Abstract
Purpose
Acute liver failure (ALF) is a rare clinical syndrome associated with a high case fatality rate. Asymptomatic primary infection with Epstein–Barr virus (EBV) is common in the general population while acute hepatitis and jaundice are much less common and ALF has been rarely reported. We reviewed the presenting features as well as clinical outcomes amongst consecutive adults with EBV-related ALF.
Methods
Amongst the 1,887 adult ALF patients enrolled into the US ALF Study Group from January 1998 to February 2012, there were four patients (0.21 %) with EBV-related ALF. Diagnostic criteria for acute EBV infection included compatible serologies and/or the detection of EBV-encoded RNA (EBER) in liver tissue.
Results
Median patient age was 30 years (range 18–44); 75 % were male, and only 25 % were immunosuppressed. The median presenting ALT was 504 IU/mL (range 156–4,920), median Alk P was 431 (range 136–1,009), and median bilirubin was 17 mg/dL (range 13–22.1). Liver biopsy findings ranged from cholestasis to submassive necrosis with EBER + staining in two of the three samples tested. Although all of the patients were treated with an antiviral agent, two died of ALF, one underwent liver transplantation (LT) and one survived with supportive care and is well at 5 years. A review of the literature identified four additional LT recipients with favorable long-term outcomes.
Conclusion
Primary EBV infection accounts for <1 % of consecutive adult ALF cases but is associated with a high case fatality rate. LT is associated with favorable short- and long-term outcomes.
Keywords: Viral hepatitis, Fulminant hepatic failure, Liver transplantation, Acyclovir
Introduction
Acute liver failure (ALF) is a rare but potentially serious illness [1]. A diagnosis of ALF in adults requires the development of coagulopathy (i.e. international normalized ratio (INR) >1.5) and encephalopathy within 26 weeks of illness onset in a patient without known prior liver disease [1]. Although infrequent in the general population, ALF is associated with substantial morbidity and mortality with a 3-week mortality rate that varies from 30 % in patients with acetaminophen overdose to nearly 100 % in subjects with Wilson disease [2]. The most frequently identified etiology of ALF in adults is acetaminophen overdose (45 %) followed by idiosyncratic drug reactions (11 %) and hepatitis B virus infection (7 %) but at least 13 % of cases are of indeterminate etiology [3]. A previously unidentified viral infection may account for some of these indeterminate ALF cases, but studies to date have been unrevealing [4–7].
Primary systemic infection with Epstein–Barr virus (EBV) is common in the general US adult population with nearly 90–95 % of adults demonstrating evidence of prior EBV infection by age 20 [8]. In addition, evidence of prior CMV infection is noted in at least 50–60 % of adult Americans [9]. However, severe primary systemic infection with these DNA viruses can infrequently be associated with clinically apparent hepatitis and jaundice in adults, as well as rare instances of progression to ALF [4, 5, 8, 10, 11]. Although some case series have suggested that immunosuppressed individuals may be at increased risk of developing severe primary EBV infection or viral reactivation, the role of host, viral, and environmental factors in these unusual cases of ALF remains unclear. The US Acute Liver Failure Study Group (ALFSG) is a consortium of 13 academic medical centers that has been prospectively studying the causes and outcomes of adult ALF patients since 1998. The aim of the current study was to describe the presenting features and clinical outcomes of consecutive adult patients with EBV-related ALF enrolled between 1998 and 2012. In addition, we conducted a literature review to improve our understanding of the outcomes with emergency liver transplantation (LT) in patients with severe EBV-related ALF.
Methods
US Acute Liver Failure Study Group
The ALFSG is a consortium of 13 US academic referral centers funded by the National Institute of Diabetes and Digestive and Kidney Diseases to conduct an ongoing prospective observational study of the etiology, clinical features, and outcomes of adult patients with ALF. Enrollment criteria include the presence of coagulopathy defined as an INR >1.5 and any level of hepatic encephalopathy within 26 weeks of illness onset in a patient with no known underlying liver disease. All centers included in the ALFSG are liver transplant centers.
Data Collection
Written informed consent was obtained from the patient's durable power of attorney or next of kin. Etiology definitions are included in the ALFSG protocol; however, sero-logical testing for unlikely causes may be incomplete in certain cases. Detailed patient demographics, medical history, clinical features and laboratory values were collected at study enrollment. Serial laboratory and clinical parameters were prospectively recorded for up to 3 weeks after enrollment. At the end of 3 weeks, short-term outcomes were classified as spontaneous survival, LT, or death. Spontaneous survivors and LT recipients were to be followed at 12 and 24 months by the site investigator. Periodic site visits were conducted by the ALFSG leadership to provide quality control. A Certificate of Confidentiality was obtained from the National Institutes of Mental Health for the entire study.
