Abstract
In India Kaposi's sarcoma is rarely seen in AIDS patients. Hence the current belief is that the incidence of human herpesvirus-8 (HHV-8) is very low in this subcontinent, most probably due to the heterosexual route of HIV transmission. However, there is a scarcity of data on the prevalence of HHV-8 in India. In India the primary mode of HIV transmission is the heterosexual route. Therefore we aimed to determine the prevalence of antibodies against HHV-8 in North Indian HIV-infected men naive of antiretroviral therapy (ART). In a prospective study, 165 Indian adult males were recruited from an ART clinic. Blood samples were collected before administering any antiretroviral drug. The sera were tested for antibodies against HHV-8 using a commercial enzyme-linked immunosorbent assay (ELISA) kit, which detects IgG antibodies to lytic antigens of HHV-8. All positive samples were confirmed for the presence of anti-HHV-8 antibodies using an indirect immunofluorescence assay (IFA). The IFA kit is intended to detect primary, latent, persistent, or reactivated infection of HHV-8. Of the 165 males, 43 (26.06%) were positive by ELISA while 26 (15.8%) were also positive by IFA. Seroprevalence decreased with increasing age (p<0.05). Factors independently associated with HHV-8 infection were younger age group and alcohol consumption. These findings suggest that even in a heterosexual population, HHV-8 can be transmitted frequently.
Introduction
Human herpesvirus-8 (HHV-8), also known as Kaposi's sarcoma-associated herpesvirus (KSHV), was discovered in 1994 by Chang and Moore.1 It is considered to be the primary etiological agent of Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman's disease (MCD).2 Since the virus was more prevalent in sexually active men, its sexual mode of transmission was hypothesized.3,4 However, it has also been reported that HHV-8 can be transmitted through nonsexual routes.5 In addition, familial clustering of HHV-8 has been observed in highly endemic regions.6 Indeed, the epidemiology of HHV-8 has long remained a puzzle. There exists a wide geographic variation in the prevalence of HHV-8 whether it is a region of high endemicity or a nonendemic region.7
HIV and HHV-8 correlate very well with each other, since the routes of transmission, risk factors, and pathogenesis are intricately related and interposed.8,9 It is thought that the seroprevalence of HHV-8 infection approximately mirrors the prevalence of KS, which is the most common neoplastic event seen in HIV-infected patients.10 Due to a scarcity of data on HHV-8 from India, the current belief is that the prevalence is quite low in this subcontinent, which is in contrast to the global epidemiology. However, a critical review of the literature indicates that more studies are needed to estimate the real prevalence of HHV-8 in men with high-risk sexual behavior with and without HIV coinfection. The association between HHV-8 and HIV seropositivity in heterosexual subjects is a debatable topic. Some studies have shown a positive association,11–13 whereas others have not.14–16 To address this gap the present study was planned and conducted.
Materials and Methods
The primary objective of this study was to assess the prevalence of HHV-8 infection in a cohort of antiretroviral therapy (ART)-naive HIV-infected adult males. The secondary objective was to investigate the characteristics of HHV-8 transmission with behavioral and demographic correlates.
The study was carried out from September 2010 to December 2012 at the Division of Clinical Microbiology and Molecular Medicine, Department of Laboratory Medicine, AIIMS, New Delhi, India. Subjects older than 18 years of age and naive of ART were included after written informed consent to participate in the study was obtained.
A structured pretested questionnaire was administered to collate demographic characteristics, sexual behavior, substance abuse including intravenous drug use (IDU), and history of sexually transmitted diseases (STDs). Sexually transmitted disease was defined as self-reported or clinically confirmed past or recent history of any one of the following: gonorrhea, syphilis, herpetic genital ulcers, and genital warts. An approximately 5-ml blood sample was collected in a sterile container without anticoagulant. Blood was centrifuged after coagulation and serum was separated, coded with a unique identification number, and stored at −80°C in a deep freezer until use.
