Abstract
Glaucoma is a complex, life-long disease that requires an individualized, multifaceted approach to treatment. Most patients will be started on topical ocular hypotensive eyedrop therapy and over time, multiple classes of drugs will be needed to control their intraocular pressure (IOP). The search for drugs with novel mechanisms of action, to treat those who do not achieve adequate IOP control with, or become refractory to, current therapeutics, is ongoing, as is the search for more efficient, targeted drug delivery methods. Gene transfer and stem cell applications for glaucoma therapeutics are moving forward. Advances in imaging technologies improve our understanding of glaucoma pathophysiology and enable more refined patient evaluation and monitoring, improving patient outcomes.
New Glaucoma Drugs in the Pipeline
Targeting the Trabecular Meshwork
Current glaucoma therapeutics lower IOP by reducing aqueous humor formation or increasing outflow of fluid through the uveoscleral pathway. A novel strategy is targeting the trabecular meshwork cytoskeleton aiming to increase fluid outflow through the trabecular meshwork (TM) /conventional outflow pathway. (1, 2) There are several targets for this approach: 1) TM – cytoskeleton-actin microfilament disruption using marine macrolides such as latrunculins (Lat-A/B) (WARF) (3–9) (FIG 1), swinholide A, jasplakinolide (10) (WARF); 2) Protein kinase inhibition using serine–threonine kinase inhibitors such as H-7 (WARF) (11), myosin light chain kinase inhibitor ML-7 (12) and rho kinase inhibitors including Y-39983/SNJ-1656/RKI-983 (Senju / Novartis) (13–15), AR-12286 (Aerie) (16,17), AR-13324 (Aerie) (18), PG324 (which is AR-13324 combined with latanoprost) (Aerie), K-115 (Kowa) (19,20), AMA0076 (Amakem) (21); 3) targeting actomyosin contractility using nonmuscle caldesmon (WARF) (22,23) or focal adhesions and cell-cell adhesions with exoenzyme C3 transferase (C3) (WARF) (24). A number of these compounds are moving through clinical trials with a mechanism of action that features relaxation of the TM, expansion of juxtacanlicular spaces (JCT), dilation of Schlemm’s canal SC and inhibition of actomyosin contractility. Although they comprise different classes, many of the above compounds can be effective at increasing conventional outflow since the real target is perturbing the overall system contractility, cell-matrix/cell-cell adhesion tension: all of which constitute a regulatory system, with efferent/afferent arms, that is likely responsive to IOP differential across the TM tissue. (25–27)
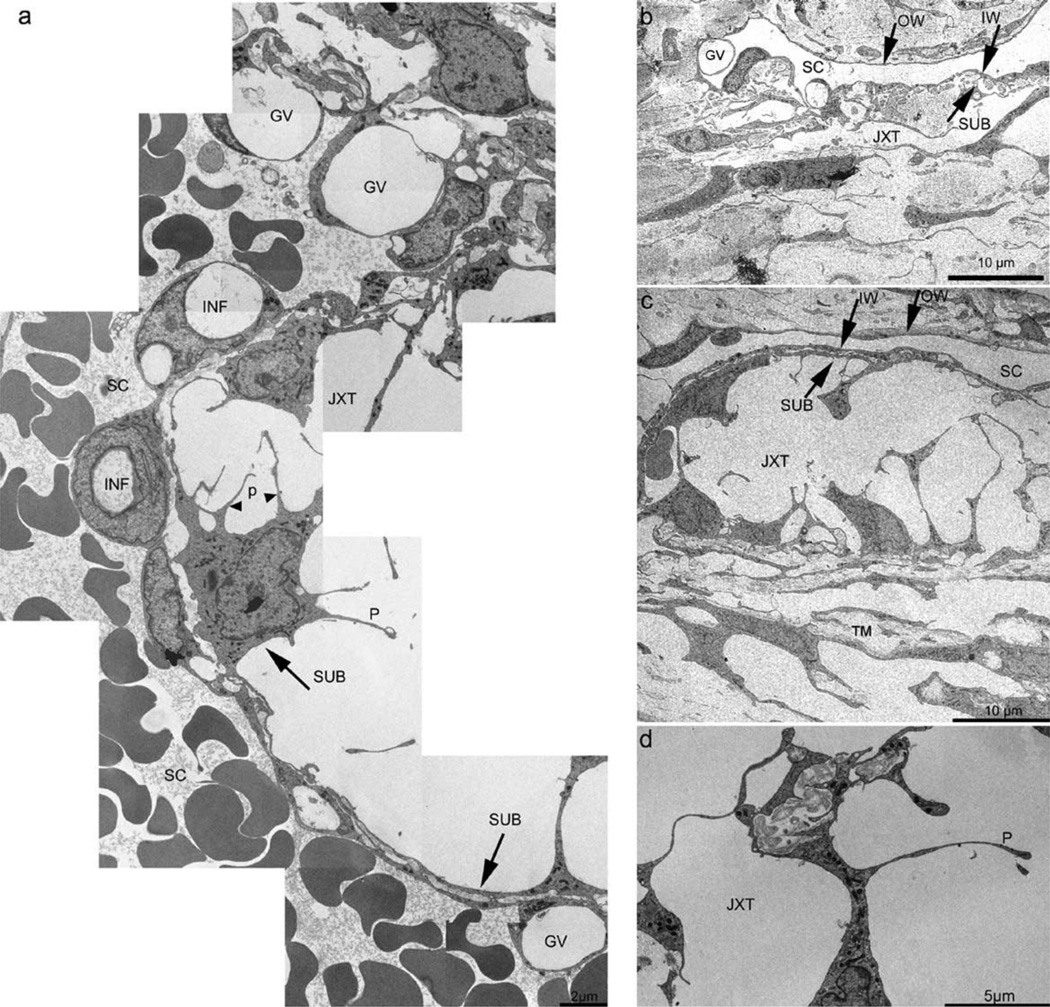
Figure 1.
Transmission EM of the trabecular meshwork (TM) following LAT-B (a, c and d [K554]) or vehicle (b [K596]). In (a), a long ‘montage’ of images is shown, depicting the IW - JXT regions of the TM following LAT-B. Panel (b) shows normal JXT region and its circumjacent structures; (c) indicates the massive ‘ballooning’ of the JXT region and the retention of close contact between IW and SUB (compare to (b)); (d) shows the absence of organelles from processes, irregular diameter of processes, and the entrapment of extracellular matrix deposits in intercellular spaces. GV, giant vacuoles; INF, membrane infoldings; IW, inner wall; JXT, juxtacanalicular region; OW, outer wall; P, cellular processes; SC, Schlemm’s canal; SUB, sub-canalicular cells. With permission from Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Latrunculin B effects on trabecular meshwork and corneal endothelial morphology in monkeys. Exp Eye Res. 2006;82(2):236–246.
Various classes of adenosine agonists may also lower IOP by increasing trabecular outflow (28), with several receptor subtypes (A1, A2A, and A3) in development as glaucoma therapeutics. Selective adenosine A1 agonist INO-8875 (Inotek) is thought to increase trabecular outflow by reducing cell volume and remodeling the extracellular matrix following secretion of matrix metalloproteinases. (29) Novel adenosine A2a receptor agonist OPA-6566 (Acucela and Otsuka Pharmaceuticals) is thought to lower IOP in human patients by stimulating aqueous humor outflow via the TM (30). A2A receptors mediate vasodilatation, coupling through G proteins to stimulate adenylyl cyclase, and may be down-regulated after chronic exposure to an agonist (31, 32). A3 / A1 receptor agonist CF-101 (Can-Fite BioPharma) is an orally administered compound that showed IOP lowering efficacy in a phase II clinical trial aimed at reducing symptoms of dry eye (33). A3 receptor agonists are thought to reduce IOP by inhibiting Cl− channels of the nonpigmented ciliary epithelial (NPE) cells at the aqueous surface of the ciliary epithelium, reducing aqueous humor production (34–36).