EBV-Related ALF
Clinical signs of systemic EBV infection (e.g. fever, sore throat, lymphadenopathy) were not used as inclusion criteria but are reported in results. EBV serologies consistent with acute infection included evidence of positive EBV viral capsid antigen (VCA) IgM with or without positive EBV VCA IgG antibody titers (see Supplemental Table 1). Eligible patients were defined as having positive EBV VCA IgM antibodies, positive serum EBV DNA by PCR, and/or evidence of EBV infection in liver tissue as determined by light microscopy or EBV-encoded RNA (EBER) positive staining of liver tissue samples. Cases were reviewed by two reviewers (J.L.M. and R.J.F.) and were defined as “definite” if both serologic and tissue studies confirmed EBV. Cases were defined as “probable” if either serologic or tissue studies, but not both, confirmed EBV.
The liver tissue samples from cases 1, 3, and 4 were interpreted by an independent expert hepatopathologist (Henry Appelman). EBER in situ immunohistochemical staining was performed using a BenchMark ULTRA IHC/ ISH Staining Module. Deparrafinized and protease treated 4-μ thick slides were hybridized with ISH probe 1 and incubated. RNA control was tested using identical reagents except that ISH probe 1 was replaced with ISH probe 2.
Literature Search
A literature search was conducted in PubMed, MEDLINE, Scopus, Web of Science, and EMBASE using the following search terms: (“Epstein–Barr” OR EBV) AND (“ALF” OR “fulminant hepatic failure” OR “liver failure” OR “hepatic failure” OR “severe hepatitis” OR “fulminant hepatitis” OR “fulminant liver failure”) AND (“liver transplant” OR “LT”). Search terms were restricted to those occurring in the title, abstract, keywords, or, in the case of Web of Science, topic subject. A search of Google Scholar was also performed. Search results were reviewed independently by a single reviewer (J.L.M.). A total of eight cases of EBV-related ALF leading to LT were identified [12–19]. Three cases were excluded due to insufficient laboratory data to confirm diagnosis [16–18] and one case was found to be reported in duplicate [19], leaving four total cases for review (Table 3).
Table 3.
Additional cases of EBV-related ALF treated with liver transplantation
| Reference # | Age (years) | Sex | Type of transplant | Symptoms |
Splenomegaly |
Admission labsa | EBV labs | EBER stain liver tissueb | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fever | LAD | Sore throat | PEX | Image | ||||||||
| 12 | 18 | Female | Cadaveric | + | NR | NR | – | NR | AST 230 ALT 130 TB 11 mg/dL |
VCA IgM +VCA IgG +EBNA +EBV PCR: 2.63 × 105 copies/mL | + | Alive at 1 year |
| 15 | 1.8 | Female | Cadaveric | + | NR | NR | – | – | WBC 22 × 103 82 % atyp lymphos AST 1777 ALT 4251 |
VCA IgM +VCA IgG +EBNA – | + | Alive at 2 years |
| 13 | 19 | Male | Cadaveric | NRc | NR | NR | NR | + | AST: 3083 ALT: 1796 Plts 3.1 × 104 |
VCA IgM + VCA IgG + EBNA + | NR (EBV-DNA PCR +) | Alive at 26 days |
| 14 | 8 | Male | Living donor | + | NR | NR | NR | NR | NR | VCA IgM + EBV PCR: 88 × 103 copies/mL | + | Alive at 8 days |
NR not reported, LAD lymphadenopathy, PE physical exam
AST and ALT reported as IU/L. Platelets and WBC reported as count/mL
All tissue studies performed on explants
Reported “flu-like” symptoms only
Data Analysis
Descriptive statistics were used to describe the presenting features and outcomes of patients and are reported as median (range) and or mean ± SD.
Results
Patient Population
There were 1,887 consecutive adult ALF patients enrolled into the ALFSG registry from January 1998 to February 2012. Amongst the enrolled patients, 4 (0.21 %) met our case definition for EBV-related ALF. The median age of these patients was 30 (Tables 1 and 2). Three patients were male and only one patient (patient #4) was immunocompromised. Although all of the patients were symptomatic at presentation, only two reported symptoms suggestive of infectious mononucleosis such as fever, sore throat, and swollen lymph nodes. The median time from symptom onset to admission was 13 days and all of the subjects were jaundiced at presentation.
