Abstract
Background
Bronchiectasis and asthma are different in many respects, but some patients have both conditions. Studies assessing the effect of bronchiectasis on asthma exacerbation are rare. The aim of this study is to investigate the effect of bronchiectasis on asthma exacerbation.
Methods
We enrolled 2,270 asthma patients who were followed up in our hospital. Fifty patients had bronchiectasis and asthma. We selected fifty age- and sex-matched controls from the 2,220 asthma patients without bronchiectasis, and assessed asthma exacerbation and its severity based on the annual incidence of total asthma exacerbation, annual prevalence of steroid use, and frequency of emergency room visits and hospitalizations due to asthma exacerbation in each group.
Results
Fifty patients (2.2%) had bronchiectasis and asthma. The annual incidence of asthma exacerbation was higher in patients with asthma and bronchiectasis than in patients with asthma alone (1.08±1.68 vs. 0.35±0.42, p=0.004). The annual prevalence of steroid use (0.9±1.54 vs. 0.26±0.36, p=0.006) and the frequency of emergency room visits (0.46±0.84 vs. 0.02±0.13, p=0.001) due to asthma exacerbation were also higher in patients with asthma and bronchiectasis than in patients with asthma alone.
Conclusion
Bronchiectasis is associated with difficult asthma control.
Keywords: Bronchiectasis, Asthma, Disease Exacerbation
Introduction
Asthma is a clinical syndrome characterized by three distinct components, such as recurrent episodes of airway obstructions, airway hyperresponsiveness, and airway inflammations. Exacerbation of asthma is indicated by episodes of progressively increasing shortness of breath, coughing, wheezing, or chest tightening, or some combination of these symptoms. Asthma exacerbations are associated with a variety of risk factors including allergens, viral infections, pollutants, drugs, obesity, emotional stress, rhinitis, sinusitis, gastroesophageal reflux, and menstruation1.
Bronchiectasis is defined as permanently dilated airways due to chronic bronchial inflammation caused by inappropriate clearance of various microorganisms and recurrent or chronic infection2. Although the true prevalence is unknown for most regions, bronchiectasis is now being diagnosed with increasing frequency in North America and around the globe3. The diagnosis of bronchiectasis is based on the review of clinical history and characteristic patterns in high-resolution computed tomography (HRCT) scan findings.
Bronchiectasis and asthma are different diseases. However, some patients have both diseases at the same time. There are rare data to determine the effects of bronchiectasis on asthma exacerbation. To our knowledge, there is only one report for the impact of coexisting bronchiectasis on asthma4. However, many clinical studies for asthma exclude bronchiectasis patients.
The aim of this study is to investigate the effects of bronchiectasis on asthma exacerbation.
Materials and Methods
Asthma diagnosis was confirmed using the Global Initiative for Asthma guideline at the time of diagnosis1. Asthma was diagnosed when patients had symptoms such as episodic breathlessness, wheezing, cough, and chest tightness, and whose spirometry showed bronchial reversibility as 12% and 200 mL from the prebronchodilator value or airway hyperresponsiveness as provocative concentration of a methacholine causing a 20% fall in forced expiratory volume in 1 second (FEV1) was below 25 mg/mL.
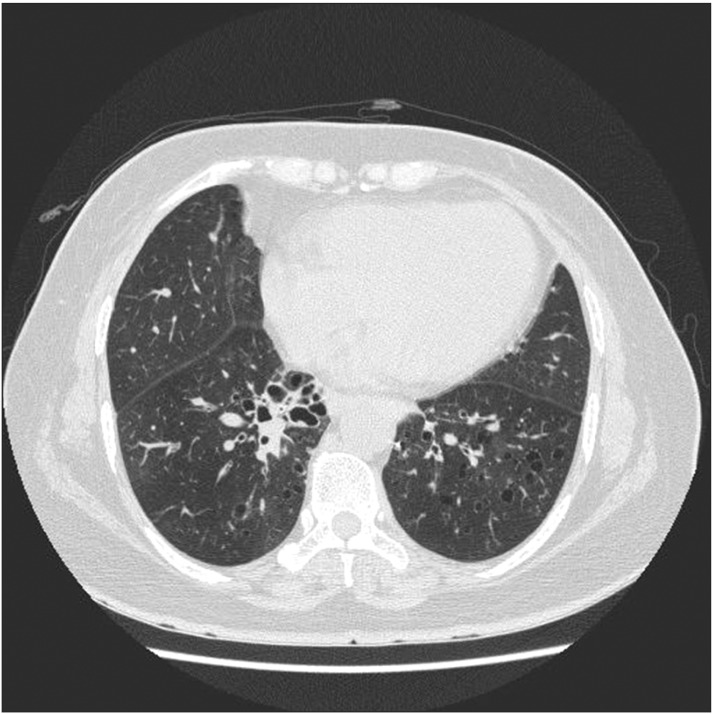
Bronchiectasis was diagnosed with HRCT of the thorax (LightSpeed Ultra 16; General Electric, Milwaukee, WI, USA), according to the criteria described by McGuinness et al.5 (Figure 1). Indications for HRCT are clinical findings consistent with bronchiectasis, such as chronic cough, sputum production, or abnormal chest X-ray results identified by a radiologist.
