Abstract
Significance: Chronic wounds commonly have high levels of bioburden and antibiotic-resistant pathogens. This review article focuses on findings from current literature related to four biophysical technologies (ultrasound, electrical stimulation, phototherapy, and negative pressure wound therapy) believed to be beneficial for managing wound bioburden and support healing.
Recent Advances and Critical Issues: Recent advances for each modality are provided as a basic synopsis of the technology followed by brief overviews of the most recent literature addressing its effectiveness for managing wound bioburden, and critical issues for each modality are provided as conclusions.
Future Directions: This review highlights the need for further clinically relevant studies examining bacterial levels in addition to healing progression for each technology.

Holly Korzendorfer, PT, PhD, CWS, FACCWS
Scope and Significance
The scope of this review article focuses on findings from current literature related to four biophysical technologies believed to be beneficial for managing wound bioburden. Given the increasing numbers of individuals suffering from chronic wounds, and the fact that most chronic wounds are colonized with pathogens, there is a need to understand what adjunctive interventions are most effective in managing bioburden. However, there is a paucity of research examining the actual bioburden effects of these technologies—most publications focus on wound healing without reporting bacterial quantities or measurements. To highlight this point, a cross-serial search of 11 databases (including CINAHL, MEDLINE, and Cochrane) for the terms “biophysical agent wound bioburden” or “biophysical agent wound bacteria” resulted in 46 articles. Searching the terms “modality wound bacteria” yielded 68 results with 5 duplicates. Searching the terms “NPWT bacteria” only yielded 47 results with 11 duplicates, several of them included silver as well and not applicable for this review. Nonetheless, ultrasound (US), electrical stimulation, phototherapy, and negative pressure wound therapy (NPWT) are the four biophysical technologies chosen as the focus of this article as important adjunctive treatment modalities for wound healing. A basic synopsis of each modality will be provided followed by a brief overview of the recent literature addressing its effectiveness for managing wound bioburden.
Translational Relevance
This review has translation relevance for those in the wound management research community as there is a need for consistent study design and reporting of results for wound bioburden to achieve a body of evidence supporting best practices with evidence-based medicine when utilizing biophysical modalities for chronic wound management.
Clinical Relevance
The clinical relevance of this article is to increase awareness and support comprehensive wound management, including health care professionals, such as physical therapists, who may provide adjunctive and augmentative treatment options for hard to heal chronic wounds needing bioburden management.
Discussion of Findings and Relevant Literature
Ultrasound
Although there is paucity in the literature addressing US and bioburden specifically, evidence does exist supporting its antimicrobial effects. Most of these studies, however, have been in vitro versus in vivo or clinical settings. Additionally, some studies have reported that kilohertz US (low-frequency ultrasound [LFUS]) not only has direct antimicrobial effects, but also works synergistically with antibiotic and antiseptic agents to enhance killing of bacteria.1,2
US is a form of mechanical energy that causes molecules within tissues to vibrate or oscillate above the limit of human hearing. US is transmitted through tissues as acoustic pressure waves and produces biophysical effects conducive for tissue and wound healing. US has been used in the United States for medicinal purposes since the 1940s.3 US is indicated for chronic or recalcitrant wounds that are clean or infected, as an adjunctive therapy when the standard of care has not made significant improvements toward healing. The current literature has shown US facilitates wound healing in various wound etiologies, including pressure ulcers, venous insufficiency ulcers, acute trauma, and recent surgically induced wounds. There are three traditional techniques described in the literature for applying high-frequency ultrasound (HFUS; 1–3 MHz) to hasten wound healing: direct application, periwound application, and immersion technique (subaqueous). Low-frequency ultrasound (LFUS; 20–120 kHz) is applied using light contact to nonviable tissues in the wound bed by coupling the probe with normal saline. LFUS can also be delivered through noncontact mode in a water bath (subaqueous). See Table 1 for an overview of acoustic pressure therapy.
Table 1.
Ultrasound: acoustic pressure wound therapy
| Ultrasound Applications | High-Intensity Ultrasound | Low-Intensity Ultrasound |
|---|---|---|
| High frequency (MHz) | Contact | Contact |
| Thermal | Nonthermal | |
| Sports medicine | Fetal monitoring | |
| Low frequency (kHz) | Contact | Noncontact |
| Thermal | Nonthermal | |
| Debridement—cutting, emulsification, fragmentation of tissue | Healing—stimulates cells, removes bacteria, assists with maintenance debridement |
Adapted from Meeting Report/Plenary Session.11
Acoustical cavitation and microstreaming are two mechanisms of action imparted by US that generate a biologic activity, impacting wounded tissues and healing. Cavitation is the vibrational effect of US on microsized gas bubbles that form due to the accumulation of dissolved gas in the path of the US beam.3 The movement and compression of the bubbles can cause changes in the activity of tissue cells in the areas subjected to the US energy. Microstreaming is created by the physical forces of sound waves that can displace small molecules and ions. The mechanical pressure created by microstreaming produces unidirectional movement of fluid along and around cell membranes.