Table 1.
Initial laboratory features of EBV-related ALF cases
| Case | Age | Sex | ALT (IU/L) | ALP (IU/L) | Total bili (mg/dL) | WBC (×103) | Lymphocyte count (%) | Hgb (g/dL) | Plts (×103) | INR |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | F | 4,920 | 147 | 22.1 | 10.6 | 33 | 14.7 | 280 | 2.8 |
| 2 | 21 | M | 674 | 136 | 12.9 | 32.5 | 34 | 9.3 | 53 | 3.6 |
| 3 | 38 | M | 156 | 715 | 27 | 2.1 | 35 | 8.8 | 53 | 1.6 |
| 4 | 18 | M | 334 | 1,009 | 11.1 | 2.1 | 13 | 10.1 | 221 | 1.9 |
| Median or % | 30 | 75 % M | 504 | 431 | 17.5 | 3.2 | 33 | 9.7 | 137 | 2.4 |
| Case | Heterophile antibody | VCA IgM | VCA IgG | EBNA Ab | EBV PCR | EBER stain of liver tissue | Case definition |
|---|---|---|---|---|---|---|---|
| 1 | ND | + | + | + | + | – | Probable |
| 2 | + | + | ND | ND | ND | ND | Probable |
| 3 | + | – | + | – | + | + | Probable |
| 4 | + | +/– | + | + | + | + | Definite |
| Median or % | 100 % | 50 % | 100 % | 66 % | 100 % |
All lab values are on admission unless otherwise indicated
VCA viral capsid antigen, EA IgG antibody to EBV early antigen, EBNA antibody to EBV nuclear antigen, ND not done
Table 2.
Presenting clinical characteristics and outcomes of EBV-related ALF cases
| Case | Symptoms |
Splenomegaly |
Treatment |
Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|
| Fever | LAD | Sore throat | Physical exam | Imaging | IV antiviralsa | NAC | Steroids | ||
| 1 | – | – | – | – | – | + | + | – | Liver transplant. Alive at 18 months |
| 2 | + | + | + | + | + | + | – | + | Death. Hospital day #4 |
| 3 | + | + | – | – | + | + | + | + | Recovered. Alive at 5 years |
| 4 | + | + | + | + | + | + | + | + | Death. Hospital day #12 |
ND not done, LAD lymphadenopathy, NAC n-acetylcysteine
IV antivirals administered: acyclovir, ganciclovir, famciclovir. See clinical vignettes for timing and dosage
Presenting and Peak Laboratory Features
The complete blood count data showed no obvious pattern with admission results ranging from a white blood cell (WBC) count of 2,100–32,500 cells/mL (Table 1). The differential of the WBC count revealed lymphocyte counts ranging from 13 to 35 % with no reported atypical lymphocytosis. The admission median serum aspartate aminotransferase (AST) level was 654 IU/L (range 192–2,690) and median serum ALT level was 504 (range 156–4,920). The median peak values for AST and ALT were 2,300 IU/L (range 451–4,248) and 3,052 IU/L (range 307–13,666), respectively. The median presenting total bilirubin was 17.5 mg/dL (range 11.1–27) with a median INR of 2.3 (range 1.6–3.6) while the median peak bilirubin level was 21 mg/day (13.4–32.4) and peak INR was 3.5 (range 1.7–17.7). Admission serum alkaline phosphatase values ranged from 136 to 1,009 IU/L with a median level of 431. Two of the subjects had a hepatocellular injury at presentation (patients #1 and #2) while two subjects had a cholestatic liver injury profile at presentation (patients #3 and #4).
EBV Serological Data
EBV serologies revealed evidence of acute EBV infection in all four patients (Table 1). EBV DNA by PCR was positive at low levels in patient #1 but subsequently became negative after 1 week of acyclovir. In addition, EBV DNA was noted to be in high titer at 1,237,000 copies/mL in patient #4 who ultimately died of extensive extranodal lymphoproliferative disorder. Patient #3 also had detectable EBV DNA by PCR. Heterophile antibody (Sure-Vue Mono™ test kit, Biokit S.A., Barcelona, Spain) was positive in all three patients that were tested.