Figure 1.
High-resolution computed tomography of an asthma patient with bronchiectasis.
We retrospectively reviewed medical records of 2,270 asthma patients who were visited to Eulji Hospital between 1999 and 2010. Bronchiectasis was diagnosed in 50 of 2,270 asthma patients during follow-up. We selected 50 age- and sex-matched controls from 2,220 asthma patients without bronchiectasis.
Exacerbations of asthma were clinically diagnosed by physician, when the patients had episodes of increase in shortness of breath, cough, wheezing, or chest tightness, or some combination of these symptoms. They were treated with steroids and rapid-acting inhaled β-agonists.
If patients with bronchiectasis had increased sputum production and viscidity of sputum, a foul odor of the sputum and the physician suspected them as only exacerbations of bronchiectasis, that were not regarded as asthma exacerbations. They were treated with antibiotics.
We assessed the severity of asthma with four variables. These include 1) total asthma exacerbations, 2) the steroid uses due to asthma exacerbation, 3) emergency room visiting due to asthma exacerbation, and 4) hospitalization due to asthma exacerbation. The steroid uses due to asthma exacerbation are the steroids uses at the out-patient department visiting, which did not include the steroid uses of emergency room visiting and hospitalization due to asthma exacerbation. The emergency room visiting due to asthma exacerbation are only emergency room visits. If the patient needs hospitalization for more treatment, the event was counted by hospitalization. The hospitalization due to asthma exacerbation includes the number of admissions via emergency room and out-patient department.
Pulmonary function was tested with a spirometer (Vmax 229 pulmonary function/cardiopulmonary exercise testing instrument; Sensor Medics Corp., Yorba Linda, CA, USA). The spirometer was calibrated once daily with a 3-L syringe, according to the operator's manual. Each patient was tested at least 3 times. The best value was expressed as a percentage of the predicted value and as an absolute value. The degree of reversibility in FEV1 which indicates a diagnosis of asthma is generally accepted as 12% and 200 mL from the pre-bronchodilator value.
Atopy was assessed with skin prick tests for 16 common allergens (Dermatophagoides pteronyssinus, Dermatophagoides farinae, Tyrophagus putrescentiae, Cladosporium herbarum, Aspergillus fumigatus, Alternaria, Trichophyton species, dog epithelium, cat fur, cockroach, mugwort, ragweed, tree mixture, grass mixture, Japanese hop, positive histamine control, and negative saline control). Skin prick test result was assessed as positive if the wheal was at least 3 mm and there was no reaction to the negative control6.
Statistical analysis was performed with SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). Values were expressed as means±SD. Continuous variables were compared with Student's t test. The chi-square or Fisher exact test was used to compare categorical variables. All p-values are from 2-tailed tests and p-values less than 0.05 were considered statistically significant.
The study protocol was approved by the Institutional Review Board of the Eulji Hospital in Seoul, Korea (IRN No. EMCIRB 12-75).
Results
We enrolled 2,270 asthma patients visiting our hospital between 1999 and 2010. Bronchiectasis was diagnosed in 50 of 2,270 asthma patients during follow-up. The prevalence of bronchiectasis among the asthma patients was 2.2%.
Baseline characteristics of subjects are summarized in Table 1. There were not different in durations of follow-up, the levels of total IgE, presences of atopy, or pulmonary function test results in two groups.
Table 1.