LFUS is believed to be clinically effective due to cavitation created on the wound surface. The implosion of microbubbles releases energy that causes fibrinolysis (debridement) thereby decreasing bioburden by fragmenting biofilms and bacteria. Thermal and nonthermal effects occur in deeper tissue layers, however, these effects are mild compared to HFUS that is capable of increasing tissue temperatures around 3°C. The main thermal effects of HFUS (1 and 3 MHz) are commonly used to enhance blood flow and increase periwound tissue temperatures. The predominant nonthermal effects of LFUS (20–40 kHz) are utilized for debridement, bactericidal effects, and to promote healing of acute and chronic wounds. Therefore, the physical effects of cavitation and microstreaming are important modes of action on the surface of wounds. HFUS creates a stable or nondestructive cavitation as shown by visible gas bubbles in fluids impacted by the US energy. Only a small number of these bubbles implode, so the cavitation effect is weak. Transient cavitation, which is more pronounced with LFUS, occurs only in a liquid medium such as normal saline. Transient cavitation is selectively destructive of fibrinous necrotic tissue, making it an effective form of debridement. It is thought that the higher viscosity of healthy tissues protects them from cavitation produced by LFUS.4 Figure 1 depicts an example of ultrasonic debridement.
Figure 1.
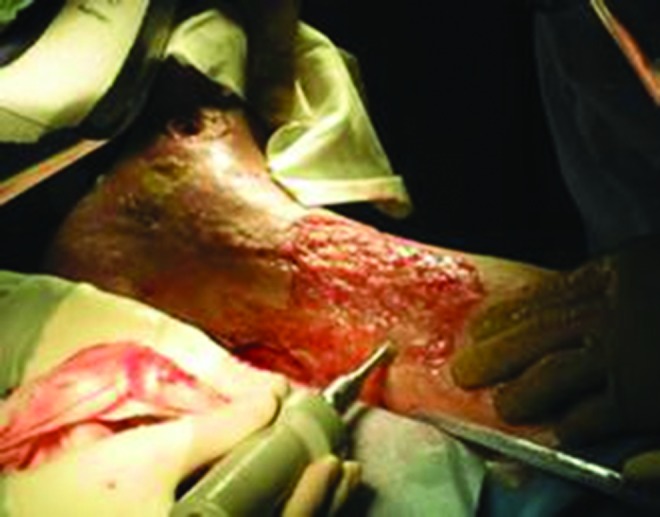
Example of LFUS debridement. The image above is a copyrighted product of AAWC (www.aawconline.org) and has been reproduced with permission. LFUS, low-frequency ultrasound. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
With respect to therapeutic US, HFUS dosage parameters, including intensity in W/cm2 and application time in minutes are important. Furthermore, the number of times per day or per week and the total number of treatments impact its therapeutic efficacy. The effects of therapeutic US are also dependent on the frequency of the probe utilized.4 Despite evidence supporting the use of LFUS for wound management and due to its more recent availability, the treatment parameters for LFUS (frequency, intensity, and series) are not yet standardized with respect to the best type of US and parameters to use to augment wound healing most effectively. It is important to follow the manufacturer's recommendations for use and to understand the various devices available, their clinical features, and their Food and Drug Administration–cleared indications (Table 2).
Table 2.
Ultrasound therapy and debridement devices
| Device and Manufacturer | Antibacterial | Fibrinolysis | Selective Debridement | Aerosolization | Pain |
|---|---|---|---|---|---|
| Ultrasonic treatment and cleaner | |||||
| MIST therapy (Celleration, Inc.) | Yes | Yes | No | Yes | No |
| Ultrasonic scalpel, cleaner and treatment | |||||
| SonicOne (Misonix, Inc.) | Yes | Yes | Yes | Yes | Yes |
| Sonoca 180 (Soring, Inc.) | Yes | Yes | Yes | Yes | Yes |
| Qoustic wound therapy system (Arobella, LLC) | Yes | Yes | Yes | Yes | No |
| Hydrosurgery, scalpel, cleaner, and suction | |||||
| Versajet (Smith & Nephew) | Yes | Yes | Yes | Yes | Yes |
The following bullet points summarize current key literature findings addressing the impact of US on bioburden.