The commercial assays used to estimate the seroprevalence of HHV-8 use two types of HHV-8 antigens: the nuclear antigen, which detects latent/persistent infection, and the cytoplasmic antigen, which detects the lytic stage of infection.17 These antigens have been used extensively to detect HHV-8 infection. All serum samples were tested for anti-HHV-8 antibodies using an enzyme-linked immunosorbent assay (ELISA) (Advanced Biotechnologies Inc., Columbia, MD) according to the manufacturer's instructions. This ELISA kit detects IgG antibodies to lytic antigens of HHV-8 in human serum or plasma. The cut-off value was calculated by taking the average reading of three negative control wells. Optical density (OD) ratios were calculated by dividing the reading of each sample well by the cut-off value. Each OD ratio was interpreted as follows: OD≤0.75 as a negative sample, OD≥1.00 as a positive sample, and OD values between 0.76 and 0.99 as equivocal or borderline and were re-tested. All samples were tested at 1:100 serum dilutions.
All positive samples were confirmed for the presence of anti-HHV-8 antibodies using an HHV-8 indirect immunofluorescence assay (IFA) (Advanced Biotechnologies Inc., Columbia, MD). The IFA kit is intended to detect primary, latent, persistent, or reactivated infection of HHV-8. A bright apple green epifluorescence in the fixed cells when examined under a fluorescence microscope (Nikon, Japan) was considered positive. In brief, Sera from HIV-infected males were incubated at a dilution of 1:40 with KS-1-infected/induced cells fixed on microscopy glass slides. Positive and negative controls provided with the IFA kit were also run along with the test sample. Primary antibody conjugation was detected using fluorescein isothiocyanate (FITC)-conjugated antihuman secondary antibody. The slides were examined using epifluorescence microscopy (Nikon, Japan). A negative fluorescence reaction is indicated by the appearance of only red cells while bright apple-green fluorescence indicates the presence of anti- HHV-8 antibodies in the sample.
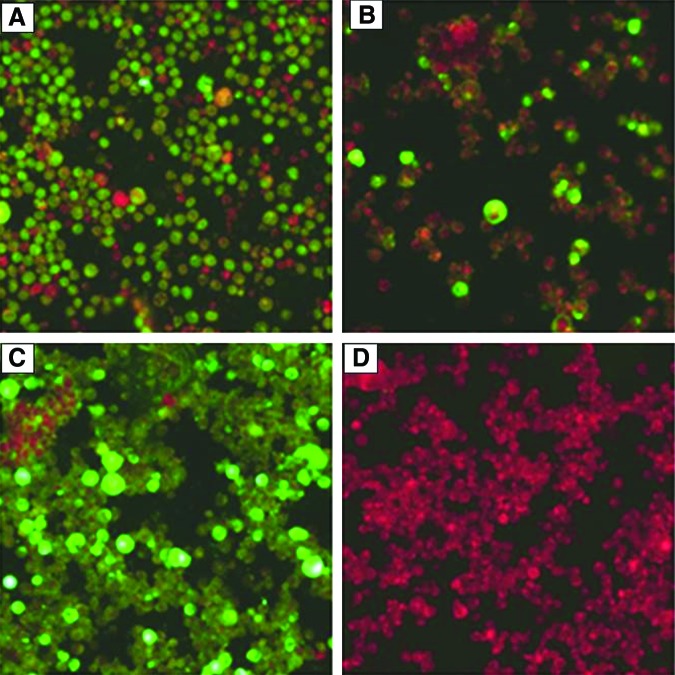
Since the IFA is a semiquantitative test, grading of positivity was done using dual criteria of brightness of the fluorescence and number of cells showing fluorescence. Arbitrary grading is done as negative, no fluorescent cells at all; 1+, a few cells show fluorescence or several cells show faint fluorescence; 2+, more than 25% of cells show bright fluorescence; 3+, more than 50% of cells show bright fluorescence; 4+, most of the cells on the slide show bright green fluorescence. The grading is made taking positive and negative control into consideration (Fig. 1).
FIG. 1.