Baseline characteristics of the subjects
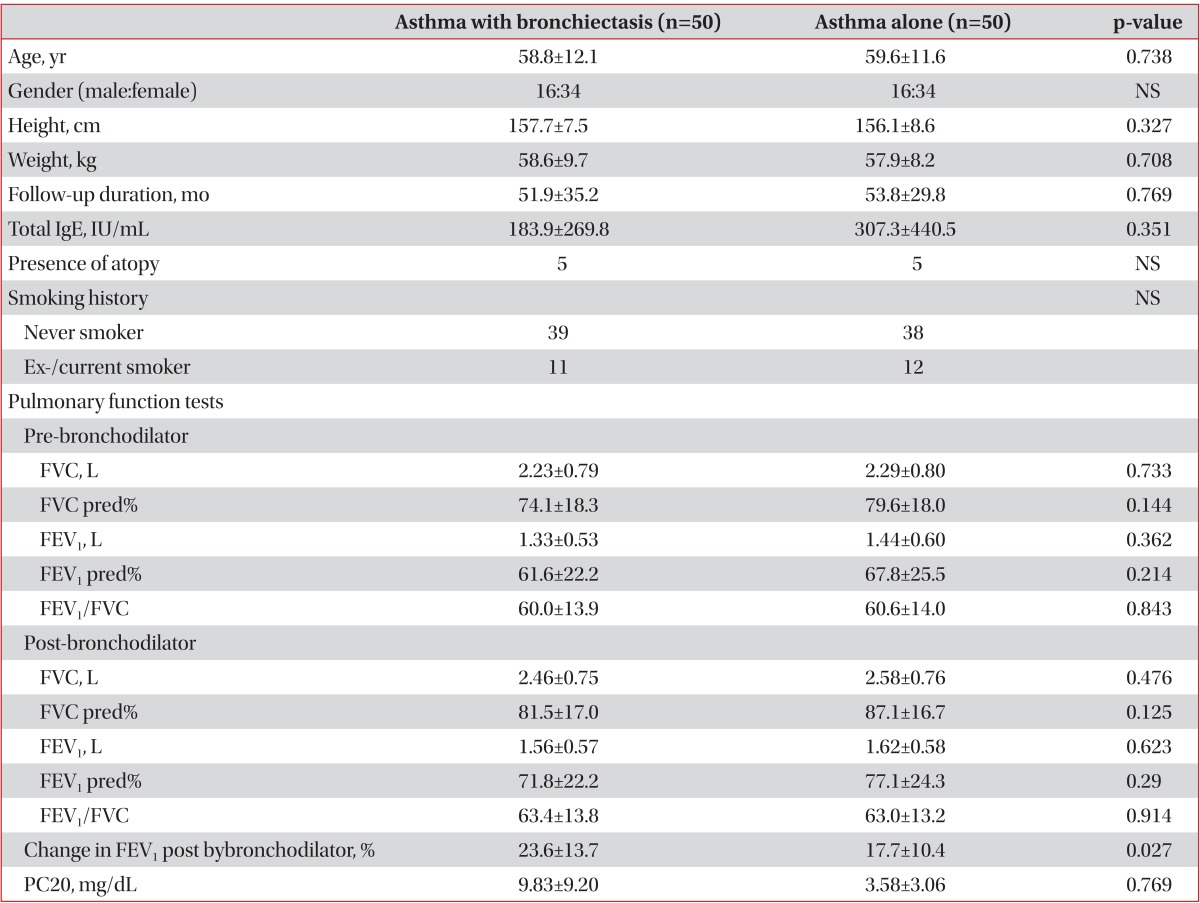
Values are expressed as means±SD.
NS: not specific; Never smoker: no smoking history ever; Ex-smoker: no current smoking status for at least 3 months with previous smoking history; FVC: forced expiratory vital capacity; FEV1: forced expiratory volume in 1 second; pred%: percent of the normal predicted value; PC20: provocative concentration of methacholine causing a 20% fall in FEV1.
The asthma alone patients used inhaled glucocorticosteroids in combination with long acting inhaled β2-agonists (ICS+LABA, 64%) and leukotriene receptor antagonist (LTRA, 24%) as the controller. The asthma patients with bronchiectasis used ICS+LABA (60%) and LTRA (14%), and theophylline (12%).
Exacerbations of asthma in subjects are summarized in Table 2. The annual incidences of asthma exacerbation, steroid use, emergency room visits due to asthma exacerbation were higher in patients with asthma and bronchiectasis than with asthma alone. Patients with asthma alone had a higher annual incidence of hospitalization due to asthma exacerbation, however this was not a statistically significant difference (p=0.4).