Liver Tissue Analysis
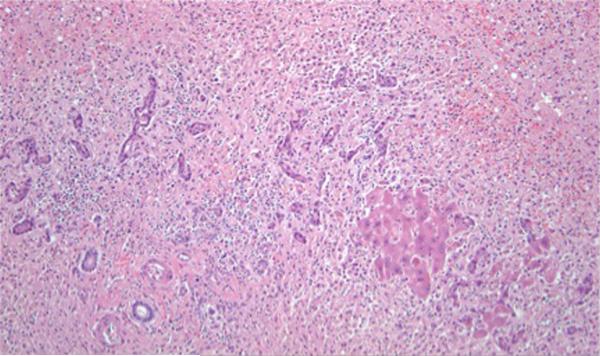
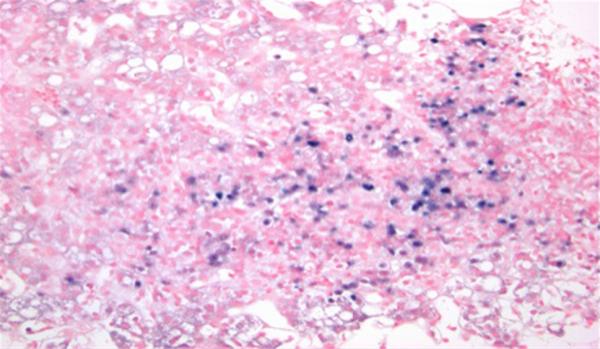
There was unequivocal evidence of EBV infection in two of the three patients with extensive sinusoidal and lobular lymphocytosis in most of them. In addition, patient #1 had evidence of massive hepatic necrosis (Fig. 1). EBER staining was completed in three of the cases and positive in two of them (Fig. 2).
Fig. 1.
Liver explant from patient #1, a 44-year-old female, with massive hepatic necrosis. The only hepatocytes in this field are the small collection to the right of center. The remainder of this field is collapsed parenchyma resulting from confluent lobular necrosis, with a portal tract at the lower left and proliferating bile ductules and neocholangioles to the left of center. (H&E stain, × 100 magnification)
Fig. 2.
Liver biopsy from patient #4, an 18-year-old male with Crohn's ileocolitis on 6-mercaptopurine. The sinusoids are full of lymphocytes, a characteristic finding in EBV hepatitis. With EBER in situ hybridization staining, these sinusoidal lymphocytes stain positive for Epstein–Barr virus encoded RNA in blue (EBER stain, × 9200 magnification). The patient died of EBV-associated lymphoproliferative disorder
Outcomes
Two of the four (50 %) patients with EBV related ALF were alive at 3 weeks after presentation. The two non-survivors died on hospital day #4 of cerebral herniation and hospital day #10 of multi-organ failure. During long-term follow-up, patient #1 was doing well at 18 months after LT and had normal liver biochemistries with no evidence of lymphoproliferative or recurrent disease. Patient #3 was also doing well at 5 years of follow-up with no long-term clinical consequences attributed to his illness. See Supplemental Materials for detailed case vignettes.
Discussion
Our study demonstrates that EBV infection is an exceedingly rare cause of ALF, accounting for only 0.21 % of consecutive adult ALF cases in a cohort of 1,887 patients. A prior study from the ALFSG also demonstrated that HSV infection was an uncommon cause of ALF and was not responsible for any of the indeterminate ALF cases [4]. In addition, prior studies from the ALFSG have failed to demonstrate evidence of SEN-V, occult HBV, or hepatitis E infection in adult patients with indeterminate ALF [4–7].
The small number of cases of EBV-related ALF enrolled in our study makes it difficult to confidently identify clinical features or potential risk factors. Overall, the EBV cases tended to occur in young adults, which is in keeping with the known epidemiology of symptomatic infectious mononucleosis in the US [20]. The hematological profile at presentation varied from mild leukopenia to mild leukocytosis with similarly variable hemoglobin and platelet counts (Table 1). While serum aminotransferase levels were highly variable, all of the subjects were overtly jaundiced and had been ill for 2–3 weeks prior to presentation. Although pre-existing immunosuppression has been implicated in more severe primary EBV infection, only one of our four cases was immunosuppressed [21, 22].
Primary EBV infection can cause a mild self-limited hepatitis which typically resolves without clinical sequelae; jaundice can be seen in 5–10 % of cases [23]. The classic triad of fever, sore throat, and lymphadenopathy describes acute infectious mono, although gastrointestinal symptoms such as abdominal pain, nausea, and diarrhea can be present. Few cases have been described documenting severe hepatitis or acute hepatic failure, but clinically significant hepatic damage from EBV infection in immunosuppressed patients, such as HIV positive patients, transplant patients, or inflammatory bowel disease patients on immunosuppressive medications has been reported [20–22]. A review of the literature produced only four additional cases in which EBV-related ALF was treated with emergency LT (Table 3). Of note, all of these patients were young (under the age of 30) and had evidence of EBV infection via EBER staining of liver tissue, and none were immunocompromised. Though extended mortality data was unavailable for these additional patients, all were reported as surviving at least to the immediate post-transplant period (range 8 days–2 years), unlike our ALFSG cohort, where two of the four cases died before transplant.