Indirect immunofluorescence assay results. Note the high fluorescence indicating a strongly (3+) positive sample in (A) and a moderately (2+) positive sample in (B); (C) is a positive (4+) serum control and (D) is a negative (no fluorescence) serum control. Magnification ×400. Color images available online at www.liebertpub.com/aid
Statistical analysis
Data were analyzed using SPSS version 19. Demographic characteristics and risk behaviors were analyzed using descriptive statistics, i.e., mean, median, and interquartile range (IQR) for continuous variables and proportions for categorical variables. HHV-8 seroprevalence was estimated using the normal approximation. Differences in variables were sought by Student's t-test or Chi-square/Fisher's exact test as appropriate. Initially univariate logistic regression analysis was conducted. Significant determinants of HHV-8 infection were adjusted in a logistic regression model expressed in odds ratio (OR) with 95% confidence interval (CI). A p-value≤0.05 was considered significant.
Results
Sociodemographic details
One hundred and sixty-five samples (one each) were collected from 165 ART-naive men accessing HIV care at the nodal ART center of AIIMS. The median age of the cohort was 31 years [IQR 26.5–36; 95% CI, 30–32]. Almost three-fourth [73.9% (122/165)] of the subjects were young individuals aged ≤35 years. Among these only 25.5% (42/165) attended a degree college or higher education (Table 1). The majority [79.4% (131/165)] of the men were married. The median age of the married men was 32 years [IQR 30–38; 95% CI, 32–34] while the median age of the single men was 24 years [IQR 22–28; 95% CI, 22–27]. Less than a quarter [23.03% (38/165)] of the males reported that they have had one or more STDs in the near past. In 88.5% (146/165) of the subjects HIV was acquired through sex whereas the nonsexual mode of transmission accounted for 11.5% (19/165), which included IDU, mother-to-child transmission (MTCT), and blood transfusion or needle prick.
Table 1.
Sociodemographic and Behavioral Correlates of Human Herpesvirus 8 Infection in Antiretroviral Treatment-Naive Indian Adult Males
| HHV-8 antibody status | ||||
|---|---|---|---|---|
| Characteristics | Negative (122) | Positive (43) | p value | Unadjusted OR |
| Age group | ||||
| 18–25 | 17 (62.96) | 10 (37.03) | 4.7 | |
| 26–35 | 67 (70.52) | 28 (29.47) | 0.032 | 3.1 |
| 36–50 | 38 (88.37) | 5 (11.62) | 1 | |
| Smoking/tobacco chewing | ||||
| No | 77 (70.64) | 32 (29.35) | 0.178 | 1 |
| Yes | 45 (80.35) | 11 (19.64) | 0.58 (0.27, 1.27) | |
| Alcohol | ||||
| No | 57 (82.61) | 12 (17.39) | 1 | |
| Yes | 65 (67.70) | 31 (32.30) | 0.031 | 2.26 (1.06, 4.82) |
| Education | ||||
| Uneducated/nonmatriculating | 53 (74.64) | 18 (25.36) | 0.112 | 1 |
| Matriculating or higher | 69 (73.40) | 25 (26.60) | 1.18 (0.57, 2.42) | |
| Socioeconomic status | ||||
| Low–income group | 65 (73.86) | 23 (26.14) | 0.981 | 1 |
| Middle–income group | 57 (74.02) | 20 (25.97) | 0.99 (0.49, 1.99) | |
| Employment status | ||||
| Unemployed | 19 (63.33) | 11 (36.66) | 0.143 | 1 |
| Employed | 103 (76.29) | 32 (36.68) | 0.5 (0.23, 1.24) | |
| Marital status | ||||
| Single | 22 (64.70) | 12 (35.29) | 0.169 | 1 |
| Married | 100 (76.33) | 31 (23.67) | 0.56 (0.25, 1.27) | |
| Sexual preference | ||||
| MSM | 12 (75.00) | 4 (25.00) | 0.919 | 1 |
| Heterosexual | 110 (73.82) | 39 (26.17) | 1.06 (0.32, 3.49) | |
| Mode of HIV acquisition | ||||
| Blood transfusion/needle prick | 10 (90.90) | 1 (9.10) | 1 | |
| MTCT | 3 (100.00) | 0 | 0 | |
| Sexual | 106 (72.60) | 40 (27.39) | 0.357 | 3.8 |
| IDU | 1 | 0 | 0 | |
| Unknown | 2 (50.00) | 2 (50.00) | 10 | |
| Past history of STD | ||||
| No | 95 (74.80) | 32 (25.20) | 0.644 | 1 |
| Yes | 27 (71.08) | 11 (29.00) | 1.20 (0.53, 2.71) | |
HHV-8, human herpesvirus 8; OR, odds ratio; MSM, men who have sex with men; MTCT, mother-co-child transmission; IDU, intravenous drug use; STD, sexually transmitted disease.