Table 2.
Asthma exacerbations in subjects
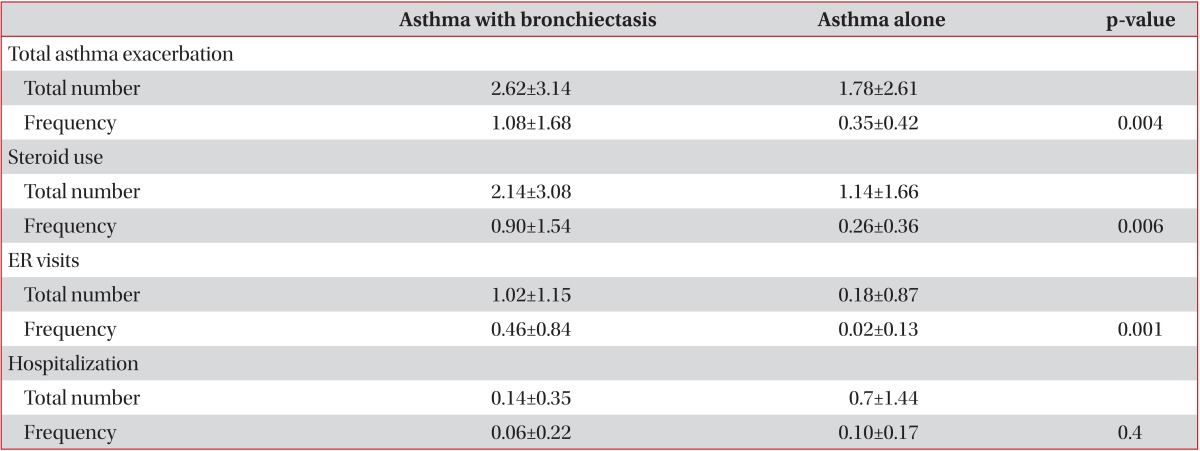
Values are presented as mean±SD.
Total asthma exacerbation means total number of steroid use, ER visits, and hospitalizations due to asthma exacerbation.
Frequency: number/yr; ER: emergency room.
None of the asthma patients with bronchiectasis had a diagnosis of allergic bronchopulmonary aspergillosis or cystic fibrosis.
Discussion
There is only one report for the impact of coexisting bronchiectasis on asthma4. In that study, asthmatics with bronchiectasis mostly had severe persistent asthma (49%), while pure asthmatics mostly had mild persistent and intermittent asthma (69.4%). Hospitalization due to severe asthma exacerbation was 49% in patients with asthma and bronchiectasis and 17.6% in patients with asthma alone, and presence of chronic respiratory failure was significantly higher in bronchiectatic group (9.8% vs. 0%). In present study, the proportion of mild persistent and intermittent asthma to asthma patients with bronchiectasis was 26% and the proportion of moderate persistent asthma was 70%, and severe persistent asthma was 4%. The proportion of mild persistent and intermittent asthma to patients with asthma alone was 44% and the proportion of moderate persistent asthma was 48%, and severe persistent asthma was 8%. The proportion of moderate and severe asthma to asthma patients with bronchiectasis was higher than patient with asthma alone.
In present study, the annual incidences of asthma exacerbation, steroid use, and emergency room visiting due to asthma exacerbation were higher in patients with asthma and bronchiectasis than patients with asthma alone. There was no statistically significant difference in the number per year of hospitalization between two groups. The patients of present study are mostly mild to moderate asthma patients, which would have an influence on the results. The subtype of bronchiectasis was not associated with asthma exacerbation in present study (Table 3).
Table 3.
