Abstract
Proteases play an essential role in normal wound healing. However, chronic wounds are characterized by abnormally high levels of proteases, in particular, the matrix metalloproteases (MMPs) and neutrophil elastase. In 2010, an international consensus panel identified elevated MMPs and human neutrophil elastase (HNE) as strong candidates for biomarkers for nonhealing wounds. A cooperative group of wound healing centers (SerenaGroup, Inc., Cambridge, MA and Systagenix, Inc., Gargrave, England) collaborated to conduct a series of laboratory and clinical trials to develop the first point-of-care diagnostic in wound healing. The first challenge was that detecting an elevated protease activity required an accurate specimen collection technique. The Levine technique utilized in the collection of bacteria for culture, using a cotton swab, failed to gather consistent levels of proteases. Extensive experimentation with collection methods and swab types finally led to the optimal procedure, The Serena Technique©. Laboratory measurements for MMPs and HNE confirmed the reliability and repeatability of the procedure. This article describes a novel method for collecting wound proteases (The Serena Technique©).

Thomas E. Serena, MD
Introduction
Proteolytic enzymes, proteases, appear in wounds as part of the normal course of healing. The family of matrix metalloproteases (MMPs) includes zinc-containing proteases. Each of the MMPs degrades a particular component of the extracellular matrix. In addition, the serine elastases, of which human neutrophil elastase (HNE) predominates, break down essential components of the extracellular matrix. After the first few days of normal wound healing, the protease levels decline as the wound progresses from the inflammatory to the proliferative stage of healing; however, in chronic and hard-to-heal wounds, it has long been known that protease levels are elevated.1 Evaluation of MMPs in fluid obtained from chronic wounds demonstrated a 30-fold greater activity in chronic wounds as opposed to acute wounds.2 Specific proteases found in excess in chronic nonhealing wound fluid include MMP-2, MMP-8, MMP-9, and HNE.3–6 Moreover, chronic wounds exhibit an imbalance between MMPs and their inhibitors: tissue inhibitors of matrix metalloproteases (TIMPs).6,7 In turn, the excessive protease activity impairs wound healing by degrading the extracellular matrix and inactivating growth factors and their receptors.6
An international consensus panel in 2010 concluded that although other biomarkers in the wound may be associated with nonhealing, assessing protease levels holds the greatest potential for the development of a wound diagnostic.1 The SerenaGroup, Inc. (Cambridge, MA) series of wound care centers partnered with Systagenix, Inc. (Gargrave, England) to develop a point-of-care diagnostic to detect elevated protease activity (EPA). The initial clinical trials focused on finding a swab type and technique that would absorb a consistent amount of wound fluid permitting a reliable and reproducible measurement of EPA.
The initial trials were conducted at a single wound center in Erie PA. The goal of these studies was to find a swab that collected a fixed amount of fluid. We discovered that polyester swabs (Puritan, Guilford, ME) consistently collect 50 μL of fluid. The next step was to determine the level of proteases in the wound fluid. We investigated a number of techniques, including the traditional Levine technique. In a multicenter trial with more than 200 patients, the best method for reliably and reproducibly measuring proteases was to moisten the wound with saline before swabbing, swabbing the entire wound until the swab head turned a tan color (this usually requires about 2 min of swabbing). The wound should not be debrided before testing because blood interferes with the assay and contains protease inhibitors. In addition, nonviable material should be avoided as particulate matter obstructs the lateral flow mechanism required to perform a point-of-care test. A follow-up multicenter trial confirmed that this technique consistently collected proteases from all wound types.
Once the swab and the technique consistently delivered results comparable to those obtained in the reference laboratory, the next challenge was defining EPA. Numerous authors in the past two decades have noted that EPA delayed wound healing; however, the exact level of EPA that inhibits healing remained elusive. The point-of-care diagnostic measures the combined activity of inflammatory proteases and, in particular, the activity levels of MMP-8, MMP-9, and HNE. EPA testing entered clinical evaluation in eight clinics across the United States with the goal of determining the level of EPA at which wound healing was impaired. The first trial of 93 patients with chronic and nonhealing wounds revealed that 28% of the wounds seen in the clinics demonstrated EPA. Wounds of all of the common etiologies (diabetic, venous, pressure, arterial, surgical, and traumatic) had similar percentages of EPA (range 22–33%). If a wound had EPA, there was a 90% probability that it was nonhealing.8 Additional trials with more than 250 patients enrolled have confirmed these findings. The results of these trials have been submitted for publication.