Prostaglandin analogs (PGs) that target the EP2 and EP4 receptors may also increase outflow through the TM pathway. A selective prostanoid EP4 receptor agonist (3,7-dithia PGE1) lowered IOP and increased total outflow facility in monkeys. No effect was seen on uveoscleral outflow or aqueous flow, suggesting that a substantial proportion of the ocular hypotensive activity was due to increased trabecular outflow facility. (37) Further studies with 3,7-dithiaPGE using human cell cultures and a whole-eye organ perfusion system showed that human SC and TM cells do express PG-EP4 receptors and their activation in the human conventional pathway results in a significantly increased outflow facility.(38) The prostanoid EP2 receptor agonist butaprost is thought to lower IOP by increasing uveoscleral outflow (39) but other EP2 receptor agonists (e.g. Taprenepag isopropyl [formerly known as PF 04217329]) appear to be additive to latanoprost, (40, 41) suggesting that there may be a different mechanism of action with this class of compounds
Combination molecules
Most new TM drugs will want to demonstrate additivity and compatibility with the currently most prescribed compounds, the PGs, which lower IOP by increasing uveoscleral outflow. Additional daily drops become burdensome for patients and adherence can decrease. Combination formulations were developed that combine 2 mechanisms, 2 targets, 2 molecules into a single drop. Initial fixed-dose combinations all contained the β-adrenergic antagonist timolol but newer combinations include prostaglandins as well. A novel combination compound in development by Bausch and Lomb is Latanoprostene Bunod, which combines 2 mechanisms and 2 targets in 1 molecule. Latanoprostene bunod (BOL-303259-X) 0.024% is a nitric oxide (NO)-donating prostaglandin F2α agonist that is rapidly metabolized in situ to latanoprost acid (to target uveoscleral outflow) and BDMN, a NO-donating moiety (to target TM outflow) (42). Aerie Pharmaceuticals has two fixed dose combination products in development. AR 13324 consists of an RKI (to target the TM) and a norepinephrine transporter inhibitor (to target aqueous humor inflow). Aerie’s PG324 compound consists of 2 molecules, 3 mechanisms, 3 targets achieved by combining AR 13324 with PG latanoprost (to target uveoscleral outflow). If the B&L and Aerie products were combined the resulting compound would have 2 molecules, 4 mechanisms, 3.5 targets.
Drug delivery
Determining the best methods for getting the drugs to the target tissue, at an effective dose, while minimizing issues of patient adherence is a complex process. Topical drops are easy for patients to use but adherence wanes over time and with increasing numbers of drugs/doses. Preservatives, such as benzalkonium chloride, used in the more cost effective multi-dose bottles, can cause ocular surface issues (43, 44) and may contribute to worsening of chronic conditions such as glaucoma. (45 – 50)
Newer topical drop formulations aim to increase ocular bioavailability through manipulation of solution viscosity or corneal penetration by polymers, collagen shields, gels, nanoparticles, microemulsions and liposomes. (51, 52) Nanoparticles developed specifically for ophthalmic use are often polymeric colloidal particles in which the therapeutic agent is either encapsulated in a polymer (nanocapsule) or dispersed in the polymer matrix (nanosphere). (53) An advantage to these systems is that they can be engineered to be relatively cell-specific.
While injections and implants are commonly used to deliver drugs to both the anterior and posterior chambers, these can be somewhat invasive procedures and do entail some risk to the patient. A variety of novel drug delivery strategies are in development. Suprachoroidal injection using hollow microneedles may be a less invasive way to target the delivery of drugs to the choroid and retina than the commonly performed intravitreal injections. (54) Encapsulated cell technology implants have been used to deliver ciliary neurotrophic factor over a period of up to 2 years in patients with retinitis pigmentosa (RP) and geographic atrophy (GA). (55) Drug levels remained stable in the eye while CNTF, anti-CNTF antibodies, and antibodies to the encapsulated cells were not detected in the serum of patients, indicating no systemic exposure response. (55,56) Minimally invasive glaucoma surgical techniques might also be used to deliver drugs directly to the relevant tissues.
Gene transfer
A longer term drug delivery option in early phase clinical trials for retinal disease is gene therapy, where the goal is to reprogram target cells to up or down regulate a biochemical / physiological process to make more or less of something. Gene therapy strategies for glaucoma include increasing conventional outflow, increasing uveoscleral outflow, decreasing aqueous humor production and neuroprotection including rescue, regeneration & targeting of retinal ganglion cell (RGC) axons and RGC soma. (57)
Gene therapy has achieved some success in retinal applications where it has been used to improve vision in people with the retinal disease Leber's congenital amaurosis (58 – 61) and recently choroideremia (62), a rare type of inherited eye disease. These trials have reported positive results, which have helped further the field of ocular gene therapy by providing much needed safety and efficacy data for viral vectors (specifically adeno-associated virus or AAV vectors), paving the way for future therapeutics. The NEI has a unit on ocular gene therapy focused on developing AAV vectors for clinical disease targets of X-linked retinoschisis, retinitis pigmentosa, and macular degeneration. (63) In addition, the FDA is continuing to develop and refine guidance documents regarding cellular and gene therapy product development. These are necessary to clarify the special considerations for monitoring and follow-up include immunogenicity, persistence, migration, shedding, and growth and development. (64)
In animal models, experiments have demonstrated that AAV and scAAV (self-complementary adeno-associated virus) viral vectors can be safely delivered to the trabecular meshwork and that expression of a GFP reporter gene can be stably expressed and monitored serially and non-invasively for 2+ years. (65, 66) (FIG 2) Experiments delivering feline immunodeficiency virus (FIV)–based lentiviral vectors encoding elements of the prostaglandin pathway (COX-2, PGFSynthase, FP receptor) resulted in long term expression and decreased IOP lasting for the 5 month duration of the experiments in cats. The combination of the COX-2co and FPRco vectors produced the largest decrease in IOP. (67) Experiments injecting viral vectors encoding the cDNA for bovine PGF synthase in monkey eyes showed a significant decrease in IOP for 5 months. (68) Further work is needed to bring this promising technology to the clinic. Issues to be addresses include viral toxicity, regulation of gene expression - turning the gene on/off, immune/inflammatory responses and localization of transfection and/or gene activity. (69)
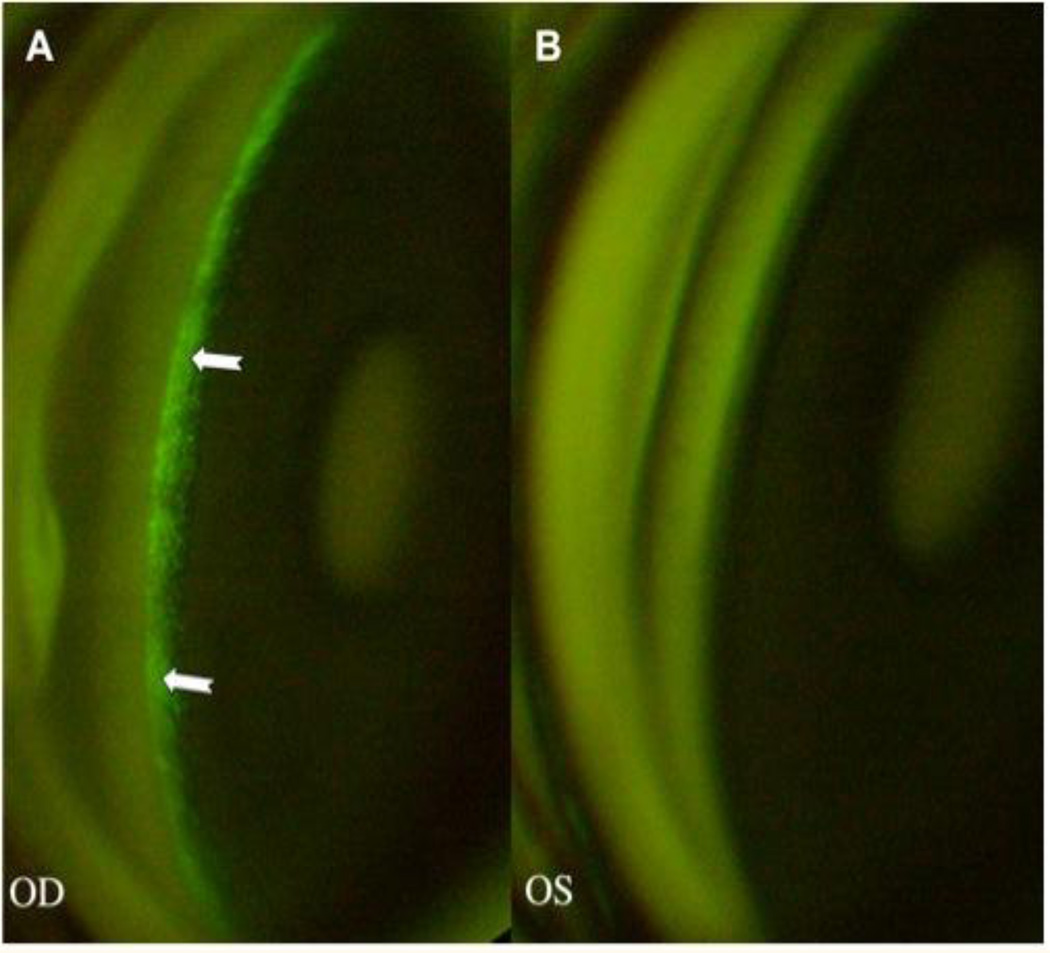
Figure 2.