Asthma exacerbation and bronchiectasis subtypes in asthma patients with bronchiectasis
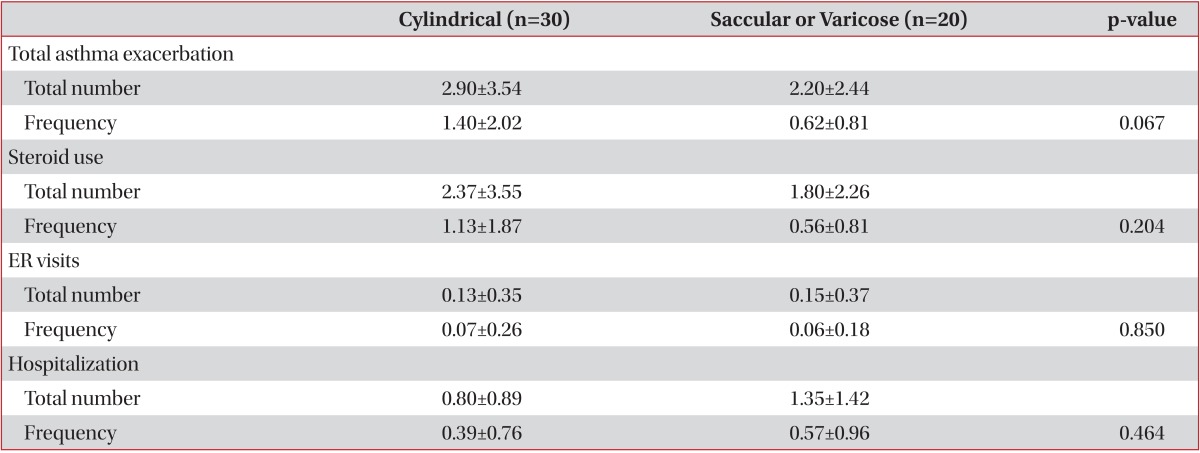
Values are presented as mean±SD.
Total asthma exacerbation means total number of steroid use, ER visits, and hospitalizations due to asthma exacerbation.
Frequency: number/year; ER: emergency room.
Studies investigating the reason that patients with bronchiectasis and asthma had more exacerbation rate than patients with asthma alone are rare. Persistent bacterial colonization of the airways in bronchiectasis is caused by impaired mucociliary transport and mucus clearance, and initiates a vicious cycle of inflammation characterized by activated neutrophils and neutrophil proteases7. The use of inhaled antibiotics in bronchiectasis patients decreases in bacterial density, airway inflammation, and exacerbation frequency8,9. The airway inflammation in bronchiectasis could be associated with asthma exacerbations.
The association between asthma and bronchiectasis has been reported in many studies. The hypothesis that asthma and atopy may have a role in the pathogenesis of bronchiectasis was first postulated in 193910. Since 1939, there have been many studies reporting positive relationships between bronchiectasis, bronchial hyperreactivity, and asthma11,12,13. While some studies could not find any relationship between bronchiectasis and atopy14,15.
The reported prevalence of asthma in patients with bronchiectasis ranges from 2.7% to 42% in different series12,16,17. However, there are only two reports in the prevalence of bronchiectasis in asthma. The one reported a bronchiectasis prevalence of 1.8% among new asthma cases13. The other one reported 3%4. In present study, the prevalence of bronchiectasis was 2.2% that was similar to previous reports.
European Respiratory Society and American Thoracic Society have released a new guideline on severe asthma18. They did not find any systematic review or primary study that investigates the results of using HRCT in screening patients with severe asthma for potential comorbid or masquerading condition. However, they placed a relatively high value on identification of alternative diagnosis, comorbidities, a relatively low value on avoiding potential complications, and cost of chest HRCT. In children and adults with severe asthma without specific indications for chest HRCT based on history, symptoms and/or results of prior investigations, it is recommended that a chest HRCT only be done when the presentation is atypical, such as excessive mucus production, rapid decline in lung function, reduced carbon monoxide transfer factor coefficient and the absence of atopy in a child with difficult asthma. Clinical symptoms of asthma and bronchiectasis may overlap significantly as symptoms of cough, sputum and dyspnea can occur in either asthma or bronchiectasis alone19. Most of the asthma patients with bronchiectasis are severe persistent asthma4. A prospective screening study with HRCT in difficult-to treat asthma patients would be needed.
Our study has limitations. First, our study is retrospective, single center, and age- and sex-matching design which could be associated with selection bias. There were no differences in height, weight, smoking history, and total IgE levels between two groups, however other factors were not compared such as duration of asthma which could have an effect on the results. Second, patients with bronchiectasis who did not have symptom or normal chest X-ray might be lost.