Acute severe hepatitis in young, immunocompetent patients has also been previously reported [24, 25]. In some of the larger case series describing EBV hepatitis, a cholestatic pattern is seen in 65 % of patients and the disease course is usually mild and self-limited [8]. Compared to other hepatotropic and non-hepatotropic viruses, symptomatic EBV hepatitis can present with a cholestatic liver injury profile [26]. Interestingly, two of our patients had a cholestatic liver injury profile at presentation (Table 1).
Diagnostic testing for EBV was heterogeneous in our patients emphasizing the difficulty in confidently establishing an early diagnosis of EBV-hepatitis [17]. Furthermore, findings on liver biopsy were also varied and nonspecific as has been reported in HSV hepatitis [4]. Portal inflammation may be observed along with sinusoidal lymphocytes that stain positive on EBER staining but sinusoidal lymphocytes may also be sparse and frequently missed in these cases [28]. A study that compared EBV DNA PCR performed on liver tissue and EBER staining demonstrated similar sensitivity between the two methods in confirming a diagnosis of EBV-related hepatitis; however, prior to performing these special studies a clinical suspicion for EBV infection is required [27]. In addition, the contribution of EBV infection to liver failure or hepatitis can be difficult to determine, particularly in immunosuppressed patients wherein the serum EBV DNA viral load may transiently increase with immunosuppression or during evolving acute infection. A high circulating viral load in the bloodstream may produce a false positive EBVDNA by PCR in liver tissue as a passive phenomenon, even when EBV has not actively infected hepatocytes. Though it could be falsely implicated as a cause, one study found that 7 of 68 liver samples from patients with “liver disease of unknown etiology” tested positive for EBV DNA PCR, suggesting a role for EBV in indeterminate acute liver injury [28]. Though different histologic patterns of liver injury have been described in EBV infection, the exact mechanism of liver injury in EBV-related ALF remains unclear [29, 30].
Rapidly establishing a diagnosis of EBV-related ALF can assist with clinical management and potential use of antiviral agents. However, treatment of severe EBV hepatitis with an antiviral agent has only rarely been described in the literature [31, 32]. In our case series, all patients were treated with acyclovir, ganciclovir, or famciclovir, with some subjects receiving more than one agent. All of the patients were treated with an intravenous antiviral but it is difficult to determine how efficacious this was since serial serum EBV DNA samples were not available. In addition, several of the patients were treated with high dose steroids in an effort to reduce the host immune response which is frequently recommended in patients with infectious mononucleosis who have marked adenopathy or splenomegaly. However, the benefit of corticosteroids in patients with severe EBV infection is difficult to determine. Currently, there are no guidelines for treatment of severe EBV hepatitis but initiation of an antiviral would appear to be prudent as is recommended for HSV hepatitis [4].
A limitation of the present study is the small number of cases identified, making it difficult to draw broad-based conclusions. Furthermore, the ALFSG includes only liver transplant centers, so patients with EBV-related ALF who recover or die prior to transfer to a transplant center may be missed. In addition, EBV serologies were incomplete in some cases making it difficult to distinguish primary infection from reactivation. EBV has been implicated as a cause of hemophagocytic lymphohistiocytosis, a multi-system syndrome resulting from uncontrolled immune activation and macrophage proliferation [33]. It remains unclear if the pathogenesis of EBV-related ALF is due to an overwhelming host immune response to EBV antigens or aberrant viral replication. Of note, both patients in our series who had clinical symptoms of infectious mononucleosis (fever, sore throat, or lymphadenopathy) died.
In summary, our data demonstrate that severe acute EBV infection is an exceedingly rare cause of ALF in adult Americans accounting for only 0.21 % of consecutive cases. Most of the afflicted patients were young and only one was immunocompromised. Patients may not have the classical symptoms of infectious mononucleosis at presentation and 50 % have a cholestatic liver injury prolife. Review of the liver tissue in three cases demonstrated highly variable histology ranging from submassive necrosis to histological cholestasis. Antiviral therapy was provided to all patients and corticosteroids were given to three of four. However, the optimal dose and duration of these interventions remains unclear. The single subject who spontaneously recovered has done well during prolonged follow-up with no evidence of residual liver disease or persistent infection. Similarly, the single LT recipient in our series and four other patients reported in the literature have done well during follow-up suggesting that LT appears to be a viable treatment option. EBV-related ALF may be under-recognized as a cause of ALF but further studies are needed. A combination of monospot testing, EBV serologies, and EBV DNA testing of serum and liver tissue may be of value in the evaluation of adult ALF patients with indeterminate ALF [19].