Important to the daily practice of wound care, our experience with more than 1,000 patients suggests that clinical examination of the wound is inaccurate in predicting EPA. In addition, a survey study of wound care practitioners in the United States confirmed that physical examination is not reliable in making the diagnosis of EPA (Fig. 1).9 Factors in the patient's history such as the size and duration of the wound could not predict the wounds with EPA.
Figure 1.
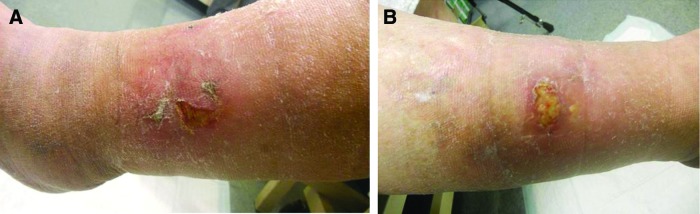
Elevated protease activity is difficult to detect on clinical examination: a patient with mirror image lateral gaiter venous leg ulcers. The left leg ulcer (A) is clean, whereas the right leg ulcer (B) contains slough. However, both are negative for elevated protease activity. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Logically, protease reduction in wounds expressing EPA would be expected to promote wound healing. Collagen/oxidized regenerated cellulose (ORC) matrix applied to chronic wounds reduced the protease activity.10 A randomized trial in chronic venous leg ulcers demonstrated decreased proteases in the wounds treated with the ORC/Collagen matrix.11 Similar findings have been reported in diabetic foot ulcers.12 Oral doxycycline has also been shown to reduce protease activity.13 Finally, there may well be other dressings and systemic therapies that decrease the protease activity.
The Serena Technique©
Before collecting the specimen, cleanse the wound with saline removing all of the loose debris and the remains of therapeutic agents. It is best not to debride the wound before swabbing. Blood interferes with the test by discoloring the test strip and blood contains TIMPs that could lead to false-negative results. If bleeding does occur, hemostasis must be achieved before obtaining the specimen.
The next step is to moisten the wound with saline such that the surface glistens. Excessive use of saline should be avoided (Fig. 2). Other wash solutions should also be avoided as they may interfere with the activity of the proteases and therefore lead to false results.
Figure 2.
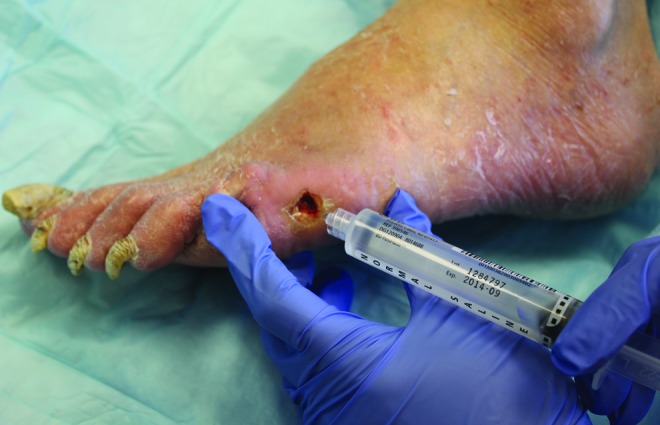
Wetting the wound surface with saline before swabbing for proteases is essential. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Use only the sterile swab provided with the kit. Press the head of the swab flat against the wound base in a parallel alignment and gently rotate or roll the swab several times while applying pressure. Continue rotating the swab with pressure in this manner until the swab head is fully coated with and discolored by the wound fluid. Areas with thick slough or necrotic or nonviable tissue should not be swabbed. Otherwise, the swab should be rolled across the entire surface of the wound. This usually takes up to 2 min (Fig. 3).
Figure 3.
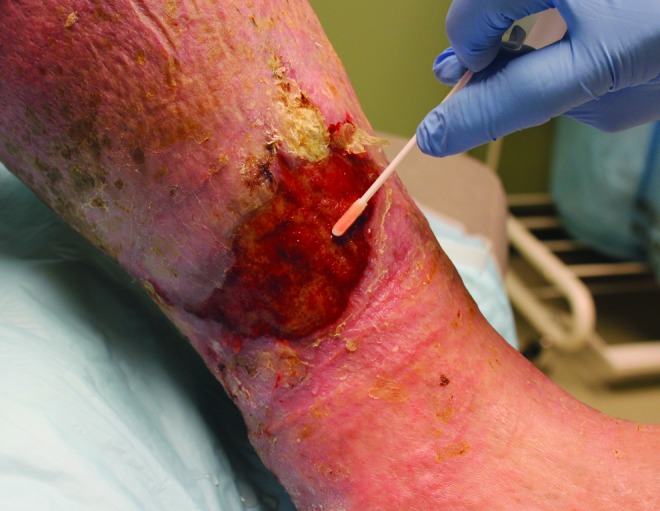
The swab is rolled over the entire surface of the wound until the head turns a tan color, which usually takes about 2 min. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Abbreviations and Acronyms
- EPA
elevated protease activity
- HNE
human neutrophil elastase
- MMP
matrix metalloprotease
- TIMPs
tissue inhibitors of matrix metalloproteases
Acknowledgments and Funding Sources
The data presented in this article was made possible through an unrestricted grant from Systagenix.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the author listed. No ghostwriters were used to write this article.
