcinogenesis[13,14].
DNMT genes are up-regulated in various human cancers, including HCC[15-20]. Significant over-expression of DNMT3B is observed in tumor tissues while over-expression of DNMT1 and DNMT3A is more modest[15,18,20].
A C-to-T transition polymorphism (C46359T, GenBank accession no. AL035071) in the promoter region of the DNMT3B gene, -149 base pairs from the transcription start site, is reported to greatly increase promoter activity. Many reports have shown that the C/T polymorphism is associated with an increased risk for lung cancer and decreased postsurgical survival in patients with small cell carcinoma of the head and neck. Carriers of T allele, particularly heterozygotes, have a significantly increased risk for such cancers[21-24].
Several polymorphic genes are reported to be correlated with modification of susceptibility to HCC. To our knowledge, the association between DNMT3B polymorphism and development of HCC has not been reported. Since the DNMT3B promoter polymorphism that is responsible for regulating genomic methylation is possibly associated with an increased risk for cancers, we evaluated the relationship between DNMT3B C46359T polymorphism and risk of HCC in a hospitalization-based case-control study in a Chinese Han nationality population.
MATERIALS AND METHODS
Subjects
One hundred case subjects were selected consecutively from Tongji Hospital (Wuhan, China) from March to November 2006. They did not receive radiotherapy or chemotherapy for newly diagnosed and histopathologically confirmed HCC. One hundred and forty control subjects having no history of cancerous or genetic diseases were healthy volunteers to Wuhan Blood Center in the same period. Frequency was matched for sex, age, alcohol consumption and cigarette smoking status of the case subjects. All the cancer patients and control subjects were unrelated Han nationality individuals from Wuhan or from its surrounding regions. Blood was taken from all recruits who consented to the epidemiology survey. Each subject was scheduled for an interview after informed consent was obtained, and a structured questionnaire was used to collect information on demographic data and risk factors, such as hepatitis B infection history and family history of any cancers.
DNMT3B genotyping
Genomic DNA was extracted from peripheral blood lymphocytes by proteinase K digestion and phenol-chloroform extraction. DNMT3B C/T polymorphism was determined by PCR-RFLP. The primers of 5'-TGCTGTGACAGGCAGAGCAG-3' (nt 46151-46170) and 5'-GGTAGCCGGGAACTCCACGG-3' (nt 46530-46511) were synthesized as previously described[21]. This 380-bp target DNA fragment contains the upstream region and the first exon of the DNMT3B gene. Amplification reaction was carried out in a 25 μL PCR mixture containing 50-200 ng of genomic DNA, 12.5 pmol of each primer (Shanghai Sangon Company), 0.1 mmol/L each deoxynucleotide triphosphate, 1 × PCR buffer (50 mmol/L KCl, 10 mmol/L Tris-HCl, and 0.1% Triton X-100), 2.0 mmol/L MgCl2, and 1.25 U Taq polymerase (Beijing Sbsbio Company). The PCR profile consisted of an initial denaturation at 95°C for 5 min, 35 cycles of denaturation at 95°C for 30 s, annealing at 65°C for 30 s and extension at 72°C for 30 s and a final extension at 72°C for 10 min. The 380-bp product was then digested with BlnI (TaKaRa Biotechnology Company) for 12-16 h in 37°C bath water. The digested product was separated by vertical electrophoresis at 200V of constant voltage for 1.5 h through 8% polyacrylamide gel (29:1) and silver staining, and then determined under ultraviolet irradiation. The variant T allele has a BlnI restriction site that results in two bands (207 bp and 173 bp), and the wild-type C allele lacks the BlnI restriction site, thus producing a single 380-bp band (Figure 1). More than 10% of the samples were randomly selected for repeated assays, and the results were 100% concordant. Restriction fragment length polymorphism (RFLP) analysis was confirmed by PCR-based sequencing with an Applied Biosystem automated sequencer (Figure 2).
Figure 1.
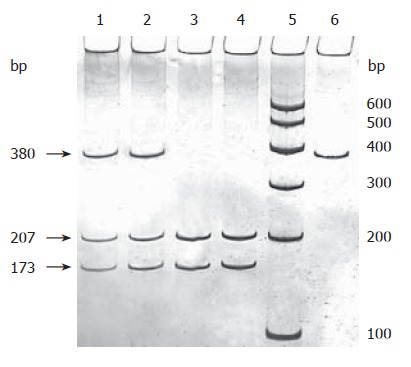
PCR-RFLP-polyacrylamide gel electrophoresis for genotyping of DNMT3B promoter C46359T. Lanes 1 and 2: CT heterozygote; lanes 3 and 4: TT genotype; lane 5: 100 bp molecular marker; lane 6: PCR product.