Fluorescence, localized in the TM (arrows), is seen 5 days post-injection in an eye that received 1.0 × 108 TUs of an eGFP-expressing FIV vector. B: The control eye, injected with an equivalent volume of saline, shows no fluorescence. With permission from Liu X, Brandt CR, Rasmussen CA, Kaufman PL. Ocular drug delivery: molecules, cells, and genes. Can J Ophthalmol. 2007 Jun;42(3):447-54. Review.
Gene therapy constructs for retinal applications are commonly delivered via subretinal injection. While not without risks, (70) the procedure delivers the vector directly to the affected area. In much the same way, gene therapy constructs for glaucoma could potentially be delivered directly to Schlemm’s canal via canaloplasty (71) or other minimally invasive surgical techniques. Benefits of this approach include the ability to use smaller volumes/doses of vector and delivering it directly to an area where resistance to outflow is known to occur. (72) Regardless of the delivery method or location, it is likely that some cells other than the target cell type will be transduced by viral vectors. Whether that is detrimental or not remains to be determined.
Stem cells
As with gene therapy, retinal applications for stem cell therapies are in a more advanced stage of development than those for glaucoma. A recent review paper lists 20+ clinical trials using a variety of cell based therapies for retinal degenerative diseases including human embryonic stem cell (hES), bone marrow stem cell (BMSC), mesenchymal stem cell (MSC), human neural stem cell (hNSC) and induced pluripotent stem cells (iPSC). (73) Some of the positive aspects of gene transfer and stem cell applications are the same – there is a relatively small area/number of cells to replace, the eye offers visualization of the transplant site allowing visualization of effects directly, serially and non-invasively, but there is a fundamental difference in approach. While gene therapy is focused on preserving the remaining cells in a degenerative disease environment (e.g. retinal ganglion cells in glaucoma or photoreceptor cells in macular degeneration), delaying the onset or progression of degeneration, stem cell therapy is intended to regenerate or replace lost tissue. Embryonic stem cells, induced pluripotent stem cells, mesenchymal stem cells and retinal stem cells have all been investigated for their potential in restoring cell types and the functionality lost in retinal diseases. (74)
Knowledge gained from the study of human embryonic stem cells and mammalian somatic cell reprogramming has led to the production of human induced pluripotent stem cells (hiPSCs). hiPSCs have potential for use in transplantation, high throughput drug screening, cell-culture disease modeling, disease gene discovery, and gene therapy testing, (75) though there are limitations on the number of cell passages that can occur before key cytological and functional attributes are lost. (76) One of the biggest allures of hiPSCs is the ability to derive patient-specific material for both clinical and research purposes. Allogeneic transplantation may avoid potential complications due to immune rejection. Based on efficacy studies in Royal College of Surgeons (RCS) rats, whose retinal dystrophy is characterized by RPE loss and secondary photoreceptor degradation, clinical trials for atrophic AMD and Stargardt macular dystrophy using subretinal injections of dissociated hESC-RPE have been initiated. Another hiPSC RPE cell trial that utilizes monolayer sheets of cells to treat exudative AMD has been announced. (77) Prefabricated RPE monolayers could also be used for combined transplantation of photoreceptors and RPE to treat diseases where both of these cell types are lost. (78, 79)
Stem cell based therapeutics for glaucoma present a formidable challenge. Replacement RGCs would need to extend axons down the optic tract to specific terminal connections in the lateral geniculate nucleus (LGN), functionally integrating into the complex circuitry of the inner retina and extending a lengthy axon capable of synapsing at precise brain targets. (74) Transplanted neural progenitors have been reported to invade the optic nerve and grow substantial distances; opening the prospect that over longer time periods transplanted RGC axons could reach their targets. (80) Highlighting the intense interest in this field the NEI Audacious Goals Initiative is to “Regenerate Neurons and Neural Connections in the Eye and Visual System”.
Intravitreal mesenchymal stem cell (MSC) transplantation can slow RGC death in a rat model of optic nerve damage. The neuroprotective effects are thought to be due to MSC secretion of factors from the platelet-derived growth factor family, indicating that the MSC implants could mediate retinal ganglion cell neuroprotection and that platelet-derived growth factor may be a target itself for realizing retinal ganglion cell neuroprotection. Autologous transplantation of MSC is a possibility since they can be isolated from bone marrow aspirates from individual patients and expanded in vitro before transplantation.(81) MSCs have also been programmed/engineered to secrete brain-derived neurotrophic factor (BDNF). When injected intravitreally in rats, they demonstrate some degree of RGC neuroprotection. (82 – 84)
A class of glial cell that has been studied for transplantation for neuroprotection is the olfactory ensheathing cell (OEC). OECs produce several neurotrophic factors that have been studied for glaucoma treatment, including BDNF, ciliary neurotrophic factor (CNTF), nerve growth factor (NGF), and glial-derived neurotrophic factor (GDNF). (85 – 87) In a rat model of optic nerve injury, OECs were transplanted into the optic nerve sheath and NTF was delivered intravitreally. This combination prolonged RGC survival and some functional benefit was noted (improved flash visual-evoked potential latency and amplitude) up to 8 weeks post-optic nerve crush. (88, 89) As noted above for MSCs, autogologus therapeutic transplantation may be possible with OECs, as they can be readily isolated from patient nasal mucosa biopsies.
Another stem cell strategy for glaucoma is to target the outflow pathway. Human TM stem cells (TMSCs) can be isolated, expanded in vitro and transplanted back into the anterior chamber where they home to the TM.(90) and exhibit phagocytic behavior. (91) After injection, TMSCs remained viable for 4 months and and reduced IOP in rats. (91)
To fully appreciate the effects of these novel treatment strategies, techniques to visualize and quantify changes in the relevant tissues/structures are needed and are being developed.
Imaging methods
Advances in imaging techniques have led to greater understanding of the disease process and how to treat it. Diagnostic imaging has added another element to the armamentarium for detecting and monitoring disease progression and the various instruments/techniques are becoming more useful to measure efficacy in clinical trials.
Structural variations that occur in glaucoma include changes in the optic nerve head, thinning of the retinal nerve fiber layer (RNFL) and ganglion cell-inner plexiform layer (GCIPL), and atrophy of the lateral geniculate nucleus (LGN) and visual cortex, which includes layer shrinkage and reduced neuron size and numbers. (92) The correlation between axon size and number and LGN atrophy has been demonstrated in experimental models of glaucoma. (93, 94) In humans, the thickness of the visual cortex, measured by magnetic resonance imaging (MRI), correlated positively with RNFL thickness, measured with a Topcon 3D OCT-1000. (95)
Neuroimaging using several types of MRI instruments has also shown LGN degeneration in human glaucoma. Using a 1.5-Tesla MRI, LGNs from glaucoma patients with bilateral visual-field defects and age-matched controls were identified and imaged. LGN heights were significantly decreased in glaucoma subjects compared to controls. (96) Using a 3.0-Tesla MRI, researchers found that LGN maximum height was negatively correlated with optic disk damage as assessed by cup-to-disc ratio. (97) An ultra-high field 7.0T MRI and a Cirrus sdOCT instrument were used to determine that LGN volumes in POAG patients were significantly smaller than those of age and gender-matched healthy controls. Furthermore, in patients, LGN volume was significantly correlated with GC-IPL thickness of the contralateral eye. (98) Together these studies indicate that LGN atrophy may have potential as a biomarker of visual system injury or glaucoma progression in some patients, especially those with media clarity issues. (99, 100)
A variety of imaging techniques were used to demonstrate the presence of lymphatic drainage channels in human, sheep and rodent eyes, including immunofluorescence with D2-40 antibodies for podoplanin, and LYVE-1 antibodies; Iodine-125 radio-labeled human serum albumin; and quantum dot tracers respectively. (101–103) It was recently determined that mice treated with latanoprost had increased lymphatic drainage from the eye by using hyperspectral imaging at multiple times following topical application of latanoprost and intracameral injection of quantum dots as a tracer. (105) This newly identified outflow pathway may be a new target for glaucoma therapeutics.