• Kavros and Schenk5 conducted an open-label, nonrandomized, baseline-controlled clinical case series utilizing noncontact LFUS delivered at 40 kHz. The authors demonstrated cell wall destruction of bacteria and damage to membranes of methicillin-resistant Staphylococcus aureus (MRSA) and improved the rate of healing and closure in recalcitrant lower extremity ulcerations.5
• Conner-Kerr et al.6 conducted an in vitro, controlled study to determine the effects of LFUS on bacterial viability, cell wall structure, and colony characteristics, including antibiotic resistance of MRSA. MRSA had reduced viability compared to the untreated MRSA (44.1–92.5%) and changes were noted in pigmentation, color, colony size, and the pattern of hemolysis in the LFUS-treated bacteria.6
• Serena et al.7 looked at the impact of noncontact, nonthermal LFUS on bacterial counts in experimental and chronic wounds. Four controlled experiments were conducted: first, US penetration in wounded and intact skin was assessed in vitro. Compared to the sham group, noncontact US penetrated farther in both wounded and intact pig skin. Second, they looked at an in vitro model to count live/dead bacteria. The findings here showed 0% of sham treated, 33% of Pseudomonas aeuroginosa, 40% of Escherichia coli, and 27% of Enterococcus faecalis were dead after one US application. Minimal effects on MRSA and S. aureus were observed. Third, using an in vivo model with tissue biopsies, the authors found that after 1 week, the overall bacterial quantity decreased with US treatment. Fourth, 11 patients with pressure ulcers and bacterial counts >105 colony-forming unit (CFU)/g of tissue were treated with 2 weeks noncontact US. The quantities of seven bacterial organisms were substantially reduced 2 weeks post-treatment. Taken together, the published treatment parameters of these four experiments indicate that noncontact US can be used to reduce bacterial quantity.7
• Escandon et al.8 conducted a prospective open-labeled pilot study on the effectiveness of noncontact LFUS therapy in refractory venous ulcers. Specifically, they evaluated 10 large venous ulcers and examined the effect of noncontact US on wound closure, bacterial counts (as determined by wound biopsies), expression of inflammatory cytokines, and pain reduction. The authors found a decline in individual and total bacterial counts, however, the differences were not statistically significant (likely due to the small sample size).8
• Ennis et al.9,10 conducted two noncomparative clinical outcome trials to examine the effects of US on recalcitrant diabetic foot ulcers and on the effectiveness of MIST (MIST Therapy® Celleration) US (a form of LFUS) for the healing of chronic wounds. In both studies, LFUS achieved healing in 40.7% and 69% of the cases, respectively. Although bacteria were not directly measured in these experiments, the authors believed wound healing could not be achieved if bacteria remained present in significant quantities. Therefore, it was determined LFUS reduces bacteria, in addition to other benefits, to enable more effective and efficient wound closure.9,10
• Ensing et al. conducted an in vitro, controlled study that demonstrated LFUS administered concurrently with antibiotics enhanced the effects of the antibiotics against bacteria and biofilms.1 This supports findings by Qian et al.2 Qian et al. reported that lower frequencies of US in the kilohertz range were more effective on biofilm viability than megahertz frequencies in enhancing the effects of antibiotics.2
In conclusion, there is increasing evidence of the impact US has on wound bioburden. The emerging literature is demonstrating the effectiveness of LFUS as an adjunctive therapy to promote wound debridement and healing. More research is needed, however, to standardize the parameters to specifically address bioburden.
Electrical stimulation
Electrical stimulation (ES) is the only biophysical agent to receive strong support as an adjunctive wound-healing modality in the 1994 Agency for Health Care Policy and Research Pressure Ulcer Guideline12 and has continued acceptance with a “strength of evidence” A rating in the updated National Pressure Ulcer Advisory Panel/European Pressure Ulcer Advisory Panel Pressure Ulcer Clinical Practice Guideline.13 ES is considered a medically justifiable treatment, eligible for Medicare reimbursement, for wounds that have failed 30 days of conventional treatment. ES involves an externally applied current (either direct or alternating) delivered through electrodes (of various styles) to the wound bed directly or via adjacent tissues (Fig. 2). Several devices are available to provide ES utilizing various wave forms, pulse rates, and voltages. The most commonly studied and published type of ES for wound management is high-voltage monophasic pulsed current (HVPC), which has twin triangular pulses of short duration delivered as pulses per second (pps). Several ES devices have preset parameters for wound healing, yet allow for the selection of polarity and pulse rate for treatment. Polarity has been reported to affect the type of cells attracted to the treatment area as a result of the energy and charge delivered by the electrodes. Table 3 summarizes the anticipated effects of polarity on several aspects of wound healing. Further detailed description of the multiple ES devices available for wound management is beyond the scope of this review.
Figure 2.