In summary, annual incidences of asthma exacerbation, steroid use due to asthma exacerbation, and emergency room visit due to asthma exacerbation were higher in patients with both asthma and bronchiectasis than asthma alone. Bronchiectasis may be a risk factor for asthma exacerbation. If asthma patient is uncontrolled, HRCT could be considered for concurrent bronchiectasis.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention 2013 [Internet] Global Initiative for Asthma; [cited 2014 Apr 1]. Available from: http://www.ginasthma.org/ [Google Scholar]
- 2.Kim C, Kim DG. Bronchiectasis. Tuberc Respir Dis. 2012;73:249–257. doi: 10.4046/trd.2012.73.5.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seitz AE, Olivier KN, Steiner CA, Montes de Oca R, Holland SM, Prevots DR. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993-2006. Chest. 2010;138:944–949. doi: 10.1378/chest.10-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oguzulgen IK, Kervan F, Ozis T, Turktas H. The impact of bronchiectasis in clinical presentation of asthma. South Med J. 2007;100:468–471. doi: 10.1097/SMJ.0b013e31802fa16f. [DOI] [PubMed] [Google Scholar]
- 5.McGuinness G, Naidich DP, Leitman BS, McCauley DI. Bronchiectasis: CT evaluation. AJR Am J Roentgenol. 1993;160:253–259. doi: 10.2214/ajr.160.2.8424327. [DOI] [PubMed] [Google Scholar]
- 6.Niggemann B, Reibel S, Wahn U. The atopy patch test (APT): a useful tool for the diagnosis of food allergy in children with atopic dermatitis. Allergy. 2000;55:281–285. doi: 10.1034/j.1398-9995.2000.00464.x. [DOI] [PubMed] [Google Scholar]
- 7.Angrill J, Agusti C, De Celis R, Filella X, Rano A, Elena M, et al. Bronchial inflammation and colonization in patients with clinically stable bronchiectasis. Am J Respir Crit Care Med. 2001;164:1628–1632. doi: 10.1164/ajrccm.164.9.2105083. [DOI] [PubMed] [Google Scholar]
- 8.Scheinberg P, Shore E. A pilot study of the safety and efficacy of tobramycin solution for inhalation in patients with severe bronchiectasis. Chest. 2005;127:1420–1426. doi: 10.1378/chest.127.4.1420. [DOI] [PubMed] [Google Scholar]
- 9.Murray MP, Govan JR, Doherty CJ, Simpson AJ, Wilkinson TS, Chalmers JD, et al. A randomized controlled trial of nebulized gentamicin in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2011;183:491–499. doi: 10.1164/rccm.201005-0756OC. [DOI] [PubMed] [Google Scholar]
- 10.Watson SH, Kibler CS. The role of allergy in bronchiectasis. J Allergy. 1939;10:364–376. [Google Scholar]
- 11.Varpela E, Laitinen LA, Keskinen H, Korhola O. Asthma, allergy and bronchial hyper-reactivity to histamine in patients with bronchiectasis. Clin Allergy. 1978;8:273–280. doi: 10.1111/j.1365-2222.1978.tb03224.x. [DOI] [PubMed] [Google Scholar]
- 12.Ip M, Lam WK, So SY, Liong E, Chan CY, Tse KM. Analysis of factors associated with bronchial hyperreactivity to methacholine in bronchiectasis. Lung. 1991;169:43–51. doi: 10.1007/BF02714140. [DOI] [PubMed] [Google Scholar]
- 13.Saynajakangas O, Keistinen T, Tuuponen T, Kivela SL. Links between hospital diagnoses of bronchiectasis and asthma. Allergy. 1997;52:1120–1122. doi: 10.1111/j.1398-9995.1997.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 14.Murphy MB, Reen DJ, Fitzgerald MX. Atopy, immunological changes, and respiratory function in bronchiectasis. Thorax. 1984;39:179–184. doi: 10.1136/thx.39.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pang J, Chan HS, Sung JY. Prevalence of asthma, atopy, and bronchial hyperreactivity in bronchiectasis: a controlled study. Thorax. 1989;44:948–951. doi: 10.1136/thx.44.11.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pang J, Chan HS, Sung JY. Prevalence of asthma, atopy, and bronchial hyperreactivity in bronchiectasis: a controlled study. Thorax. 1989;44:948–951. doi: 10.1136/thx.44.11.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahous J, Cartier A, Pineau L, Bernard C, Ghezzo H, Martin RR, et al. Pulmonary function tests and airway responsiveness to methacholine in chronic bronchiectasis of the adult. Bull Eur Physiopathol Respir. 1984;20:375–380. [PubMed] [Google Scholar]
- 18.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 19.Ip MS, So SY, Lam WK, Yam L, Liong E. High prevalence of asthma in patients with bronchiectasis in Hong Kong. Eur Respir J. 1992;5:418–423. [PubMed] [Google Scholar]