Early identification of cellular degeneration in glaucoma, perhaps even before irreversible vision loss occurs, and monitoring disease progression are key goals for several systems aimed at imaging apoptosis in RGCs. One system uses fluorescently labeled annexin V to non-invasively visualize single retinal cells undergoing apoptosis in vivo using a wide-angle confocal laser scanning ophthalmoscope (cLSO). This has been given the acronym DARC (Detection of Apoptosing Retinal Cells). (105) A range of in vivo studies using experimental models has been performed using DARC technology for the determination of RGC apoptosis. (106) (FIG 3) Modifications of the cSLO instrument enabled simultaneous detection of multiple, spectrally distinct markers. This allowed identification and quantification of nerve cells in the early and late phases of apoptosis and necrosis in different disease models: an Ab model of RGC death (intravitreal Ab25_35), recently shown to induce RGC apoptosis in rodent eyes (107); a model of staurosporine (SSP), induced neuronal apoptosis (108); an experimental glaucoma rat model of ocular hypertension (OHT) (109) and a triple transgenic Alzheimer’s disease (AD) model (3xTg-AD) (110). The latter is a model of AD, which overexpresses APPSwe and tauP301L, as well as carries a PS1M146V knock-in mutation, and is currently the only existing transgenic model with both Ab and tau neuropathology. DARC will soon be tested in a glaucoma Phase I clinical trial (ISRCTN59484478) where the number of cells undergoing apoptosis at a given time point will be measured, with the goal of determining whether repeated imaging is useful as an indicator of disease progression.
Figure 3.
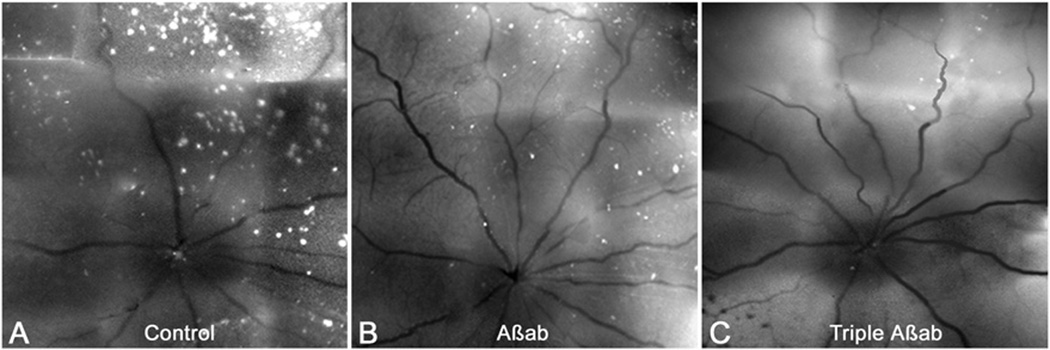
Effects of combination Aβ-targeting therapy on RGC apoptosis. Compared to nontreatment control (A), DARC imaging shows triple therapy (C, Aβab+CR+βSI) was more effective than Aβab alone (B) in reduction of RGC apoptosis in an OHT model. With permission from Guo L1, Cordeiro MF Assessment of neuroprotection in the retina with DARC. Prog Brain Res. 2008;173:437-50. doi: 10.1016/S0079-6123(08)01130-8.
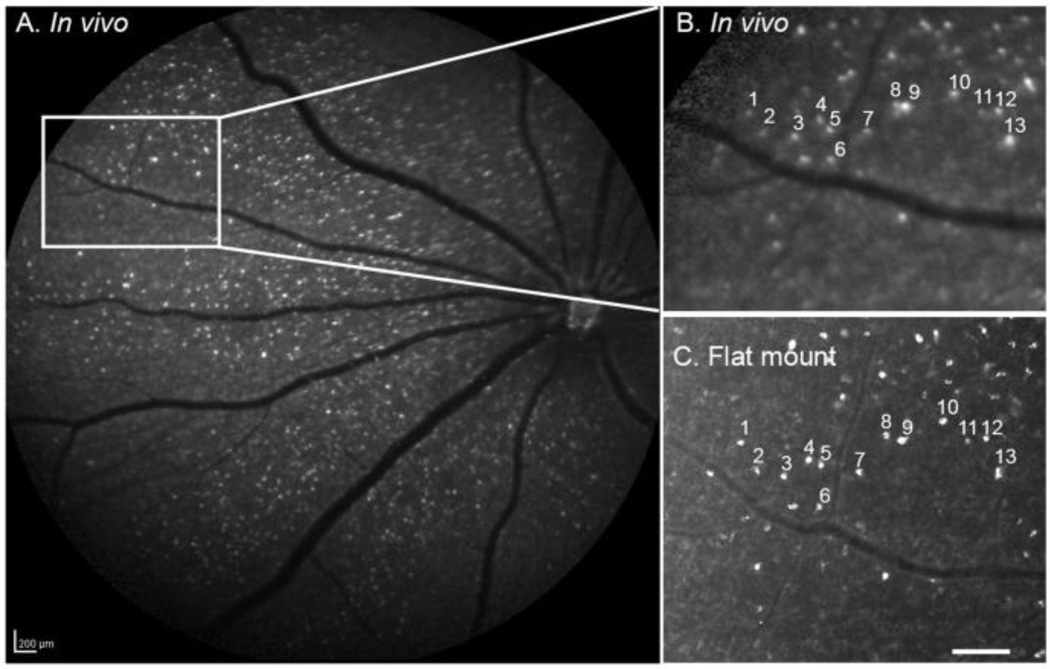
A second system in development for serial, non-invasive imaging of apoptosis is TcapQ488, which uses a cell-penetrating caspase- activatable peptide probe. (111) Initial ex-vivo studies validated highly specific uptake by RGCs following intravitreal injection of fluorophores conjugated to a modified cell-penetrating peptide sequence and subsequent localization of apoptosing cells using retinal flat mounts from a rat model of NMDA-induced RGC degeneration. (112, 113) (FIG 4) In subsequent in-vivo studies using the same rat model and a confocal scanning laser ophthalmoscope (CSLO), probe activation was characterized. Sequential non-invasive fluorescence fundus imaging of individual animals showed that the time course of the probe activation signal reached near maximal at 12 hours and remained steady to 72 hours post injection. Electroretinogram (ERG) test showed no evidence of probe toxicity. An advantage of these cell penetrating peptides is that they can be modified to deliver other molecular imaging probes to RGCs or deliver probes with enhanced uptake by other retinal cell types. (114)
Figure 4.
(A) Fluorescent fundus image obtained in vivo using the CSLO (28 hours post-probe injection) from a rat eye pretreated with NMDA followed by TcapQ488. Strong, punctate fluorescent signals were detected in the retina ganglion cell (RGC) layer. (B) Higher magnification of the boxed area in A in which prominent fluorescent signals are highlighted. (C) Ex vivo flat mount of the same retina showed excellent correspondence with in vivo images in A and B, indicating that real time images reflect single cell resolution of probe activation. Scale bar: A, 200 µm; C, 100 µm. With permission from Qiu X, Johnson JR, Wilson BS, Gammon ST, Piwnica-Worms D, Barnett EM. Single-cell resolution imaging of retinal ganglion cell apoptosis in vivo using a cell-penetrating caspase-activatable peptide probe. PLoS One. 2014 Feb 21;9(2): :e88855. doi: 10.1371/journal.pone.0088855. eCollection 2014.
The results from both imaging systems demonstrate the potential of this type of technique, not only for direct assessment of retinal ganglion cell health in neurodegenerative diseases such as glaucoma and Alzheimers, where increases and decreases in apoptotic activity can help guide treatment decisions and aiding the tracking of disease, but also to provide an assessment of the potential neuroprotective effects of novel drug candidates and their therapeutic efficacy. The development of a new and meaningful clinical endpoint would help fill an unmet need in glaucoma research and in the development of therapeutics. (115)
Conclusion
Advances in techniques that further our understanding of glaucoma pathophysiology (including continuous modeling of IOP) help inform development of novel therapeutics (including biodegradable implants) for glaucoma patients. Better drugs, better delivery methods and better patient evaluation and monitoring can enhance patient outcomes by giving physicians better tools to refine and personalize treatment strategies for this multifaceted disease.
Acknowledgments
Supported by grants from the National Institutes of Health/National Eye Institute (University of Wisconsin- Madison Core Grant for Vision Research (P30 EY016665) and P51 RR000167); Research to Prevent Blindness, Inc., New York, NY, unrestricted departmental and Physician-Scientist awards; Ocular Physiology Research and Education Foundation; and Walter Helmerich Chair from the Retina Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement
C.A. Rasmussen: No competing financial interests exist.