One hundred and forty nine (90.3%) men stated that they were heterosexual while sixteen (9.7%) identified themselves as having sex with men (either homosexual or bisexual). This difference was highly significant (p<0.001). Most married men [91.6% (120/131); p<0.001] were heterosexual and only 8.4% (11/131) were bisexual. A tendency to homosexual behavior was slightly more (14.7%) in unmarried men. As many as 69.1% (114/165) men had addiction or substance abuse in the form of alcohol, smoking/chewing of tobacco, or IDU. Of these 114 males, 96 (84.21%) regularly consumed alcohol, 39 (34.21%) were chronic smokers along with alcohol consumption, 17 (14.91%) were smokers with no alcohol consumption, and only one (0.87%) male was an intravenous drug user (p<0.001).
HHV-8 seroprevalence and confounding factors
Out of 165 subjects 43 (26.0%) tested positive for HHV-8, of whom 26 (60.5%) were positive for antibodies against both lytic and latent HHV-8 antigens and 17 (39.5%) were positive for HHV-8 lytic antigen only. In the univariate analysis, there was a tendency toward a high risk of HHV-8 positivity in younger individuals aged 18–25 years [OR: 4.32; 95% CI, 1.27–14.78] and alcohol consumption [OR: 2.26; 95% CI, 1.06–4.82]. Table 1 shows that seroprevalence decreased with the increase in age from 37.03% (10/27) in men aged 18–25 years to only 11.6% (5/43) in men older than 35 years of age (p<0.05). Interestingly no significant difference was observed in the prevalence of anti-HHV-8 antibodies between heterosexual men (26.17%) and men who have sex with men (MSM) (25%) (Table 1). The median CD4+ T cells count was 358/μl [IQR 250–491.5; 95% CI, 332–404 cells/μl].
Discussion
This study was carried out with the basic aim of assessing HHV-8 prevalence in Indian males with high-risk sexual behavior. In the present study, the seroprevalence of HHV-8 was found to be 26.1% in HIV-infected Indian males. The prevalence of HHV-8 is reported to be higher in the homosexual population than the heterosexual population.17–22 Thus, the prevalence rate reported in the present study may seem to be very high for the heterosexual population included in this study.23 However, HIV infection among MSM is increasingly being reported from India.23 In the present study, we found a notable proportion (12/16; 75%) of men with bisexual orientation, or in other words these were heterosexually married men. This implies that significant numbers of Indian men are bisexually active but are not identified as an open MSM group, and that these men could be a potential source of HHV-8 transmission.
A wide variation in HHV-8 seroprevalence has previously been observed in East Asian countries, viz. Japan (11.7%), China (12.7–43.2%), and Thailand (1–28.1%). 17–22 However, to date except for India, there is no published literature regarding the seroprevalence of HHV-8 from countries of the Indian subcontinent such as Pakistan, Afghanistan, Bangladesh, Sri Lanka, and Nepal. In addition, in India only a few studies on this subject have been published. The very first study was published in 1999, which showed a seroprevalence of 2.4%.24 But their sample size was very small (42; 36 males and 12 females). Another study carried out on 87 HIV-infected subjects from South India also reported a prevalence of <5%.25 In both these studies the reported prevalence was much lower than what we have found. No study with a comparable sample size has been carried from India; hence, it is difficult to compare our data with other's data and to ascertain the reasons for the variations in seroprevalence rates.