Figure 2.
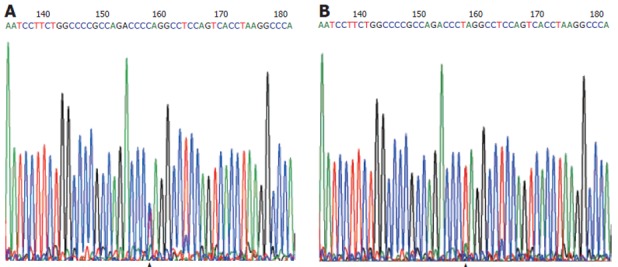
CT genotype (A) and TT genotype (B) Sequencing-confirmed.
Statistical analysis
Data were presented as mean ± SD. A database was developed by Epi Info 6.0 and the analysis of data was accomplished using SPSS 10.0 software. The difference in frequency distributions of genotypes and allelotypes between the two groups was detected by chi-square test. P values of less than 0.05 were considered statistically significant.
RESULTS
The characteristics of 100 HCC case patients and 140 control subjects are summarized in Table 1. In the case group, the age ranged 18-70 years, the mean age was (55.86 ± 10.12) years, and the gender ratio was 4:1 while the mean time of alcohol consumption and cigarette smoking was (10.01 ± 5.21) years and (11.26 ± 6.25) years, respectively. In the control group, the age ranged was 15-68 years, the mean age was (57.12 ± 10.01) years, and the gender ratio was 4.4:1 while the mean time of alcohol consumption and cigarette smoking was (13.58 ± 5.09) years and (14.89 ± 7.56) years, respectively. The two groups had a similar frequency of distribution in age, sex, alcohol consumption and cigarette smoking status. In addition, 95% of the case patients had a chronic infection with HBV but others had no infection with any hepatitis virus.
Table 1.
Frequency distributions of selected variables in HCC patients and control subjects, n (%)
| Variable | Case (n = 100) | Control (n = 140) |
| Sex | ||
| Male | 80 (80) | 114 (81.4) |
| Female | 20 (20) | 26 (18.6) |
| Age (yr) | 55.86 ± 10.12 | 57.12 ± 10.01 |
| Smoking status | ||
| Current smoker | 60 (60.0) | 92 (65.7) |
| Former smoker | 21 (21.0) | 26 (18.6) |
| Non smoker | 19 (19.0) | 22 (15.7) |
| Pack/year | 11.26 ± 6.25 | 14.89 ± 7.56 |
| Alcohol consumption | ||
| Current drinker | 55 (55.0) | 70 (50.0) |
| Former drinker | 19 (19.0) | 29 (20.7) |
| Non drinker | 26 (26.0) | 41 (29.3) |
| Alcohol/year | 10.01 ± 5.21 | 13.58 ± 5.09 |
The DNMT3B T allele frequency and genotype distributions in the case patients and control subjects are summarized in Table 2. Only one genotype of CT was found in 140 control subjects (0.7%) and three in 100 case subjects (3.0%), respectively. The variant C allele frequency was 1.5% for the case patients and 0.4% for the control subjects. The CC genotype was not detected in both HCC patients and controls. Although the frequency of CT genotype and C allele was higher in HCC patients than in control subjects, but there was no significant difference (P > 0.05).
Table 2.
DNMT3B genotype and allele frequencies in case patients and control subjects and their association with HCC, n (%)
| Genotypes | Case patients (n = 100) | Control subjects (n = 140) |
| CT | 3 (3) | 1 (0.7) |
| TT | 97 (97) | 139 (99.3) |
| C allele | 3 (1.5) | 1 (0.4) |
| T allele | 197 (98.5) | 279 (99.6) |
χ2 = 1.86, P = 0.17 (genotype); χ2 = 1.84, P = 0.17 (allele).