P.L. Kaufman: AGTC (C, R), Lens AR, Inc. (F), WARF (F, P), Z Lens, LLC (F), Alcon (C, R), Allergan (C, R), Altheos, Inc. (C, R), Bausch & Lomb (C, R), Amakem Therapeutics (C, R), Johnson & Johnson (C, R), Merck (C, R, F), Pfizer (C, R), Santen (F, C, R), Refocus (C, R). C, consultant; R, honoraria, F, financial support; P, patent.
References
- 1.Tian B, Gabelt BT, Geiger B, Kaufman PL. The role of the actomyosin system in regulating trabecular fluid outflow. Exp Eye Res. 2009 Apr;88(4):713–717. doi: 10.1016/j.exer.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian B, Kaufman PL. Comparisons of actin filament disruptors and Rho kinase inhibitors as potential antiglaucoma medications. Expert Rev Ophthalmol. 2012 Apr;7(2):177–187. doi: 10.1586/eop.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson JA, Tian B, Geiger B, Kaufman PL. Effect of latrunculin-B on outflow facility in monkeys. Exp Eye Res. 2000 Mar;70(3):307–313. doi: 10.1006/exer.1999.0797. [DOI] [PubMed] [Google Scholar]
- 4.Peterson JA, Tian B, McLaren JW, Hubbard WC, Geiger B, Kaufman PL. Latrunculins’ effects on intraocular pressure, aqueous humor flow, and corneal endothelium. Invest Ophthalmol Vis Sci. 2000;41(7):1749–1758. [PubMed] [Google Scholar]
- 5.Okka M, Tian B, Kaufman PL. Effect of low-dose latrunculin B on anterior segment physiologic features in the monkey eye. Arch Ophthalmol. 2004 Oct;122(10):1482–1488. doi: 10.1001/archopht.122.10.1482. [DOI] [PubMed] [Google Scholar]
- 6.Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Latrunculin B effects on trabecular meshwork and corneal endothelial morphology in monkeys. Exp Eye Res. 2006;82(2):236–246. doi: 10.1016/j.exer.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Ethier CR, Read AT, Chan DW. Effects of latrunculin-B on outflow facility and trabecular meshwork structure in human eyes. Invest Ophthalmol Vis Sci. 2006;47(5):1991–1998. doi: 10.1167/iovs.05-0327. [DOI] [PubMed] [Google Scholar]
- 8.Ritch R, Schiewe M, Zink RC, et al. Latrunculin B (INS115644) reduces intraocular pressure (IOP) in ocular hypertension (OHT) and primary open angle glaucoma (POAG) Invest Ophthalmol Vis Sci. 2010;51 ARVO E-Abstract and Poster 6432. [Google Scholar]
- 9.Okka M, Tian B, Kaufman PL. Effects of latrunculin B on outflow facility, intraocular pressure, corneal thickness, and miotic and accommodative responses to pilocarpine in monkeys. Trans Am Ophthalmol Soc. 2004;102:251–259. [PMC free article] [PubMed] [Google Scholar]
- 10.Tian B, Kiland JA, Kaufman PL. Effects of the marine macrolides swinholide A and jasplakinolide on outflow facility in monkeys. Invest Ophthalmol Vis Sci. 2001 Dec;42(13):3187–3192. [PubMed] [Google Scholar]
- 11.Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Functional and structural reversibility of H-7 effects on the conventional aqueous outflow pathway in monkeys. Exp Eye Res. 2004 Jan;78(1):137–150. doi: 10.1016/j.exer.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Tian B, Brumback LC, Kaufman PL. ML-7, chelerythrine and phorbol ester increase outflow facility in the monkey Eye. Exp Eye Res. 2000 Dec;71(6):551–566. doi: 10.1006/exer.2000.0919. [DOI] [PubMed] [Google Scholar]
- 13.Tokushige H, Waki M, Takayama Y, Tanihara H. Effects of Y-39983, a selective Rho-associated protein kinase inhibitor, on blood flow in optic nerve head in rabbits and axonal regeneration of retinal ganglion cells in rats. Curr Eye Res. 2011 Oct;36(10):964–970. doi: 10.3109/02713683.2011.599106. [DOI] [PubMed] [Google Scholar]
- 14.Tokushige H, Inatani M, Nemoto S, Sakaki H, Katayama K, Uehata M, Tanihara H. Effects of topical administration of y-39983, a selective rho-associated protein kinase inhibitor, on ocular tissues in rabbits and monkeys. Invest Ophthalmol Vis Sci. 2007 Jul;48(7):3216–3222. doi: 10.1167/iovs.05-1617. [DOI] [PubMed] [Google Scholar]
- 15.Tanihara H, Inatani M, Honjo M, Tokushige H, Azuma J, Araie M. Intraocular pressure-lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch Ophthalmol. 2008;126(3):309–315. doi: 10.1001/archophthalmol.2007.76. [DOI] [PubMed] [Google Scholar]
- 16.Kopczynski C, Novack GD, Swearingen D, van Haarlem T. Ocular hypotensive efficacy, safety and systemic absorption of AR-12286 ophthalmic solution in normal volunteers. Br J Ophthalmol. 2013 May;97(5):567–572. doi: 10.1136/bjophthalmol-2012-302466. [DOI] [PubMed] [Google Scholar]
- 17.Williams RD, Novack GD, van Haarlem T, Kopczynski C AR-12286 Phase 2A Study Group. Ocular hypotensive effect of the Rho kinase inhibitor AR-12286 in patients with glaucoma and ocular hypertension. Am J Ophthalmol. 2011 Nov;152(5):834–841. doi: 10.1016/j.ajo.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Kopczynski CC, Epstein DL. Emerging Trabecular Outflow Drugs. J Ocul Pharmacol Ther. 2014 Mar 1;30(2–3):85–87. doi: 10.1089/jop.2013.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanihara H, Inoue T, Yamamoto T, Kuwayama Y, Abe H, Araie M K-115 Clinical Study Group. Phase 2 randomized clinical study of a Rho kinase inhibitor, K-115, in primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 2013 Oct;156(4):731–736. doi: 10.1016/j.ajo.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Tanihara H, Inoue T, Yamamoto T, Kuwayama Y, Abe H, Araie M. K-115 Clinical Study Group. Phase 1 clinical trials of a selective Rho kinase inhibitor, K-115. JAMA Ophthalmol. 2013 Oct;131(10):1288–1295. doi: 10.1001/jamaophthalmol.2013.323. [DOI] [PubMed] [Google Scholar]
- 21.Van de Velde S, Van Bergen T, Sijnave D, Hollanders K, Castermans K, Defert O, Leysen D, Vandewalle E, Moons L, Stalmans I. AMA0076, a novel, locally acting Rho kinase inhibitor, potently lowers intraocular pressure in New Zealand white rabbits with minimal hyperemia. Invest Ophthalmol Vis Sci. 2014 Feb 18;55(2):1006–1016. doi: 10.1167/iovs.13-13157. [DOI] [PubMed] [Google Scholar]
- 22.Grosheva I, Vittitow JL, Goichberg P, Gabelt BT, Kaufman PL, Borrás T, Geiger B, Bershadsky AD. Caldesmon effects on the actin cytoskeleton and cell adhesion in cultured HTM cells. Exp Eye Res. 2006 Jun;82(6):945–958. doi: 10.1016/j.exer.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Gabelt BT, Hu Y, Vittitow JL, Rasmussen CR, Grosheva I, Bershadsky AD, Geiger B, Borrás T, Kaufman PL. Caldesmon transgene expression disrupts focal adhesions in HTM cells and increases outflow facility in organ-cultured human and monkey anterior segments. Exp Eye Res. 2006 Jun;82(6):935–944. doi: 10.1016/j.exer.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Hu Y, Filla MS, Gabelt BT, Peters DM, Brandt CR, Kaufman PL. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol Vis. 2005 Dec 13;11:1112–1121. [PubMed] [Google Scholar]
- 25.Kaufman PL. Enhancing trabecular outflow by disrupting the actin cytoskeleton, increasing uveoscleral outflow with prostaglandins, and understanding the pathophysiology of presbyopia interrogating Mother Nature: asking why, asking how, recognizing the signs, following the trail. Exp Eye Res. 2008;86:3–17. doi: 10.1016/j.exer.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stamer WD, Lei Y, Boussommier-Calleja A, Overby DR, Ethier CR. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011 Dec 9;52(13):9438–9444. doi: 10.1167/iovs.11-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman PL, Rasmussen CA. Advances in glaucoma treatment and management: outflow drugs. Invest Ophthalmol Vis Sci. 2012 May 4;53(5):2495–2500. doi: 10.1167/iovs.12-9483m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian B, Gabelt BT, Crosson CE, Kaufman PL. Effects of adenosine agonists on intraocular pressure and aqueous humor dynamics in cynomolgus monkeys. Exp Eye Res. 1997;64:979. doi: 10.1006/exer.1997.0296. [DOI] [PubMed] [Google Scholar]