Supplementary Material
Acknowledgments
We gratefully acknowledge the support provided by the members of The Acute Liver Failure Study Group. This study was funded by the National Institute of Diabetes, Digestive and Kidney Diseases (DK U-01-58369). Additional funding was provided by the Tips Fund of Northwestern Medical Foundation and the Jeanne Roberts and Rollin and Mary Ella King Funds of the Southwestern Medical Foundation. Additionally, Dr. Melllinger is supported by the T32 DK62708-01, NIDDK Training Grant in Gastrointestinal Epidemiology, and a Clinical and Translational Science Award from the Michigan Institute for Clinical and Health Research.
Abbreviations
- ALF
Acute liver failure
- ALFSG
Acute Liver Failure Study Group
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CMV
Cytomegalovirus
- EBER
Epstein–Barr encoded RNA
- EBV
Epstein–Barr virus
- HSV
Herpes simplex virus
- INR
International normalized ratio
- NA
Nuclear antigen
- LT
Liver transplantation
- ULN
Upper limit of normal
- VCA
Viral capsid antigen
- WBC
White blood count
Appendix
Members and institutions participating in the Acute Liver Failure Study Group 1998–2010 are as follows: W.M. Lee, M.D. (Principal Investigator), George A. Ostapowicz, M.D., Frank V. Schiødt, M.D., Julie Polson, M.D., University of Texas Southwestern, Dallas, TX; Anne M. Larson, M.D., Iris Liou, M.D., University of Washington, Seattle, WA; Timothy Davern, M.D., University of California, San Francisco, CA (current address: California Pacific Medical Center, San Francisco, CA), Oren Fix, M.D., University of California, San Francisco; Michael Schilsky, M.D., Mount Sinai School of Medicine, New York, NY (current address: Yale University, New Haven, CT); Timothy McCashland, M.D., University of Nebraska, Omaha, NE; J. Eileen Hay, M.B.B.S., Mayo Clinic, Rochester, MN; Natalie Murray, M.D., Baylor University Medical Center, Dallas, TX; A. Obaid S. Shaikh, M.D., University of Pittsburgh, Pittsburgh, PA; Andres Blei, M.D., Northwestern University, Chicago, IL (deceased), Daniel Ganger, M.D., Northwestern University, Chicago, IL; Atif Zaman, M.D., University of Oregon, Portland, OR; Steven H.B. Han, M.D., University of California, Los Angeles, CA; Robert Fontana, M.D., University of Michigan, Ann Arbor, MI; Brendan McGuire, M.D., University of Alabama, Birmingham, AL; Raymond T. Chung, M.D., Massachusetts General Hospital, Boston, MA; Alastair Smith, M.B., Ch.B., Duke University Medical Center, Durham, NC; Robert Brown, M.D., Cornell/Columbia University, New York, NY; Jeffrey Crippin, M.D., Washington University, St Louis, MO; Edwin Harrison, Mayo Clinic, Scottsdale, AZ; Adrian Reuben, M.B.B.S., Medical University of South Carolina, Charleston, SC; Santiago Munoz, M.D., Albert Einstein Medical Center, Philadelphia, PA; Rajender Reddy, M.D., University of Pennsylvania, Philadelphia, PA; R. Todd Stravitz, M.D., Virginia Commonwealth University, Richmond, VA; Lorenzo Rossaro, M.D., University of California Davis, Sacramento, CA; Raj Satyanarayana, M.D., Mayo Clinic, Jacksonville, FL; and Tarek Hassanein, M.D., University of California, San Diego, CA. The University of Texas Southwestern Administrative Group included Grace Samuel, Ezmina Lalani, Carla Pezzia, and Corron Sanders, Ph.D., Nahid Attar, the Statistics and Data Management Group included Joan S. Reisch, Ph.D., Linda S. Hynan, Ph.D., Janet P. Smith, Joe W. Webster and Mechelle Murray, and the Medical University of South Carolina Data Coordination Unit included Valerie Durkalski, Ph.D., Wenle Zhao, Ph.D., Catherine Dillon, and Tomoko Goddard.
Footnotes
On behalf of the US Acute Liver Failure Study Group.