DISCUSSION
Alterations in DNA methylation can cause changes in gene transcription patterns and also promote mutational events leading to malignant tumors. Recent studies have shown that several mechanisms, including DNA hypomethylation on pericentromeric satellite regions, DNA hypermethylation on CpG islands of genes such as p16, E-cadherin, and HIC-1 (hypermethylated-in-cancer), over-expression of DNA methyltransferases, and reduced expression of methyl-CpG-binding proteins play a role in hepatocarcinogenesis[25-27]. It was reported that genetic disruption of both DNMT1 and DNMT3B in human cancer cells results in global and gene-specific demethylation, and abrogation of silencing of tumor suppressor genes. Both the wild type and catalytically inactive DNMT3B mutant can suppress rDNA promoter irrespective of its methylation status[28,29], suggesting that altered activities of DNMTs contribute to the generation of aberrant methylation in cancer.
Single nucleotide polymorphisms (SNPs) are the most common form of human genetic variation. SNPs in the promoter region of genes may affect either the expression or the activity of enzymes and therefore may be mechanistically associated with cancer risk. The DNMT3B gene contains a C-to-T transition polymorphism (C46359T) in a novel promoter region, -149 bp from the transcription start site, which in in vitro assays confers a 30% increase in promoter activity[23]. It was postulated that the T variant might up-regulate DNMT3B expression, resulting in a predisposition towards aberrant de novo methylation of CpG islands in tumor suppressor and DNA repair genes. These findings encouraged us to examine the relationship between a novel polymorphism in the human DNMT3B promoter and risk of HCC.
To select China as a research field to analyze the relationship between DNMT3B genetic polymorphisms and HCC is a new attempt. In this study, the DNMT3B genetic polymorphism was not susceptible to HCC. The CC genotype was not detectable in both HCC patients and controls, while the T allele was predominant. In control subjects, the frequency of TT and CT genotypes was 99.3% and 0.7% respectively, while that of T and C alleles was 99.6% and 0.4% respectively. The frequency of CT genotype was higher in HCC (3.0%), while that of T and C alleles was 98.5% and 1.5%. Although the frequency of CT genotype and C allele was higher in HCC patients than in control subjects, but there was no significant difference.
The frequency of DNMT3B genotypes is much different from the reported frequency in Caucasians[21]. In the control group, the frequency of TT, CT and CC was 23.2%, 41.8% and 35.0% respectively, while the T allele frequency was 44.1%. C allele was very common. The frequency of TT, CT and CC in lung cancer patients was 21.0%, 56.7% and 22.3% respectively, indicating that a different race has a different genetic composition.
The DNMT3B allele frequency of people in North China is slightly different from that of people in other areas of China[30]. In the present study, the CC genotype was also not detectable. In control subjects, the frequency of TT and CT genotypes was 94.9% and 5.1% respectively, while that of T and C alleles was 97.4% and 2.6% respectively, suggesting that DNMT3B genetic polymorphism is variable in the same race living in different geographic areas. Only the TT genotype was detectable in all control subjects as compared with other ethnic groups such as Japanese[31], also suggesting that a different ethnic group has a different genetic composition.
The PCR product was digested with BlnIfor 12-16 h to complete the reaction of restriction enzyme. Since the sensibility of electrophoresis through polyacrylamide gel is much higher than that of electrophoresis through agarose gel, we chose the former to separate the DNA fragments.
In our study, only one of the case subjects with CT genotype had a family history of HCC, but HBsAg, HBeAb and HBcAb in the other case subjects remained positive for a period of over 10 years without any treatment. Although we designed experiments to assess the correlation of the distribution of DNMT3B genotypes to the transcription and expression of DNMT3B and HBV infection status in selected tumor and normal hepatic tissues, we could not carry out the experiments due to the insufficient number of samples with CT genotype in our study.
The very similar distribution of DNMT3B genotypes in HCC patients and healthy controls suggested that the C/T polymorphism of DNMT3B gene might not independently affect the risk for HCC. A study in North China showed that DNMT3B SNPs are not associated with the susceptibility to gastric cardiac adenocarcinoma[30], although this polymorphism has been demonstrated to be associated with the susceptibility to cancers of the lung, head, neck and breast.
Recently, several candidate SNPs in the DNMT3B gene have been deposited in public databases (http://www.ncbi.nlm.nih.gov/SNP). Although the functional effects of these polymorphisms have not been elucidated, we hypothesize that some of these variants, particularly their haplotypes, may influence DNMT3B activity on DNA methylation, thereby modulating the susceptibility to HCC. The C/T SNP (C46359T) in the promoter of the DNMT3B gene may not be associated with up-regulation of DNMT3B and an increased risk of HCC as observed in this study, but probably there are other polymorphisms in DNMT3B which are susceptible to HCC.