- 29.Zhong Y, Yang Z, Huang WC, Luo X. Adenosine, adenosine receptors and glaucoma: an updated overview. Biochim. Biophys. Acta. 2013;1830:2882–2890. doi: 10.1016/j.bbagen.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Runyan SA, Robinson MR. Novel ocular antihypertensive compounds in clinical trials. Clin Ophthalmol. 2011;5:667–677. doi: 10.2147/OPTH.S15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantell S, Jones R, Trevethick M. Design and application of locally delivered agonists of the adenosine A(2A) receptor. Expert Rev Clin Pharmacol. 2010 Jan;3(1):55–72. doi: 10.1586/ecp.09.57. [DOI] [PubMed] [Google Scholar]
- 32.Webb RL, Sills MA, Chovan JP, Peppard JV, Francis JE. Development of tolerance to the antihypertensive effects of highly selective adenosine A2a agonists upon chronic administration. J Pharmacol Exp Ther. 1993;267(1):287–295. [PubMed] [Google Scholar]
- 33.Avni I, Garzozi HJ, Barequet IS, Segev F, Varssano D, Sartani G, Chetrit N, Bakshi E, Zadok D, Tomkins O, Litvin G, Jacobson KA, Fishman S, Harpaz Z, Farbstein M, Yehuda SB, Silverman MH, Kerns WD, Bristol DR, Cohn I, Fishman P. Treatment of dry eye syndrome with orally administered CF101: data from a phase 2 clinical trial. Ophthalmology. 2010 Jul;117(7):1287–1293. doi: 10.1016/j.ophtha.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fishman P, Cohen S, Bar-Yehuda S. Targeting the A3 adenosine receptor for glaucoma treatment (review) Mol Med Rep. 2013 Jun;7(6):1723–1725. doi: 10.3892/mmr.2013.1413. Epub 2013 Apr 4. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Do CW, Avila MY, Peterson-Yantorno K, Stone RA, Gao ZG, Joshi B, Besada P, Jeong LS, Jacobson KA, Civan MM. Nucleoside-derived antagonists to A3 adenosine receptors lower mouse intraocular pressure and act across species. Exp Eye Res. 2010 Jan;90(1):146–154. doi: 10.1016/j.exer.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell CH, Peterson-Yantorno K, Carré DA, et al. A3 adenosine receptors regulate Cl- channels of nonpigmented ciliary epithelial cells. Am J Physiol. 1999;276(3):C659–C666. doi: 10.1152/ajpcell.1999.276.3.C659. [DOI] [PubMed] [Google Scholar]
- 37.Woodward DF, Nilsson SF, Toris CB, Kharlamb AB, Nieves AL, Krauss AH. Prostanoid EP4 receptor stimulation produces ocular hypotension by a mechanism that does not appear to involve uveoscleral outflow. Invest. Ophthalmol. Vis. Sci. 2009;50:3320–3328. doi: 10.1167/iovs.08-3031. [DOI] [PubMed] [Google Scholar]
- 38.Millard LH, Woodward DF, Stamer WD. The Role of the Prostaglandin EP4 Receptor in the Regulation of Human Outflow Facility. Invest Ophthalmol Vis Sci. 2011 Jun 1;52(6):3506–3513. doi: 10.1167/iovs.10-6510. Print 2011. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson SF, Lütjen-Drecoll E, Toris CB, Krauss AH, Kharlamb A, Nieves A, Guerra T, Woodward DF. The prostanoid EP2 receptor agonist butaprost increases uveoscleral outflow in the cynomolgus monkey. Invest. Ophthalmol. Vis. Sci. 2006;47:4042–4049. doi: 10.1167/iovs.05-1627. [DOI] [PubMed] [Google Scholar]
- 40.Schachar RA, Raber S, Courtney R, Zhang M. A Phase 2, Randomized, Dose-Response Trial of EP2 Receptor Agonist Taprenepag Isopropyl (PF-04217329) Versus Latanoprost 0.005% in Open-Angle Glaucoma and Ocular Hypertension. Current Eye Research. 2011 Sep;36(9):809–817. doi: 10.3109/02713683.2011.593725. [DOI] [PubMed] [Google Scholar]
- 41.Prasanna G, Carreiro S, Anderson S, Gukasyan H, Sartnurak S, Younis H, Gale D, Xiang C, Wells P, Dinh D, Almaden C, Fortner J, Toris C, Niesman M, Lafontaine J, Krauss A. Effect of PF-04217329 a prodrug of a selective prostaglandin EP2 agonist on intraocular pressure in preclinical models of glaucoma. Exp Eye Res. 2011 Sep;93(3):256–264. doi: 10.1016/j.exer.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Krauss AH, Impagnatiello F, Toris CB, Gale DC, Prasanna G, Borghi V, Chiroli V, Chong WK, Carreiro ST, Ongini E. Ocular hypotensive activity of BOL-303259-X, a nitric oxide donating Prostaglandin F2α agonist, in preclinical models. Exp Eye Res. 2011 Mar 9; doi: 10.1016/j.exer.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355. doi: 10.1097/IJG.0b013e31815c5f4f. [DOI] [PubMed] [Google Scholar]
- 44.Fechtner RD, Godfrey DG, et al. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29(6):618–621. doi: 10.1097/ICO.0b013e3181c325b2. [DOI] [PubMed] [Google Scholar]
- 45.Baudouin C, Denoyer A, Desbenoit N, Hamm G, Grise A. In Vitro and in Vivo Experimental Studies on Trabecular Meshwork Degeneration Induced by Benzalkonium Chloride (An American Ophthalmological Society Thesis) Trans Am Ophthalmol Soc. 2012 Dec;110:40–63. [PMC free article] [PubMed] [Google Scholar]
- 46.Samples JR, Binder PS, Nayak S. The effect of epinephrine and benzalkonium chloride on cultured corneal endothelial and trabecular meshwork cells. Exp Eye Res. 1989;49(1):1–12. doi: 10.1016/0014-4835(89)90071-7. [DOI] [PubMed] [Google Scholar]
- 47.Ammar DA, Kahook MY. Effects of benzalkonium chloride- or polyquad-preserved fixed combination glaucoma medications on human trabecular meshwork cells. Mol Vis. 2011;17:1806–1813. Epub 2011 Jul 2. [PMC free article] [PubMed] [Google Scholar]
- 48.Brignole-Baudouin F, Desbenoit N, Hamm G, et al. A new safety concern for glaucoma treatment demonstrated by mass spectrometry imaging of benzalkonium chloride distribution in the eye, an experimental study in rabbits. PLoS One. 2012;7(11):e50180. doi: 10.1371/journal.pone.0050180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hopes M, Broadway D. Preservative-free Treatment in Glaucoma Is a Sensible and Realistic Aim for the Future. European Ophthalmic Review. 2010;4:23–28. [Google Scholar]
- 50.Rasmussen CA, Kaufman PL, Kiland JA. Benzalkonium chloride and glaucoma. J Ocul Pharmacol Ther. 2014 Mar-Apr;30(2–3):163–169. doi: 10.1089/jop.2013.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, Brandt CR, Rasmussen CA, Kaufman PL. Ocular drug delivery: molecules, cells, and genes. Can J Ophthalmol. 2007 Jun;42(3):447–454. [PubMed] [Google Scholar]
- 52.Barbu E, Sarvaiya I, Green KL, Nevell TG, Tsibouklis J. Vinylpyrrolidone-co-(meth)acrylic acid inserts for ocular drug delivery: synthesis and evaluation. J Biomed Mater Res A. 2005 Sep 15;74(4):598–606. doi: 10.1002/jbm.a.30329. [DOI] [PubMed] [Google Scholar]
- 53.Cai X, Conley SM, Nash Z, Fliesler SJ, Cooper MJ, Naash MI. Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J. 2010 Apr;24(4):1178–1191. doi: 10.1096/fj.09-139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel SR, Berezovsky DE, McCarey BE, Zarnitsyn V, Edelhauser HF, Prausnitz MR. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci. 2012 Jul 1;53(8):4433–4441. doi: 10.1167/iovs.12-9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kauper K, McGovern C, Sherman S, Heatherton P, Rapoza R, Stabila P, Dean B, Lee A, Borges S, Bouchard B, Tao W. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Invest Ophthalmol Vis Sci. 2012 Nov 1;53(12):7484–7491. doi: 10.1167/iovs.12-9970. [DOI] [PubMed] [Google Scholar]
- 56.Birch DG, Weleber RG, Duncan JL, Jaffe GJ, Tao W Ciliary Neurotrophic Factor Retinitis Pigmentosa Study Groups. Randomized trial of ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for retinitis pigmentosa. Am J Ophthalmol. 2013 Aug;156(2):283–292.e1. doi: 10.1016/j.ajo.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu X, Rasmussen CA, Gabelt BT, Brandt CR, Kaufman PL. Gene therapy targeting glaucoma: where are we? Surv Ophthalmol. 2009 Jul-Aug;54(4):472–486. doi: 10.1016/j.survophthal.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cideciyan AV, Jacobson SG, Beltran WA, et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci USA. 2013 Feb 5;110(6):E517–E525. doi: 10.1073/pnas.1218933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. PubMed. [DOI] [PubMed] [Google Scholar]
- 61.Jacobson SG, Cideciyan AV, Ratnakaram R, et al. Gene therapy for Leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012;130:9–24. doi: 10.1001/archophthalmol.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.MacLaren RE, Groppe M, Barnard AR, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014 doi: 10.1016/S0140-6736(13)62117-0. published online Jan 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. [accessed Apr 26, 2014]; http://www.nei.nih.gov/intramural/ocular_gene.asp.