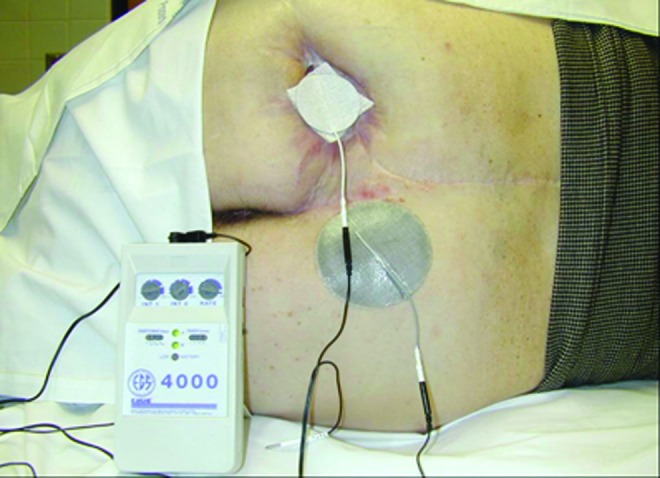
Electrical stimulation on patient. The image above is a copyrighted product of AAWC (www.aawconline.org) and has been reproduced with permission. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Table 3.
| Negative Polarity | Positive Polarity |
|---|---|
| Increase blood flow | Hemostasis |
| Stimulation granulation and collagen production | Denatures protein |
| Fibroblast proliferation and activated neutrophil migration | Macrophage and neutrophil migration |
| Decreased edema and necrotic tissue | Decreases number of mast cells |
| Epidermal migration |
The following bullet points summarize key findings of two recent studies that specifically reported the impact ES has on wound bioburden.
-
• Merriman et al.14 looked at the zone of inhibition, pH and polarity effects of four types of ES utilizing clinical parameters. The types of ES applied to inoculated (with S. aureus) Petri dishes for 1 h daily for 3 days during this study were
1. continuous microamperage direct current (μADC)
2. HVPC
3. low-voltage monophasic milliamperage pulsed current
4. low-voltage biphasic milliamperage pulsed current
The HVPC zone of inhibition was significantly more than all the treatment conditions and the control. Additionally, the zone of inhibition was significantly greater for continuous μADC and HVPC (at both poles each day) than for the control or other two low-voltage ES conditions, which did not have significant bacterial inhibition. No significant differences were found among days 1–3 or between positive or negative polarity. pH was found to be acidic at the anode (+) and alkaline at the cathode (−) for both HVPC and continuous μADC.14
• Daeschlein et al.15 examined the antibacterial effect of monophasic low-voltage pulsed current (LVPC) polarity on three gram-positive and three gram-negative pathogens inoculated onto sterile 100% cotton covered with electrodes and a sterile glass slide. The gram-positive bacteria utilized were S. aureus, S. epidermidis, and E. faecium. The gram-negative bacteria were E. coli, P. aeruginosa, and Klebsiella pneumonia. The control conditions had no antibacterial effects. Both polarities of the LVPC ES significantly (p<0.01) reduced all tested bacteria levels over controls and there was a significant difference in the amount of decreased bacteria between positive and negative polarity (p=0.02). Positive polarity achieved the highest level of bacteria reduction. There was no significant difference between the reduction of gram-positive or gram-negative bacteria for positive polarity ES.15
Both the aforementioned in vitro studies examined the effects of monophasic LVPC on bacteria; however, they did not find significant bactericidal or polarity effects. Merriman et al.14 found no significant bacterial inhibition nor difference between positive and negative polarity using LVPC with a wavelength of 120 μs at 30 mA, 128 pps for 60-min treatments. Daeschlein et al.15 found significant bacterial inhibition over controls and polarity differences using LVPC with a wavelength of 140 μs, an intensity of 42 mA at 128 pps for 30 min. The contrary results of these two studies may be due to methodology or the type of analysis of antibacterial activity or the different total amounts of energy delivered during the treatments. Kloth and Zhao16 have summarized older in vitro and in vivo studies with slightly varied results, yet the overall conclusion is that ES is bactericidal. However, the variations in treatment parameters and study design make it difficult to build a body of evidence in this field.
In conclusion, while ES has several published studies supporting it as an effective biophysical modality for wound healing, the best type of ES and parameters for most effective wound healing are not fully established. Additionally, the evidence supporting ES's role in bioburden management is lacking clinically relevant studies.