In the present study, the DNMT3B gene was not negligible in the study of hepatocarcinogenesis in different areas. Whether DNMT3B SNPs are associated with different tumor types needs to be further studied in China. To clarify the role of DNMT3B SNP in the development of HCC, investigations in other populations need to be performed as well. The potential usefulness of DNMT3B genotyping needs further studies on a larger scale.
Saito[32] has found that over-expression of a splice variant of DNMT3B, DNMT3B4, which may lack DNA methyltransferase activity and compete with DNMT3B3 for targeting pericentromeric satellite regions, results in DNA hypomethylation on these regions, even at precancerous stages, and plays a critical role in the development of human HCC because of chromosomal instability and aberrant expression of cancer-related genes. Further investigation on the mechanism of aberrant expression of DNMT3B in tumors, including HCC, should be performed.
ACKNOWLEDGMENTS
The authors thank Ping Xiong (Department of Immunology, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China) for the technical advice on genotyping assays, Dr. Wan-Guang Zhang (Hepatic Surgery Center, Tongji Hospital) for the assistance in recruiting study subjects.
COMMENTS
Background
Genetic and epigenetic factors are important in determining individuals' susceptibility to hepatocellular carcinoma (HCC). Alterations in DNA methylation can cause changes in gene transcription patterns and also promote mutational events leading to malignant tumors. DNA methylation is mediated by DNA methyltransferases (DNMTs), of which three active forms have been identified: DNMT1, DNMT3A and DNMT3B. DNMT3B, regarded as a de novo DNA methyltransferase, is thought to play an important role in the generation of aberrant methylation in carcinogenesis.
Research frontiers
Studies have shown that significant over-expression of DNMT3B is observed in tumor tissues while over-expression of DNMT1 and DNMT3A is more modest. A C-to-T transition polymorphism (C46359T) in the promoter region of the DNMT3B gene, -149 base pairs from the transcription start site, greatly increases promoter activity and is associated with an increased risk of cancers, such as cancer of the lung, head and neck. Carriers of the T allele, particularly heterozygotes, have a significantly increased risk foe such cancers.
Innovations and breakthroughs
Since the DNMT3B promoter polymorphism that is responsible for regulating genomic methylation is possibly associated with an increased risk for cancer, we evaluated the relationship between DNMT3B C46359T polymorphism and risk of HCC using PCR-RFLP and sequencing analysis.The PCR product was digested with BlnIfor 12-16 h to complete the reaction of restriction enzyme. Since the sensibility of electrophoresis through polyacrylamide gel is much higher than that of electrophoresis through agarose gel, we chose the former to separate the DNA fragments.
Applications
C/T polymorphism of the DNMT3B gene may not independently affect the risk for HCC and probably there are other polymorphisms in DNMT3B which are susceptible to HCC. Whether DNMT3B SNPs are associated with different tumor types and different races needs to be further studied. The potential usefulness of DNMT3B genotyping needs further studies in a large scale.
Terminology
Single nucleotide polymorphisms (SNPs) represent a natural genetic variability at a high density in the human genome. A synonymous expression is “biallelic marker” corresponding to the two alleles that may differ in a given nucleotide position of diploid cells. A single SNP represents an alternative nucleotide in a given and defined genetic location at a frequency exceeding 1% in a given population. This definition does not include other types of genetic variability like insertions and deletions, and variability in copy number of repeated sequences. SNPs are considered the major genetic source to phenotypic variability that differentiates individuals from one another within a given species. Restriction fragment length polymorphism (RFLP) is a technique by which organisms may be differentiated by analysis of patterns derived from cleavage of their DNA. If two organisms differ in the distance between sites of cleavage of a particular restriction endonuclease, the length of fragments produced differs when DNA is digested with a restriction enzyme. The similarity of patterns generated can be used to differentiate species (and even strains) from one another.
Peer review
Dr. Wu and co-investigators looked at C/T SNP in the promoter region of DNMT3B. They could not demonstrate a significant difference in C/T polymorphism between HCC patients and normal Chinese population. However, DNMT3B gene is not negligible in study of HCC in different races, ethnic groups or geographic areas as well as in study of different tumor types.