Members and institutions participating in the Acute Liver Failure Study Group are presented in “Appendix”.
Electronic supplementary material The online version of this article (doi:10.1007/s10620-014-3029-2) contains supplementary material, which is available to authorized users.
Conflict of interest None.
Contributor Information
Jessica L. Mellinger, Division of Gastroenterology and Hepatology, Department of Internal Medicine, University of Michigan Health System, 3912 Taubman Center, 1500 E. Medical Center Drive, SPC 5362, Ann Arbor, MI 48109-0362, USA
Lorenzo Rossaro, Division of Gastroenterology and Hepatology, Davis Medical Center, University of California, Sacramento, CA, USA.
Willscott E. Naugler, Department of Medicine, Oregon Health Sciences University, Portland, OR, USA
Satish N. Nadig, Division of Transplant Surgery, University of Michigan Health System, Ann Arbor, MI, USA
Henry Appelman, Department of Pathology, University of Michigan Health System, Ann Arbor, MI, USA.
William M. Lee, Division of Digestive and Liver Diseases, Department of Internal Medicine, University of Texas Southwestern, Dallas, TX, USA
Robert J. Fontana, Division of Gastroenterology and Hepatology, Department of Internal Medicine, University of Michigan Health System, 3912 Taubman Center, 1500 E. Medical Center Drive, SPC 5362, Ann Arbor, MI 48109-0362, USA
References
- 1.Polson J, Lee WM. American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 2.Lee WM, Squires RH, Nyberg SL, et al. Acute liver failure: summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 4.Levitsky J, Duddempudi AT, Lakeman FD, et al. Detection and diagnosis of herpes simplex virus infection in adults with acute liver failure. Liver Transpl. 2008;14:1498–1504. doi: 10.1002/lt.21567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davern TJ, Chalasani N, Fontana RJ, et al. Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011;141:1665–1672. doi: 10.1053/j.gastro.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen EY, Baum K, Collins W, et al. Hepatitis E masquerading as drug-induced liver injury. Hepatology. 2012;56:2420–2423. doi: 10.1002/hep.26158. [DOI] [PubMed] [Google Scholar]
- 7.Huang RJ, Varr BC, Triadafilopoulos G. Acute fulminant hepatic failure associated with parvovirus B19 infection in an immuno-competent adult. Dig Dis Sci. 2012;57:2811–2813. doi: 10.1007/s10620-012-2110-y. [DOI] [PubMed] [Google Scholar]
- 8.Kofteridis DP, Koulentaki M, Valachis A, et al. Epstein Barr virus hepatitis. Eur J Intern Med. 2011;22:73–76. doi: 10.1016/j.ejim.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis. 2010;50:1439–1447. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umemura T, Tanaka E, Ostapowicz G, et al. Investigation of SEN virus infection in patients with cryptogenic acute liver failure, hepatitis-associated aplastic anemia, or acute and chronic non-A–E hepatitis. J Infect Dis. 2003;188:1545–1552. doi: 10.1086/379216. [DOI] [PubMed] [Google Scholar]
- 11.Lee WM, Brown KE, Young NS, et al. Brief report: no evidence for parvovirus B19 or hepatitis E virus as a cause of acute liver failure. Dig Dis Sci. 2006;51:1712–1715. doi: 10.1007/s10620-005-9061-5. [DOI] [PubMed] [Google Scholar]
- 12.Dumortier J, Mekki Y, Rimmelé T, et al. Hépatite fulminanteà virus Epstein–Barr:évolution favorable après transplantation hépatique. Gastroentérologie Clinique et Biologique. 2007;31:725–728. doi: 10.1016/s0399-8320(07)91934-x. [DOI] [PubMed] [Google Scholar]
- 13.Park HW, Lee SG, Hwang SK, et al. Epstein–Barr virus associated fulminant hepatitis requiring liver transplantation (case report) [abstract]. HPB Off J Int Hepato Pancreato Biliary Assoc. 2011;13:174–244. [Google Scholar]
- 14.Tohyama T, Takada Y, Waianabe J, et al. ABO incompatible living donor liver transplantation saved a child with fulminant hepatic failure caused by primary EBV infection [abstract]. Liver Transpl. 2011;S1:S146. [Google Scholar]