Phototherapy
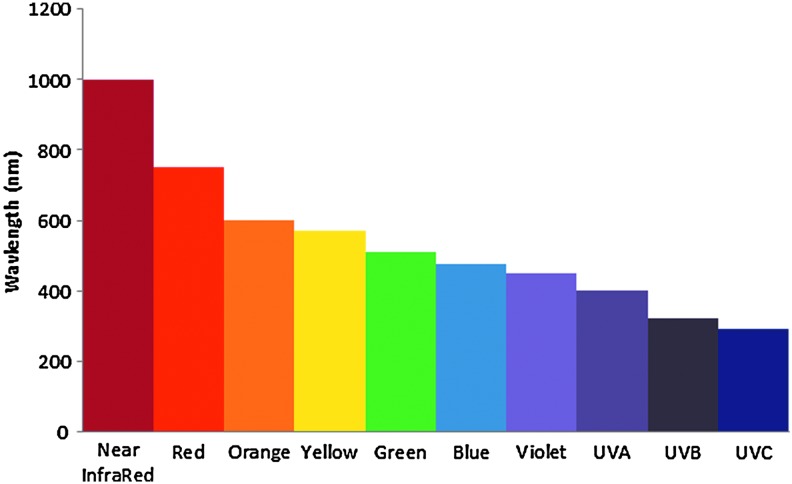
The third biophysical technology is phototherapy consisting of modalities providing treatment utilizing energy from the electromagnetic light spectrum. Phototherapy consists of visible light, ultraviolet (UV) rays and laser therapy. Wavelengths in the visible light spectrum of electromagnetic energy are ∼400–800 nm. UV wavelengths are on the low end and below this spectrum, consisting of UVA, UVB, and UVC rays, all of which are reported to be bactericidal.17 Devices providing only UVC light (with wavelengths in the range of ∼100–290 nm) treatment provide more targeted bactericidal effects with shorter treatment times (Fig. 3). Figure 4 shows general light wavelengths, but please note there is a slight variation in the ranges for the light spectrum, including UV, among various organizations. Laser is another phototherapy treatment that consists of a unidirectional beam of light at one wavelength. Lasers for wound treatment are low-level or cold lasers that utilize lower intensities so as to not heat the tissues.
Figure 3.

Example of an ultraviolet-C device. The image above is a copyrighted product of AAWC (www.aawconline.org) and has been reproduced with permission. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 4.
Wavelengths of light (nm). Approximate wavelength ranges: near-infrared=700–1500 nm, visible light=400–800 nm, UVC=100–290 nm. UV, ultraviolet light. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The following bullet points summarize key findings of recent studies that specifically reported the impact phototherapy has on wound bioburden.
- • Rao et al.18 performed a prospective in vitro study to examine the bactericidal effect of direct UVC and UVC filtered through a 0.15-mm-thick transparent film dressing on multiple gram-positive cocci. The bacteria were inoculated onto agar plates, incubated, and then exposed to a UV lamp device emitting 74% UVC at a wavelength of 254 nm, 5% UVB, 2.5% UVA, and 18.5% visible light. The light therapy was applied at a standard 10-cm distance with the following energy output parameters by time:
- ○ 1.59 J/m2 for 5 s
- ○ 3.18 J/m2 for 10 s
- ○ 4.77 J/m2 for 15 s
- ○ 6.36 J/m2 for 20 s
- ○ 7.95 J/m2 for 25 s
- ○ 9.54 J/m2 for 30 s
There were 18 experimental cultures (three for each time exposure) and a control not exposed to UVC. The authors found that direct UVC resulted in 100% eradication of all gram-positive cocci with 15 s of exposure (range 5–15 s) and no bactericidal effect for the filtered UVC.18
• Thai et al.19 published a case study to evaluate the role of UVC in bioburden management of chronic ulcers infected with MRSA. The case study consisted of three MRSA-infected chronic ulcers (present for at least 3 months) determined by a positive swab and clinical signs of infection. The UVC (200–290 nm) device was warmed for 5 min before application, and then applied 1 inch away from and perpendicular to the wound for a treatment time of 180 s per wound site. All three cases had decreased wound bioburden and clinical signs of infection after either seven daily treatments (two cases) or seven treatments in 14 days (one case) determined via semiquantitative swabs and wound assessments.19
• Baffoni et al.20 evaluated the effect of near-infrared (NIR) laser in vitro on mono- and polymicrobial biofilms created from two strains of bacteria isolated from a chronic venous leg ulcer (VLU). The laser treatment was applied to the biofilm plates after a 24-h maturation period at a distance of 5 cm, using a wavelength of 980 nm at 10 W for a total energy density of 148 J/cm2. The treatment time was not reported. The authors reported no significant differences in biomass reduction or cell viability in both mono- and polymicrobial samples compared to controls. Qualitative live/dead images showed a modification of compactness of treated biofilms compared to controls, particularly for P. aeruginosa and the polymicrobial biofilm. Bacterial growth on treated sessile and planktonic cells was observed in some cases, with the CFU count for S. aureus (sessile and planktonic) significantly lower in the treatment group versus the controls as well as for the treated planktonic polymicrobial biofilm. No reduction in bacterial growth was noted for P. aeruginosa.20
• An expert review by Wollina and Geinig21 reports that white light (400–800 nm) at 120 J/cm2 produces a reactive oxygen species that results in phototoxicity, which may decrease colony counts of bacteria from chronic wounds based on a 2008 study.