Footnotes
S- Editor Liu Y L- Editor Wang XL E- Editor Lu W
References
- 1.Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaniushin BF. DNA methylation and epigenetics. Genetika. 2006;42:1186–1199. [PubMed] [Google Scholar]
- 3.Shames DS, Minna JD, Gazdar AF. DNA methylation in health, disease, and cancer. Curr Mol Med. 2007;7:85–102. doi: 10.2174/156652407779940413. [DOI] [PubMed] [Google Scholar]
- 4.Turek-Plewa J, Jagodziński PP. The role of mammalian DNA methyltransferases in the regulation of gene expression. Cell Mol Biol Lett. 2005;10:631–647. [PubMed] [Google Scholar]
- 5.Siedlecki P, Zielenkiewicz P. Mammalian DNA methyltransferases. Acta Biochim Pol. 2006;53:245–256. [PubMed] [Google Scholar]
- 6.Robertson KD. DNA methylation, methyltransferases, and cancer. Oncogene. 2001;20:3139–3155. doi: 10.1038/sj.onc.1204341. [DOI] [PubMed] [Google Scholar]
- 7.Esteller M, Herman JG. Cancer as an epigenetic disease: DNA methylation and chromatin alterations in human tumours. J Pathol. 2002;196:1–7. doi: 10.1002/path.1024. [DOI] [PubMed] [Google Scholar]
- 8.Szyf M. Targeting DNA methylation in cancer. Bull Cancer. 2006;93:961–972. [PubMed] [Google Scholar]
- 9.Esteller M. Epigenetics provides a new generation of oncogenes and tumour-suppressor genes. Br J Cancer. 2007;96 Suppl:R26–R30. [PubMed] [Google Scholar]
- 10.Yoshikawa H. DNA methylation and cancer. Gan To Kagaku Ryoho. 2007;34:145–149. [PubMed] [Google Scholar]
- 11.Wilson AS, Power BE, Molloy PL. DNA hypomethylation and human diseases. Biochim Biophys Acta. 2007;1775:138–162. doi: 10.1016/j.bbcan.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Kondo Y, Shen L, Suzuki S, Kurokawa T, Masuko K, Tanaka Y, Kato H, Mizuno Y, Yokoe M, Sugauchi F, et al. Alterations of DNA methylation and histone modifications contribute to gene silencing in hepatocellular carcinomas. Hepatol Res. 2007;37:974–983. doi: 10.1111/j.1872-034X.2007.00141.x. [DOI] [PubMed] [Google Scholar]
- 13.Eng C, Herman JG, Baylin SB. A bird's eye view of global methylation. Nat Genet. 2000;24:101–102. doi: 10.1038/72730. [DOI] [PubMed] [Google Scholar]
- 14.Jair KW, Bachman KE, Suzuki H, Ting AH, Rhee I, Yen RW, Baylin SB, Schuebel KE. De novo CpG island methylation in human cancer cells. Cancer Res. 2006;66:682–692. doi: 10.1158/0008-5472.CAN-05-1980. [DOI] [PubMed] [Google Scholar]
- 15.Robertson KD, Uzvolgyi E, Liang G, Talmadge C, Sumegi J, Gonzales FA, Jones PA. The human DNA methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic Acids Res. 1999;27:2291–2298. doi: 10.1093/nar/27.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin CH, Hsieh SY, Sheen IS, Lee WC, Chen TC, Shyu WC, Liaw YF. Genome-wide hypomethylation in hepatocellular carcinogenesis. Cancer Res. 2001;61:4238–4243. [PubMed] [Google Scholar]
- 17.Jin F, Dowdy SC, Xiong Y, Eberhardt NL, Podratz KC, Jiang SW. Up-regulation of DNA methyltransferase 3B expression in endometrial cancers. Gynecol Oncol. 2005;96:531–538. doi: 10.1016/j.ygyno.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 18.Park HJ, Yu E, Shim YH. DNA methyltransferase expression and DNA hypermethylation in human hepatocellular carcinoma. Cancer Lett. 2006;233:271–278. doi: 10.1016/j.canlet.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt WM, Sedivy R, Forstner B, Steger GG, Zöchbauer-Müller S, Mader RM. Progressive up-regulation of genes encoding DNA methyltransferases in the colorectal adenoma-carcinoma sequence. Mol Carcinog. 2007;46:766–772. doi: 10.1002/mc.20307. [DOI] [PubMed] [Google Scholar]