We found a statistically significant association between age and HHV-8 positivity. The young adults showed a higher prevalence than older males. The most plausible explanation could be that active transmission of HHV-8 in this part of India has occurred only in the past few decades. Alcohol consumption, which is a well-known factor mitigating high-risk sexual behavior,16 was independently associated with increased HHV-8 prevalence in our study subjects. Therefore, it is conceivable that the prevalence was higher in those who consumed alcohol regularly and risked sexually transmitted diseases including HHV-8.
The most important observation of the study was the comparable rates of HHV-8 infection in heterosexual men and MSM. This observation suggests that heterosexual men could potentially transmit HHV-8 to their female partners. Hence, it would be most prudent to carry out well-designed studies in sexually active females as well as in school going young children to determine the baseline prevalence of this virus.
From this study, however, we cannot ascertain the clinical significance of HHV-8 infection in our population, as clinically confirmed KS cases are rarely reported from India, in spite of the high prevalence of the infection. This may be due to the fact that the genotype(s) of HHV-8 circulating in India are avirulent or due to a lack of some other unknown cofactors.26
Acknowledgments
The authors wish to acknowledge the skillful help of Mrs. Veena Balooni, Mrs. Shalini Singhal, and Mr. Shakil Ahmad, from the Division of Clinical Microbiology, Department of Laboratory Medicine, AIIMS, New Delhi.
The study was financially supported by the Indian Council of Medical Research, New Delhi, India (grant Indo-US/69/9/2010-ECD-II).
The ethics committee of the All India Institute of Medical sciences (AIIMS), New Delhi approved this prospective study (Ref. No. IEC/NP-260/2010).
Arshi Munawwar recruited study subjects, collected data, performed the experiments, and drafted the initial manuscript. S.K. Sharma and Somesh Gupta guided in the recruitment of subjects, provided treatment and clinical details, and helped in the writing of this manuscript. Sarman Singh conceptualized and supervised the study, arranged funds for chemicals and reagents, and prepared the final draft of the manuscript. All authors read and approved the final draft.
Part of this work was previously presented at the Eleventh International Congress on Drug Therapy in HIV Infection: Munawwar A, et al. J Int AIDS Soc 2012;15(Suppl 4):18120.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Chang Y, Cesarman E, Pessin MS, et al. : Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 1994;266:1865–1869 [DOI] [PubMed] [Google Scholar]
- 2.Moore PS. and Chang Y: Kaposi's sarcoma (KS), KS-associated herpesvirus, and the criteria for causality in the age of molecular biology. Am J Epidemiol 1998;147:217–221 [DOI] [PubMed] [Google Scholar]
- 3.Casper C, Meier AS, Wald A, et al. : Human herpesvirus 8 infection among adolescents in the REACH cohort. Arch Pediatr Adolesc Med 2006;160:937–942 [DOI] [PubMed] [Google Scholar]
- 4.Grulich AE, Cunningham P, Munier ML, et al. : Sexual behaviour and human herpesvirus 8 infection in homosexual men in Australia. Sex Health 2005;2:13–18 [DOI] [PubMed] [Google Scholar]
- 5.Vieira J, Huang ML, Koelle DM, et al. : Transmissible Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in saliva of men with a history of Kaposi's sarcoma. J Virol 1997;71:7083–7087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guttman-Yassky E, Cohen A, Kra-Oz Z, et al. : Familial clustering of classic Kaposi sarcoma. J Infect Dis 2004;189:2023–2026 [DOI] [PubMed] [Google Scholar]
- 7.Tanzi E, Zappa A, Caramaschi F, et al. : Human herpesvirus type 8 infection in an area of northern Italy with high incidence of classical Kaposi's sarcoma. J Med Virol 2005;76:571–575 [DOI] [PubMed] [Google Scholar]