In conclusion, phototherapy can be delivered by devices emitting various forms of light therapy, including but not limited to white light, NIR laser, a combination of UV rays and white light, or UVC rays only. The varied treatment parameters utilized by multiple types of phototherapy devices make it difficult to establish a body of evidence supporting phototherapy technology for bioburden management. Although it appears to be effective with in vitro studies, more clinically relevant studies are needed.
Negative-pressure wound therapy
The final biophysical modality reviewed for its role in bioburden management is NPWT also known as topical negative pressure (TNP). This technology consists of creating a closed system between the wound and a device capable of generating a suction (either constant or intermittent) to create TNP at the wound. The interface with the wound may be a foam dressing (several types with varying composition and pore size) gauze or another material to which, a noncompressible tubing apparatus is applied, and then covered with a sealed transparent film-type dressing. The exact mechanism of action as to how or why this technology augments wound healing is unknown and it is not agreed upon in the wound-healing community whether or not NPWT significantly improves healing of chronic wounds.
The following bullet points summarize key findings of recent studies that specifically reported the impact NPWT has on wound bioburden.
• Mouës et al.22 examined if vacuum-assisted closure (VAC—a specific NPWT brand by Kinetic Concepts, Inc.) had an effect on the bacterial balance of treated wounds, specifically gram-negative nonfermentative rods, Staphylococcus aureus, gram-negative members of Enterobacteriacae and anaerobes. Fifty-four subjects were randomly assigned and wounds were stratified as early or late treated. The treatment group (29 subjects) received NPWT using a polyurethane foam (pore size 400–600 μm) and continuous negative pressure (−125 mmHg) changed every 48 h. The conventional therapy group (25 subjects) received standard moist gauze therapy two or more times daily consisting of either 0.9% saline, 0.2% nitrofuralam, 1% acetic acid solution, or 2% sodium hypochlorite. Debridement was performed before the start of therapy and as needed for both groups. The wound surface area was measured directly after debridement and during therapy via tracing that was copied and scanned. Bacterial load was determined by aseptic condition biopsies using a scalpel to obtain viable tissue from the center of the wound. The authors looked at the time it took to be “ready for surgical therapy”—that is, a clean red granulating wound bed and found no significant difference when comparing the groups.22
The authors measured the wound surface area on 28 of the subjects (15 NPWT; 13 conventional) and found that 100% of the NPWT subjects had reduced surface area measurements compared to 77% of the conventional subjects, with both treatments significantly reducing the surface area compared to initial measurements. The authors found that NPWT reduced surface area significantly (p<0.05) more than the conventional treatment.22
Bacterial load findings were that NPWT decreased nonfermentative-negative rods (significantly p<0.05), while there was no significant effect for the conventional group. However, NPWT (VAC) increased S. aureus (significantly p<0.05), while there was no significant effect for the conventional group. The number of Enterobacteriacae and anaerobes did not change significantly for either group and neither group had a significant effect on the total amount of bacteria. This finding in the conventional group is interesting to note as antimicrobial moist gauze therapy options could be used, while there was not an antimicrobial option for the treatment group.22
• Weed et al.23 performed a retrospective chart review to quantitatively assess and monitor bacteria bioburden of acute and chronic wounds using NPWT (via the VAC system). Results were reported for 25 subjects (26 wounds). All necrotic tissue was removed from the wounds before application of NPWT immediately after surgery in 14 cases and onto healthy granulating wounds in 12 cases. Quantitative swabs were obtained after the wound was wiped with saline gauze, a calcium alginate applicator was pressed onto a 1-cm2 area firmly enough to exude wound fluid, and the applicator was then placed into a sterile container with 5 mL of sterile saline. A statistically significant increase in bacterial bioburden was found during NPWT treatment compared to before (p=0.000) and after (p=0.003). There was no statistically significant difference in pre- or post-NPWT bioburden levels. When those two scenarios were combined as an off VAC sample, there was again a statistically significant (p=0.000) increase in bacterial bioburden on VAC versus off. There was no statistical significant change in bacterial bioburden with NPWT (VAC), but a trend was noted: 43% increase bacterial bioburden; 35% no change; and 22% decreased bacterial bioburden. Nineteen percent of the wounds were closed surgically, another 19% healed, while undergoing NPWT treatment, and 12% failed NPWT therapy (increased the wound size or necrotic tissue developed). Lastly, the quantitative cultures did not correlate with healing failure or progression noted in the charts.23
• Wollina et al.4 report on a prospective open trial of NPWT's effect on the microbiology of chronic, noninfected VLU of seven patients receiving compression therapy. Each VLU was swabbed and NPWT was applied at −125 mmHg continuous pressure for 6 days total. Standard methods for bacteriological sampling and wound surface measurements were applied at baseline and at NPWT dressing changes on day 3 and 6. The log 10 CFU on day 1 were 305, on day 3 were 4.7, and on day 6 were 5.1, indicating a significant increase in bacterial colonization between day 1 and 6 (p<0.02). There was no change in microbiological species.4
In conclusion, NPWT does not appear to help reduce wound bioburden clearance despite the active withdrawal of wound exudate into the collecting compartment of the device. Furthermore, NPWT may actually increase bacterial burden during treatment, however, there is not enough evidence to firmly draw that conclusion at this time. Lastly, it is not known why healing occurred in the aforementioned studies despite the instances of increased bacterial burden with NPWT.