- 64. [accessed Apr 26, 2014]; http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guida/CellularandGeneTherapy/UCM359073.
- 65.Barraza RA, Rasmussen CA, Loewen N, Cameron JD, Gabelt BT, Teo WL, Kaufman PL, Poeschla EM. Prolonged transgene expression with lentiviral vectors in the aqueous humor outflow pathway of nonhuman primates. Hum Gene Ther. 2009 Mar;20(3):191–200. doi: 10.1089/hum.2008.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buie LK, Rasmussen CA, Porterfield EC, Ramgolam VS, Choi VW, Markovic-Plese S, Samulski RJ, Kaufman PL, Borrás T. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Invest Ophthalmol Vis Sci. 2010 Jan;51(1):236–248. doi: 10.1167/iovs.09-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barraza RA, McLaren JW, Poeschla EM. Prostaglandin pathway gene therapy for sustained reduction of intraocular pressure. Mol Ther. 2010 Mar;18(3):491–501. doi: 10.1038/mt.2009.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee ES, Rasmussen CA, Filla MS, Slauson SR, Kolb AW, Peters DM, Kaufman PL, Gabelt BT, Brandt CR. Prospects for Lentiviral Vector Mediated Prostaglandin F Synthase Gene Delivery in Monkey Eyes In vivo. Curr Eye Res. 2014 Feb 21; doi: 10.3109/02713683.2014.884593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rasmussen CA, Kaufman PL. Novel therapeutic approaches for glaucoma. Drugs Fut. 2011;36(4):287. ISSN 0377-8282. [Google Scholar]
- 70.Nork TM, Murphy CJ, Kim CB, Ver Hoeve JN, Rasmussen CA, Miller PE, Wabers HD, Neider MW, Dubielzig RR, McCulloh RJ, Christian BJ. Functional and anatomic consequences of subretinal dosing in the cynomolgus macaque. Arch Ophthalmol. 2012 Jan;130(1):65–75. doi: 10.1001/archophthalmol.2011.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aktas Z, Tian B, McDonald J, Yamamato R, Larsen C, Kiland J, Kaufman PL, Rasmussen CA. Application of canaloplasty in glaucoma gene therapy: where are we? J Ocul Pharmacol Ther. 2014 Mar-Apr;30(2–3):277–282. doi: 10.1089/jop.2013.0203. Epub 2014 Feb 10…. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tian B, Kaufman PL. A Potential Application of Canaloplasty in Glaucoma Gene Therapy. Transl Vis Sci Technol. 2013 Jan 31;2(1):2. doi: 10.1167/tvst.2.1.2. pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lewallen M, Xie T. Cell-based therapies for retinal degenerative diseases: a thousand strategies. J Glaucoma. 2013 Jun-Jul;22(Suppl 5):S42–S45. doi: 10.1097/IJG.0b013e3182934b45. Review. [DOI] [PubMed] [Google Scholar]
- 74.Eveleth DD. Cell-based therapies for ocular disease. J Ocul Pharmacol Ther. 2013 Dec;29(10):844–854. doi: 10.1089/jop.2013.0028. Epub 2013 Sep 19. [DOI] [PubMed] [Google Scholar]
- 75.Wright LS, Phillips MJ, Pinilla I, Hei D, Gamm DM. Induced pluripotent stem cells as custom therapeutics for retinal repair: Progress and rationale. Exp Eye Res. 2014 Feb 14; doi: 10.1016/j.exer.2013.12.001. pii: S0014-4835(13)00345-X. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh R, Phillips MJ, Kuai D, Meyer J, Martin JM, Smith MA, Perez ET, Shen W, Wallace KA, Capowski EE, Wright LS, Gamm DM. Functional analysis of serially expanded human iPS cell-derived RPE cultures. Invest Ophthalmol Vis Sci. 2013 Oct 17;54(10):6767–6778. doi: 10.1167/iovs.13-11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cyranoski D. Stem cells cruise to clinic. Nature. 2013 Feb 28;494(7438):413. doi: 10.1038/494413a. [DOI] [PubMed] [Google Scholar]
- 78.Rowland TJ, Blaschke AJ, Buchholz DE, Hikita ST, Johnson LV, Clegg DO. Differentiation of human pluripotent stem cells to retinal pigmented epithelium in defined conditions using purified extracellular matrix proteins. J Tissue Eng Regen Med. 2013 Aug;7(8):642–653. doi: 10.1002/term.1458. [DOI] [PubMed] [Google Scholar]
- 79.Hynes SR, Lavik EB. A tissue-engineered approach towards retinal repair: scaffolds for cell transplantation to the subretinal space. Graefes Arch Clin Exp Ophthalmol. 2010 Jun;248(6):763–778. doi: 10.1007/s00417-009-1263-7. [DOI] [PubMed] [Google Scholar]
- 80.Chen G, et al. Application of human persistent fetal vasculature neural progenitors for transplantation in the inner retina. Cell Transplant. 2012;21:2621–2634. doi: 10.3727/096368912X647153. [DOI] [PubMed] [Google Scholar]
- 81.Johnson TV, DeKorver NW, Levasseur VA, Osborne A, Tassoni A, Lorber B, Heller JP, Villasmil R, Bull ND, Martin KR, Tomarev SI. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain. 2014;137:503–519. doi: 10.1093/brain/awt292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levkovitch-Verbin H, Sadan O, Vander S, Rosner M, Barhum Y, Melamed E, Offen D, Melamed S. Intravitreal injections of neurotrophic factors secreting mesenchymal stem cells are neuroprotective in rat eyes following optic nerve transection. Invest Ophthalmol Vis Sci. 2010;51:6394–6400. doi: 10.1167/iovs.09-4310. [DOI] [PubMed] [Google Scholar]
- 83.Harper MM, Grozdanic SD, Blits B, Kuehn MH, Zamzow D, Buss JE, Kardon RH, Sakaguchi DS. Transplantation of bdnf secreting mesenchymal stem cells provides neuroprotection in chronically hypertensive rat eyes. Invest Ophthalmol Vis Sci. 2011;52:4506–4515. doi: 10.1167/iovs.11-7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson TV1, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010 Apr;51(4):2051–2059. doi: 10.1167/iovs.09-4509. Epub 2009 Nov 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Woodhall E, West AK, Chuah MI. Cultured olfactory ensheathing cells express nerve growth factor, brain-derived neurotrophic factor, glia cell line-derived neurotrophic factor and their receptors. Brain Res Mol Brain Res. 2001;88:203–213. doi: 10.1016/s0169-328x(01)00044-4. [DOI] [PubMed] [Google Scholar]
- 86.Lipson AC, Widenfalk J, Lindqvist E, Ebendal T, Olson L. Neurotrophic properties of olfactory ensheathing glia. Exp Neurol. 2003;180:167–171. doi: 10.1016/s0014-4886(02)00058-4. [DOI] [PubMed] [Google Scholar]
- 87.Johnson TV, Bull ND, Martin KR. Neurotrophic factor delivery as a protective treatment for glaucoma. Exp Eye Res. 2011;93:196–203. doi: 10.1016/j.exer.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, Gong Z, Liu L, Sun H. Combined effect of olfactory ensheathing cell (OEC) transplantation and glial cell line-derived neurotrophic factor (GDNF) intravitreal injection on optic nerve injury in rats. Mol Vis. 2010;16:2903–2910. [PMC free article] [PubMed] [Google Scholar]
- 89.Wu MM, Fan DG, Tadmori I, Yang H, Furman M, Jiao XY, Young W, Sun D, You SW. Death of axotomized retinal ganglion cells delayed after intraoptic nerve transplantation of olfactory ensheathing cells in adult rats. Cell Transplant. 2010;19:159–166. doi: 10.3727/096368910X492625. [DOI] [PubMed] [Google Scholar]