- 8.Gallo R: Some aspects of the pathogenesis of HIV-associated Kaposi's sarcoma. J Natl I Monogr 1998; 23:55–57 [DOI] [PubMed] [Google Scholar]
- 9.Viejo BA. and Schutz T: KSHV: Key aspects of epidemiology and pathogenesis. AIDS Rev 2003;5:222–229 [PubMed] [Google Scholar]
- 10.Serraino D, Toma L, Andreoni M, et al. : A seroprevalence study of human herpesvirus type 8 (HHV8) in eastern and Central Africa and in the Mediterranean area. Eur J Epidemiol 2001;17(9):871–876 [DOI] [PubMed] [Google Scholar]
- 11.Reinheimer C, Allwinn R, and Sturmer M: Do fewer cases of Kaposi's sarcoma in HIV-infected patients reflect a decrease in HHV8 seroprevalence? Med Microbiol Immunol 2011;3:161–164 [DOI] [PubMed] [Google Scholar]
- 12.Nuvor SV, Katano H, Ampofo WK, et al. : Higher prevalence of antibodies to human herpesvirus 8 in HIV-infected individuals than in the general population in Ghana, West Africa. Eur J Clin Microbiol Infect Dis 2001;3:362–364 [DOI] [PubMed] [Google Scholar]
- 13.Klaskala W, Brayfield BP, Kankasa C, et al. : Epidemiological characteristics of human herpesvirus-8 infection in a large population of antenatal women in Zambia. J Med Virol 2005;3:93–100 [DOI] [PubMed] [Google Scholar]
- 14.Biryahwaho B, Dollard SC, Pfeiffer RM, et al. : Sex and geographic patterns of human herpesvirus 8 infection in a nationally representative population-based sample in Uganda. J Infect Dis 2010;3:1347–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He J, Bhat G, Kankasa C, et al. : Seroprevalence of human herpesvirus 8 among Zambian women of childbearing age without Kaposi's sarcoma (KS) and mother-child pairs with KS. J Infect Dis 1998;3:1787–1790 [DOI] [PubMed] [Google Scholar]
- 16.Baeten JM, Chohan BH, Lavreys L, et al. : Correlates of human herpesvirus 8 seropositivity among heterosexual men in Kenya. AIDS 2002;3:2073–2078 [DOI] [PubMed] [Google Scholar]
- 17.Katano H, Yokomaku Y, Fukumoto H, et al. : Seroprevalence of Kaposi's sarcoma-associated herpesvirus among men who have sex with men in Japan. J Med Virol 2013;85:1046–1052 [DOI] [PubMed] [Google Scholar]
- 18.Zhang T, Lin H, Minhas V, et al. : Prevalence and correlates of Kaposi's sarcoma-associated herpesvirus infection in a sample of men who have sex with men in Eastern China. Epidemiol Infect 2013;141(9):1823–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayuthaya PI, Katano H, Inagi R, et al. : The seroprevalence of human herpesvirus 8 infection in the Thai population. Southeast Asian J Trop Med Public Health 2002;33:297–305 [PubMed] [Google Scholar]
- 20.Chen N, Nelson KE, Jenkins FJ, et al. : Seroprevalence of human herpesvirus 8 infection in Northern Thailand. Clin Infect Dis 2004;39:1052–1058 [DOI] [PubMed] [Google Scholar]
- 21.Zhang TJ, He N, Ding YY, et al. : Antibody responses to lytic and latent human herpesvirus 8 antigens among HIV-infected patients in central China. Biosci Trends 2012;6:122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Liu J, Dilimulati, Li L, et al. : Seroprevalence of Kaposi's sarcoma-associated herpesvirus and risk factors in Xinjiang, China. J Med Virol 2009; 81:1422–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National AIDS Control Organization, Government of India, New Delhi: Available at www.naco.gov.in/NACO/National_AIDS_Control_Program/10711/ Accessed on January5, 2014
- 24.Ablashi D, Chatlynne L, Cooper H, et al. : Seroprevalence of human herpesvirus-8 (HHV-8) in countries of Southeast Asia compared to the USA, the Caribbean and Africa. Br J Cancer 1999;81:893–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sachithanandham J, Kannangai R, Abraham AM, et al. : Human herpes virus-8 infections among subjects with human immunodeficiency virus infection and normal healthy individuals in India. Intervirology 2013;56:253–257 [DOI] [PubMed] [Google Scholar]
- 26.Cassar O, Blondot ML, Mohanna S, et al. : Human herpesvirus 8 genotype E in patients with Kaposi sarcoma, Peru. Emerg Infect Dis 2010;16(9):1459–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]