About the Author
Dr. Thomas E. Serena, MD, FACS, FACHM, MAPWCA, Founder and Medical Director, Serena Group, is a Phi Beta Kappa graduate of The College of William and Mary and Penn State Medical School. He completed his residency in Surgery at The Milton S. Hershey Medical Center with fellowship training in Plastic and Reconstructive Surgery at Southern Illinois University. He has opened and operates wound care centers across the United Sates and globally. Dr. Serena has been the lead or Principal Investigator in over 75 clinical trials. In 2011, he developed a diagnostic technique that now bears his name (The Serena Technique©). He is recognized internationally as an expert in the field of wound healing: He has more than 100 published articles and has given more than 350 invited lectures throughout the world. He was a member of the Board of Directors of the Wound Healing Society and Association for the Advancement of Wound Care. He is currently the Vice President of the American College of Hyperbaric Medicine and President of American Professional Wound Care Association (APWCA). Dr. Serena has done extensive medical relief work with Health Volunteers Overseas and serves as the Chairman of the AAWC Global Volunteers/HVO Steering Committee. He consults for the government of Rwanda on AIDS prevention research.
References
- 1.International Consensus. The Role of Proteases in Wound Diagnostics. An Expert Working Group Review. London, United Kingdom: Wounds International, 2011 [Google Scholar]
- 2.Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen 1996;4:321–325 [DOI] [PubMed] [Google Scholar]
- 3.Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol 1993;101:64–68 [DOI] [PubMed] [Google Scholar]
- 4.Nwomeh BC, Liang HX, Cohen IK, Yager DR. MMP-8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res 1999;81:189–195 [DOI] [PubMed] [Google Scholar]
- 5.Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol 2008;158:951–961 [DOI] [PubMed] [Google Scholar]
- 6.Yager DR, Chen SM, Ward SI, Olutoye OO, Diegelmann RF, Kelman Cohen I. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen 1997;5:23–32 [DOI] [PubMed] [Google Scholar]
- 7.Bullen EC, Longaker MT, Updike DL, et al. . Tissue inhibitor of metalloproteinases-1 is decreased and activated gelatinases are increased in chronic wounds. J Invest Dermatol 1995;104:236–240 [DOI] [PubMed] [Google Scholar]
- 8.Serena T, et al. . Protease Activity Levels Associated with Healing Status of Chronic Wounds. Poster, Wounds UK, 2011 [Google Scholar]
- 9.Snyder R, Cullen B, Nisbet L, Serena T. A Survey: The Importance of Proteases in Wound Healing and Wound Assessment. Presented at Wounds UK, Harrogate, 2011 [Google Scholar]
- 10.Cullen B, Kemp L, Essler L, Wallenfang-Sohle K, Stadler R. Rebalancing wound biochemistry improves healing: a clinical study examining effect of PROMOGRAN®. Wound Repair Regen 2004;12:A4 [Google Scholar]
- 11.Smeets R, Ulrich D, Unglaub F, Wöltje M, Pallua N. Effect of oxidised regenerated cellulose/collagen matrix on proteases in wound exudate of patients with chronic venous ulceration. Int Wound J 2008;5:195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulrich D, Smeets R, Unglaub F, Wöltje M, Pallua N. Effect of oxidized regenerated cellulose/collagen matrix on proteases in wound exudate of patients with diabetic foot ulcers. J Wound Ostomy Continence Nurs 2011;38:522–528 [DOI] [PubMed] [Google Scholar]
- 13.Stechmiller J, Cowan L, Schultz G. The role of doxycycline as a matrix metalloproteinase inhibitor for the treatment of chronic wounds. Biol Res Nurs 2010;11:226–244 [DOI] [PubMed] [Google Scholar]