Take-Home Messages.
• The clinically relevant evidence for the roles of US, ES, phototherapy, and/or NPWT in bioburden management is inadequate.
• Future wound-healing research related to these modalities should measure and report bacteria inhibition findings.
• Consistent bioburden research and treatment protocols and parameters are needed for each of the modalities.
• LFUS, ES, and UVC appear to have bactericidal effects based on available research to date.
Conclusion
Limited research exists reporting specifically on the effect certain biophysical agents have on wound bioburden. The studies reviewed for this article, indicate that there is limited evidence to support the use of US (LFUS vs. HFUS), ES, and phototherapy to assist in the management and/or reduction of wound bioburden. The literature does not currently support the use of NPWT specifically for wound bioburden management. Given the increasing numbers of individuals suffering from chronic wounds, and the fact that most chronic wounds are highly colonized, there is a need to understand what interventions (beyond topical and systemic medications) are most effective in managing bioburden. Further research is warranted to understand the effectiveness of biophysical agents on bioburden. In addition, protocols, parameters, and treatment regimens need to be determined for optimal resource utilization.
Abbreviations and Acronyms
- μADC
continuous microamperage direct current
- CFU
colony-forming unit
- ES
electrical stimulation
- HFUS
high-frequency ultrasound
- HVPC
high-voltage monophasic pulsed current
- LFUS
low-frequency ultrasound
- LVPC
low-voltage biphasic milliamperage pulsed current
- MRSA
methicillin-resistant Staphylococcus aureus
- NIR
near infrared
- NPWT
negative pressure wound therapy
- TNP
topical negative pressure
- US
ultrasound
- UV
ultraviolet
- VAC
vacuum-assisted closure
- VLU
venous leg ulcer
Acknowledgments and Funding Sources
None to report.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Holly Korzendorfer is the Vice President of Business and Clinical Development at DermaRite Industries, a skin and wound care product manufacturer. Her responsibilities include management of the sales force, while collaborating with executives and clinical services for the development of internal/external educational materials and expansion of DermaRite's offerings to the skin and wound care community. She is also an author/faculty for Present WOC online education and lectures for a local DPT program. Holly serves on the ABWM exam committee, assists with the WHS education committee, is an editorial board member for OWM, and has published various wound-related articles. Heather Hettrick is an Associate Professor in the Department of Physical Therapy at Nova Southeastern University. Her clinical expertise resides in wound, burn, and lymphedema management. Heather is currently on the Board of the Association for the Advancement of Wound Care and is a Past President of the American Board of Wound Management. Heather presents, publishes, and conducts research on a variety of topics pertaining to integumentary dysfunction and is actively involved in numerous professional organizations.
References
- 1.Ensing GT, Neut D, van Horn JR, van der Mei HC, and Busscher HJ: The combination of ultrasound with antibiotics released from bone cement decreases the viability of planktonic and biofilm bacteria: an in vitro study with clinical strains. J Antimicrob Chemother 2006; 58:1287. [DOI] [PubMed] [Google Scholar]
- 2.Qian Z, Sagers RD, and Pitt WG: The effect of ultrasound frequency upon enhanced killing of Pseudomonas aeuroginosa biofilm. Ann Biomed Eng 1997; 25:69. [DOI] [PubMed] [Google Scholar]
- 3.Kloth L. and Niezgoda J: Ultrasound for wound debridement and healing. In: Wound Healing Evidence Based Management, 4th edition, edited by McCulloch J. and Kloth L. Philadelphia, PA: FA Davis, 2010, pp. 545–575 [Google Scholar]
- 4.Wollina U, Heinig B, and Kloth K: The use of biophysical technologies in chronic wound management. In: Measurements in Wound Healing, edited by Mani R, Romanelli M, and Shukla V. London: Springer-Verlag, 2012, pp. 313–354 [Google Scholar]
- 5.Kavros SJ. and Schenk EC: Use of noncontact low frequency ultrasound in the treatment of chronic foot and leg ulcerations: a 51-patient analysis. J Am Podiatr Med Assoc 2007; 97:95. [DOI] [PubMed] [Google Scholar]