- 20.Oh BK, Kim H, Park HJ, Shim YH, Choi J, Park C, Park YN. DNA methyltransferase expression and DNA methylation in human hepatocellular carcinoma and their clinicopathological correlation. Int J Mol Med. 2007;20:65–73. [PubMed] [Google Scholar]
- 21.Shen H, Wang L, Spitz MR, Hong WK, Mao L, Wei Q. A novel polymorphism in human cytosine DNA-methyltransferase-3B promoter is associated with an increased risk of lung cancer. Cancer Res. 2002;62:4992–4995. [PubMed] [Google Scholar]
- 22.Wang L, Rodriguez M, Kim ES, Xu Y, Bekele N, El-Naggar AK, Hong WK, Mao L, Oh YW. A novel C/T polymorphism in the core promoter of human de novo cytosine DNA methyltransferase 3B6 is associated with prognosis in head and neck cancer. Int J Oncol. 2004;25:993–999. [PubMed] [Google Scholar]
- 23.Montgomery KG, Liu MC, Eccles DM, Campbell IG. The DNMT3B C->T promoter polymorphism and risk of breast cancer in a British population: a case-control study. Breast Cancer Res. 2004;6:R390–R394. doi: 10.1186/bcr807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones JS, Amos CI, Pande M, Gu X, Chen J, Campos IM, Wei Q, Rodriguez-Bigas M, Lynch PM, Frazier ML. DNMT3b polymorphism and hereditary nonpolyposis colorectal cancer age of onset. Cancer Epidemiol Biomarkers Prev. 2006;15:886–891. doi: 10.1158/1055-9965.EPI-05-0644. [DOI] [PubMed] [Google Scholar]
- 25.Tchou JC, Lin X, Freije D, Isaacs WB, Brooks JD, Rashid A, De Marzo AM, Kanai Y, Hirohashi S, Nelson WG. GSTP1 CpG island DNA hypermethylation in hepatocellular carcinomas. Int J Oncol. 2000;16:663–676. doi: 10.3892/ijo.16.4.663. [DOI] [PubMed] [Google Scholar]
- 26.Soejima H, Zhao W, Mukai T. Epigenetic silencing of the MGMT gene in cancer. Biochem Cell Biol. 2005;83:429–437. doi: 10.1139/o05-140. [DOI] [PubMed] [Google Scholar]
- 27.Tannapfel A. Methylation and other new concepts for the origin of hepatocellular carcinoma. Pathologe. 2006;27:284–288. doi: 10.1007/s00292-006-0837-y. [DOI] [PubMed] [Google Scholar]
- 28.Majumder S, Ghoshal K, Datta J, Smith DS, Bai S, Jacob ST. Role of DNA methyltransferases in regulation of human ribosomal RNA gene transcription. J Biol Chem. 2006;281:22062–22072. doi: 10.1074/jbc.M601155200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Lin RK, Hsu HS, Chang JW, Chen CY, Chen JT, Wang YC. Alteration of DNA methyltransferases contributes to 5'CpG methylation and poor prognosis in lung cancer. Lung Cancer. 2007;55:205–213. doi: 10.1016/j.lungcan.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 30.Wang YM, Wang R, Wen DG, Li Y, Guo W, Wang N, Wei LZ, He YT, Chen ZF, Zhang XF, et al. Single nucleotide polymorphism in DNA methyltransferase 3B promoter and its association with gastric cardiac adenocarcinoma in North China. World J Gastroenterol. 2005;11:3623–3627. doi: 10.3748/wjg.v11.i23.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aung PP, Matsumura S, Kuraoka K, Kunimitsu K, Yoshida K, Matsusaki K, Nakayama H, Yasui W. No evidence of correlation between the single nucleotide polymorphism of DNMT3B promoter and gastric cancer risk in a Japanese population. Oncol Rep. 2005;14:1151–1154. [PubMed] [Google Scholar]
- 32.Kanai Y, Saito Y, Ushijima S, Hirohashi S. Alterations in gene expression associated with the overexpression of a splice variant of DNA methyltransferase 3b, DNMT3b4, during human hepatocarcinogenesis. J Cancer Res Clin Oncol. 2004;130:636–644. doi: 10.1007/s00432-004-0586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