- 15.Feranchak AP, Tyson RW, Narkewicz MR, et al. Fulminant Epstein–Barr viral hepatitis: orthotopic liver transplantation and review of the literature. Liver Transpl Surg. 1998;4:469–476. doi: 10.1002/lt.500040612. [DOI] [PubMed] [Google Scholar]
- 16.Silfeler I, Kurnaz H, Acar Y, et al. EBV-induced fulminant hepatic failure treated with liver transplantation. Pak J Med Sci. 2010;26:971–972. [Google Scholar]
- 17.Suh MY, Wang K, Gutweiler JR, et al. Liver transplantation for Epstein Barr virus (EBV)-related hepatic failure using a graft from hepatitis B core antibody positive (HBcAb?) donor [abstract]. Pediatr Transpl. 2007;11:249. [Google Scholar]
- 18.Matsusaki T, Morimatsu H, Matsumi J, et al. Two cases of fulminant hepatitis due to Epstein Barr virus [abstract]. Anesth Analg. 2010;110:S286. [Google Scholar]
- 19.Bakay HK, Silfeler I, Hamilcikan B, et al. EBV-induced fulminant hepatic failure treated with liver transplantation [abstract]. Acta Paediatr. 2008;97:1–259. [Google Scholar]
- 20.Cohen JI. Epstein–Barr virus infection. N Engl J Med. 2000;343:481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 21.Posthuma EF, Westendorp RG, van der Sluys Veer A, et al. Fatal infectious mononucleosis: a severe complication in the treatment of Crohn's disease with azathioprine. Gut. 1995;36:311–313. doi: 10.1136/gut.36.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.N'guyen Y, Andreoletti L, Patey M, et al. Fatal Epstein–Barr virus primo infection in a 25-year-old man treated with azathioprine for Crohn's disease. J Clin Microbiol. 2009;47:1252–1254. doi: 10.1128/JCM.02052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crum NF. Epstein Barr virus hepatitis: case series and review. South Med J. 2006;99:544–547. doi: 10.1097/01.smj.0000216469.04854.2a. [DOI] [PubMed] [Google Scholar]
- 24.Ader F, Chatellier D, Le Berre R, et al. Fulminant Epstein–Barr virus (EBV) hepatitis in a young immunocompetent subject. Med Mal Infect. 2006;36:396–398. doi: 10.1016/j.medmal.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Pagidipati N, Obstein KL, Rucker-Schmidt R, et al. Acute hepatitis due to Epstein–Barr virus in an immunocompetent patient. Dig Dis Sci. 2009;55:1182–1185. doi: 10.1007/s10620-009-0835-z. [DOI] [PubMed] [Google Scholar]
- 26.Vine LJ, Shepherd K, Hunter JG, et al. Characteristics of Epstein–Barr virus hepatitis among patients with jaundice or acute hepatitis. Aliment Pharmacol Ther. 2012;36:16–21. doi: 10.1111/j.1365-2036.2012.05122.x. [DOI] [PubMed] [Google Scholar]
- 27.Suh N, Liapis H, Misdraji J, et al. Epstein–Barr virus hepatitis: diagnostic value of in situ hybridization, polymerase chain reaction, and immunohistochemistry on liver biopsy from immunocompetent patients. Am J Surg Pathol. 2007;31:1403–1409. doi: 10.1097/PAS.0b013e31802ffdd5. [DOI] [PubMed] [Google Scholar]
- 28.Drebber U, Kasper HU, Krupacz J, et al. The role of Epstein–Barr virus in acute and chronic hepatitis. J Hepatol. 2006;44:879–885. doi: 10.1016/j.jhep.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Markin RS. Manifestations of Epstein–Barr virus-associated disorders in liver. Liver. 1994;14:1–13. doi: 10.1111/j.1600-0676.1994.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 30.Markin RS, Linder J, Zuerlein K, et al. Hepatitis in fatal infectious mononucleosis. Gastroenterology. 1987;93:1210–1217. doi: 10.1016/0016-5085(87)90246-0. [DOI] [PubMed] [Google Scholar]
- 31.Adams LA, Deboer B, Jeffrey G, et al. Ganciclovir and the treatment of Epstein–Barr virus hepatitis. J Gastroenterol Hepatol. 2006;21:1758–1760. doi: 10.1111/j.1440-1746.2006.03257.x. [DOI] [PubMed] [Google Scholar]
- 32.Pisapia R, Mariano A, Rianda A, et al. Severe EBV hepatitis treated with valganciclovir. Infection. 2013;41:251–254. doi: 10.1007/s15010-012-0303-0. [DOI] [PubMed] [Google Scholar]
- 33.Liapis K, Apostolidis J, Delimpasis S. EBV-associated hemophagocytic syndrome. Am J Hematol. 2011;86:422. doi: 10.1002/ajh.21811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.