- 90.Du Y, Yun H, Yang E, Schuman JS. Stem cells from trabecular meshwork home to TM tissue in vivo. Invest Ophthalmol Vis Sci. 2013 Feb 19;54(2):1450–1459. doi: 10.1167/iovs.12-11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Du Y, Roh DS, Mann MM, Funderburgh ML, Funderburgh JL, Schuman JS. Multipotent stem cells from trabecular meshwork become phagocytic TM cells. Invest Ophthalmol Vis Sci. 2012;53:1566–1575. doi: 10.1167/iovs.11-9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yücel YH, et al. Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch Ophthalmol. 2000;118:378–384. doi: 10.1001/archopht.118.3.378. [DOI] [PubMed] [Google Scholar]
- 93.Weber AJ, et al. Experimental glaucoma and cell size, density, and number in the primate lateral geniculate nucleus. Invest Ophthalmol Vis Sci. 2000;41:1370–1379. [PubMed] [Google Scholar]
- 94.Yücel YH, et al. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. 2003;22:465–481. doi: 10.1016/s1350-9462(03)00026-0. [DOI] [PubMed] [Google Scholar]
- 95.Yu L1, Xie B, Yin X, Liang M, Evans AC, Wang J, Dai C. Reduced cortical thickness in primary open-angle glaucoma and its relationship to the retinal nerve fiber layer thickness. PLoS One. 2013 Sep 3;8(9):e73208. doi: 10.1371/journal.pone.0073208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gupta N, Greenberg G, de Tilly LN, Gray B, Polemidiotis M, Yücel YH. Atrophy of the lateral geniculate nucleus in human glaucoma detected by magnetic resonance imaging. Br J Ophthalmol. 2009;93:56–60. doi: 10.1136/bjo.2008.138172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Z1, Wang J, Lin F, Dai H, Mu K, Zhang H. Correlation between lateral geniculate nucleus atrophy and damage to the optic disc in glaucoma. J Neuroradiol. 2013 Oct;40(4):281–287. doi: 10.1016/j.neurad.2012.10.004. Epub 2013 Feb 19. [DOI] [PubMed] [Google Scholar]
- 98.Lee JY1, Jeong HJ, Lee JH, Kim YJ, Kim EY, Kim YY, Ryu T, Cho ZH, Kim YB. An Investigation of Lateral Geniculate Nucleus Volume in Patients with Primary Open-angle Glaucoma using 7 Tesla Magnetic Resonance Imaging. Invest Ophthalmol Vis Sci. 2014 Apr 10; doi: 10.1167/iovs.14-13902. pii: iovs.14-13902v1. [DOI] [PubMed] [Google Scholar]
- 99.Yücel Y. Central nervous system changes in glaucoma. J Glaucoma. 2013 Jun-Jul;22(Suppl 5):S24–S25. doi: 10.1097/IJG.0b013e3182934a55. [DOI] [PubMed] [Google Scholar]
- 100.Zhang YQ1, Li J, Xu L, Zhang L, Wang ZC, Yang H, Chen CX, Wu XS, Jonas JB. Anterior visual pathway assessment by magnetic resonance imaging in normal-pressure glaucoma. Acta Ophthalmol. 2012 Jun;90(4):e295–e302. doi: 10.1111/j.1755-3768.2011.02346.x. Epub 2012 Apr 10. [DOI] [PubMed] [Google Scholar]
- 101.Yücel YH, Johnston MG, Ly T, Patel M, Drake B, Gümüş E, Fraenkl SA, Moore S, Tobbia D, Armstrong D, Horvath E, Gupta N. Identification of lymphatics in the ciliary body of the human eye: a novel "uveolymphatic" outflow pathway. Exp Eye Res. 2009 Nov;89(5):810–819. doi: 10.1016/j.exer.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 102.Tam AL, Gupta N, Zhang Z, Yücel YH. Quantum dots trace lymphatic drainage from the mouse eye. Nanotechnology. 2011 Oct 21;22(42):425101. doi: 10.1088/0957-4484/22/42/425101. Epub 2011 Sep 21. [DOI] [PubMed] [Google Scholar]
- 103.Kim M, Johnston MG, Gupta N, Moore S, Yücel YH. A model to measure lymphatic drainage from the eye. Exp Eye Res. 2011 Nov;93(5):586–591. doi: 10.1016/j.exer.2011.07.006. Epub 2011 Jul 27. [DOI] [PubMed] [Google Scholar]
- 104.Tam AL1, Gupta N, Zhang Z, Yücel YH. Latanoprost Stimulates Ocular Lymphatic Drainage: An In Vivo Nanotracer Study. Transl Vis Sci Technol. 2013 Aug;2(5):3. doi: 10.1167/tvst.2.5.3. Epub 2013 Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cordeiro MF, Guo L, Luong V, Harding G, Wang W, Jones HE, et al. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc Natl Acad Sci USA. 2004;101:13352–13356. doi: 10.1073/pnas.0405479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cordeiro MF1, Migdal C, Bloom P, Fitzke FW, Moss SE. Imaging apoptosis in the eye. Eye (Lond) 2011 May;25(5):545–553. doi: 10.1038/eye.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guo L, Salt TE, Luong V, Wood N, Cheung W, Maass A, et al. Targeting amyloid-beta in glaucoma treatment. Proc Natl Acad Sci USA. 2007;104:13444–13449. doi: 10.1073/pnas.0703707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maass A, von Leithner PL, Luong V, Guo L, Salt TE, Fitzke FW, et al. Assessment of rat and mouse RGC apoptosis imaging in vivo with different scanning laser ophthalmoscopes. Curr Eye Res. 2007;32:851–861. doi: 10.1080/02713680701585872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guo L, Salt TE, Maass A, Luong V, Moss SE, Fitzke FW, et al. Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo. Invest Ophthalmol Vis Sci. 2006;47:626–633. doi: 10.1167/iovs.05-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sensi SL, Rapposelli IG, Frazzini V, Mascetra N. Altered oxidant-mediated intraneuronal zinc mobilization in a triple transgenic mouse model of Alzheimer’s disease. Exp Gerontol. 2008;43:488–492. doi: 10.1016/j.exger.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 111.Barnett EM, Elangovan B, Bullok KE, Piwnica-Worms D. Selective cell uptake of modified tat peptide-fluorophore conjugates in rat retina in ex vivo and in vivo models. Invest Ophthalmol Vis Sci. 2006;47:2589–2595. doi: 10.1167/iovs.05-1470. [DOI] [PubMed] [Google Scholar]
- 112.Barnett EM, Zhang X, Maxwell D, Chang Q, Piwnica-Worms D. Single cell imaging of retinal ganglion cell apoptosis with a cell-penetrating, activatable peptide probe in an in vivo glaucoma model. Proc Natl Acad Sci U S A. 2009;106:9391–9396. doi: 10.1073/pnas.0812884106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maxwell D, Chang Q, Zhang X, Barnett E, Piwnica-Worms D. An improved cell-penetrating, caspase-activatable, near-infrared fluorescent peptide for apoptosis imaging. Bioconjug Chem. 2009;20:702–709. doi: 10.1021/bc800516n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qiu X, Johnson JR, Wilson BS, Gammon ST, Piwnica-Worms D, Barnett EM. Single-cell resolution imaging of retinal ganglion cell apoptosis in vivo using a cell-penetrating caspase-activatable Peptide probe. PLoS One. 2014 Feb 21;9(2):e88855. doi: 10.1371/journal.pone.0088855. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weinreb RN, Kaufman PL. Glaucoma research community and FDA look to the future, II: NEI/FDA Glaucoma Clinical Trial Design and Endpoints Symposium: measures of structural change and visual function. Invest Ophthalmol Vis Sci. 2011 Oct 4;52(11):7842–7851. doi: 10.1167/iovs.11-7895. [DOI] [PMC free article] [PubMed] [Google Scholar]