- 6.Conner-Kerr T, Alston G, Stovall A, Vernon T, Winter D, Meixner J, Grant K, and Kute T: The effects of low frequency ultrasound (35 kHz) on methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Ostomy Wound Manage 2010; 56:32. [PubMed] [Google Scholar]
- 7.Serena T, Lee K, Lam K, Attar P, Meneses P, and Ennis W: The impact of noncontact, nonthermal, low frequency ultrasound on bacterial counts in experimental and chronic wounds. Ostomy Wound Manage 2009; 55:22. [PubMed] [Google Scholar]
- 8.Escandon J, Vivas A, Perez R, Kirsner R, and Davis S: A prospective pilot study of ultrasound therapy effectiveness in refractory venous ulcers. Int Wound J 2012; 9:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ennis WJ, Foremann P, Mozen N, Massey J, Conner-Kerr T, and Meneses P: Ultrasound therapy for recalcitrant diabetic foot ulcers: results of a randomized, double-blind, controlled, multicenter study. Ostomy Wound Manage 2005; 51:24. [PubMed] [Google Scholar]
- 10.Ennis WJ, Valdes W, Gainer M, and Meneses P: Evaluation of clinical effectiveness of MIST ultrasound therapy for the healing of chronic wounds. Adv Skin Wound Care 2006; 19:437. [DOI] [PubMed] [Google Scholar]
- 11.Meeting Report/Plenary Session Wounds UK, 2010, Cutting K, Unger P, Norris R, and Driver V: MIST ultrasound therapy: the science and the benefits. Wounds UK 2011; 7:130 [Google Scholar]
- 12.Bergstrom N, Allman RM, Alvarez OM, Bennett MA, Carlson CE, Frantz RA, Garber SL, Jackson BS, Kaminski MV, Jr, Kemp MG, Krouskop TA, Lewis VL, Jr, Maklebust J, Margolis DJ, Marvel EM, Reger SI, Rodeheaver GT, Salcido R, Xakellis GC, and Yarkony GM: Treatment of Pressure Ulcers. Clinical Practice Guideline, No. 15. Rockville, MD: U.S. Department of Health and Human Services. Public health Service, Agency for Health Care Policy and Research; AHCPR Publication No. 95-0652 [Google Scholar]
- 13.National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisor Panel. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Washington DC: National Pressure Ulcer Advisory Panel, 2009 [Google Scholar]
- 14.Merriman HL, Hegyi CA, Albright-Overton CR, Carlos J, Jr, Putnam RW, and Mulcare JA: A comparison of four electrical stimulation types on Staphylococcus aureus growth in vitro. J Rehabil Res Dev 2004; 41:139. [DOI] [PubMed] [Google Scholar]
- 15.Daeschlein G, Assadian O, Kloth LC, Meinl C, Ney F, and Kramer A: Antibacterial activity of positive and negative polarity low voltage pulsed current (LVPC) on six typical Gram-positive and Gram-negative bacterial pathogens of chronic wounds. Wound Repair Regen 2007; 15:399. [DOI] [PubMed] [Google Scholar]
- 16.Kloth L. and Zhao M: Endogenous and exogenous electrical fields for wound healing. In: Wound Healing Evidence Based Management, 4th edition, edited by McCulloch J. and Kloth L. Philadelphia, PA: FA Davis, 2010, pp. 450–513 [Google Scholar]
- 17.Conner-Kerr T: Light therapies. In: Wound Healing Evidence Based Management, 4th edition, edited by McCulloch J. and Kloth L. Philadelphia, PA: FA Davis, 2010, pp. 576–593 [Google Scholar]
- 18.Rao BK, Kumar P, Rao S, and Gurung B: Bactericidal effect of ultraviolet C (UVC), direct and filtered through transparent plastic, on Gram-positive cocci: an in vitro study. Ostomy Wound Manage 2011; 57:46. [PubMed] [Google Scholar]
- 19.Thai TP, Houghton PE, Campbell KE, and Woodbury MG: Ultraviolet C in the treatment of chronic wounds with MRSA: a case study. Ostomy Wound Manage 2002; 48:52. [PubMed] [Google Scholar]
- 20.Baffoni M, Bessa LJ, Grande R, Di Giulio M, Mongelli M, Ciarelli A, and Cellini L: Laser irradiation effect on Staphylococcus aureus and Pseudomonas aeruginosa biofilms isolated from venous leg ulcer. Int Wound J 2012; 9:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wollina U. and Geinig B: Novel therapies developed for the treatment of leg ulcers: focus on physical therapies. Expert Rev Dermatol 2012; 7:419 [Google Scholar]
- 22.Mouës CM, Vos MC, van den Bemd GJCM, Stijnen T, and Hovius SE: Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen 2004; 12:11. [DOI] [PubMed] [Google Scholar]
- 23.Weed T, Ratliff C. and Drake DB: Quantifying bacterial bioburden during negative pressure wound therapy; does the wound VAC enhance bacterial clearance? Ann Plast Surg 2004; 52:276. [DOI] [PubMed] [Google Scholar]