Abstract
The present study investigated the immunological pathogenesis of immune reconstitution inflammatory syndrome (IRIS) in acquired immunodeficiency syndrome (AIDS) patients undergoing highly active antiretroviral therapy (HAART). A total of 238 patients with AIDS who received initial HAART were included in this prospective cohort study. Blood samples were collected immediately, at baseline, at week 12, and at week 24 after initial HAART and at the onset of IRIS. Lymphocyte subsets, Th1 and Th2 cytokines, and interleukin (IL)-7 levels were measured by flow cytometry or ELISA. Among the 238 patients with AIDS who received HAART, 47 patients developed IRIS. The percentages of CD4+ and CD8+ naive, memory, and activated cells exhibited no significant differences between AIDS patients with and without IRIS 24 weeks after initial HAART. The percentage of CD4+CD25+Foxp3+ regulatory T cells was lower in IRIS patients than in non-IRIS patients before HAART, 12 weeks after HAART, 24 weeks after HAART, and at the onset of IRIS. IL-2 and interferon (IFN)-γ levels were significantly higher at week 4 and at the onset of IRIS in IRIS patients than in non-IRIS patients. In contrast, IL-4 and IL-10 levels were significantly lower at week 4 and at the onset of IRIS in IRIS patients than in non-IRIS patients. Plasma IL-7 decreased gradually with the progression of HAART. The level of IL-7 was higher in IRIS patients than in non-IRIS patients at all follow-up time points. An imbalance of Th1/Th2 cytokines, a consistently low CD+CD25+Fox3+ percentage, and a high IL-7 level may be crucial in the pathogenesis of IRIS in AIDS patients who had received HAART.
Introduction
Highly active antiretroviral therapy (HAART) is currently the most effective way to treat acquired immunodeficiency syndrome (AIDS). HAART can dramatically suppress the replication of human immunodeficiency virus (HIV), rebuild the immune function of infected patients, and reduce the incidence of opportunistic infections.1,2 However, shortly after the initiation of HAART, about 10–30% of patients receiving HAART contract more severe illnesses, opportunistic infections, or infections from new pathogens. This phenomenon was described in literature as immune reconstruction disease (IRD) or immune reconstruction inflammation syndrome (IRIS).3,4 IRIS is a life-threatening syndrome. Preventing the development of IRIS is of great significance to improving the prognosis of AIDS.
Despite the progress made in understanding the manifestations of IRIS, the pathogenesis of IRIS in HAART-treated AIDS patients remains largely unknown. The treatment efficacy of HAART is mainly evaluated by the decrease in HIV-1 viral load and the degree of reconstitution of the immune system.5 However, the implications of the rebuilding of immune function for the development of IRIS in AIDS patients have not been widely reported. In particular, the immunopathogenesis of IRIS is rarely elucidated. In this study, we investigated the T lymphocyte subsets, Th1 and Th2 cytokines, and interleukin (IL)-7 levels at weeks 0, 12, and 24 after HAART treatment and at the onset of IRIS in AIDS patients who developed IRIS compared to patients who did not develop IRIS 24 weeks after initial HAART.
Subjects and Methods
Study design
This is a prospective observational study. All patients were treated with the conventional HARRT regimen: lamivudine, 300 mg per dose, once per day; zidovudine, 300 mg per dose, twice per day; and nevirapine, 200 mg per dose, once per day, which were changed to twice per day 2 weeks later if no side effects were observed. Patients who developed IRIS were allocated to the IRIS group, while patients who did not develop IRIS until 24 weeks were assigned to the non-IRIS group. Blood samples were collected from 47 IRIS cases and 191 non-IRIS cases for the detection of lymphocyte subsets, Th1 and Th2 cytokines, and IL-7 level at weeks 0, 12, and 24 post-HAART and at the onset of IRIS.
Subjects
A total of 238 patients with AIDS (163 male and 65 female) who received HAART in the Hunan Provincial Center for Disease Control and Prevention (CDC) from October 2007 to September 2009 were enrolled in this study. This study was preapproved by the medical ethics committee of Second Xiangya Hospital. Patients were informed of the purpose of the study, the possible side effects, and other considerations. Written informed consent forms were obtained from all patients. All AIDS patients were diagnosed according to the “AIDS Diagnosis and Treatment Guidelines” (National Ministry of Health, China, 2006). All patients were confirmed to be HIV-1 positive and met the requirements of “National AIDS antiviral treatment manuals” (second edition) for initiating HAART. The patients were followed up for 24 weeks. The average age of the patients was 38.3 years with a range from 29.5 to 45.3 years. The assessment of IRIS and non-IRIS during follow-up was based on the widely accepted IRIS criteria.6 Thirty-one healthy students and employees (20 male and 11 female) with ages that ranged from 23.1 years to 43.7 years were recruited as healthy controls. These healthy controls were HIV negative and had no history of other severe infectious diseases or systemic diseases.
Sample collection
Five milliliters of fasting blood was collected in an anticoagulant tube from each subject. Plasma and peripheral blood mononuclear cells (PBMCs) were separated with lymphocyte separation reagents. Plasma was frozen at −80°C.
FACS analysis of T cell subsets
Two- to three-color flow cytometry was applied to quantify T cell subsets in fresh blood samples. The absolute counts (cells/μl) of lymphocytes in erythrocyte lysed whole blood were determined by FACS Calibur flow cytometry as previously described.7 The monoclonal antibodies including PE-CD4, PE-CD8, FITC-CD45RA, FITC-CD45RO, APC-62L, PerCP-CD38, and APC-CD25 were purchased from BD Corporation (USA).
ELISA detection of cytokines
Plasma IL-2, interferon (IFN)-γ, IL-4, IL-7, and IL-10 levels were detected by ELISA kits. Sample absorbance was obtained with a microplate reader, and data were analyzed with CurveExpert1.3 software (Microsoft, Redmond, WA). ELISA kits for IL-2, IFN-γ, IL-4, IL-10, and IL-7 were purchased from Shenzhen Jingmei biotechnology company (Shenzhen, China). An MK 2 ELISA analyzer was purchased from Labsystems Dragon (Wellscan, Finland).
Statistical analysis
The lymphocyte subsets and cytokines were compared between IRIS patients, non-IRIS patients, and healthy controls at weeks 0, 12, and 24 and at the onset of IRIS. The results were analyzed with SPSS version 13.0 statistical software packages. Data were expressed as mean values±standard deviation (SD). The parametric data were analyzed using a t test. Nonparametric data were analyzed using the Wilcoxon rank-sum test. A p<0.05 was considered statistically significant.
Results
Comparison of lymphocyte subsets
Among the 238 patients with AIDS who received HAART, 47 patients developed IRIS. Of the 47 patients with IRIS, 29 patients were infected with tuberculosis (29/47, 61.7%), 3 patients were infected with fungal infections (3/47, 6.3%), and 15 patients were infected with a virus (15/47, 32.0%). The average number of days before IRIS was observed was 28 days (9–36 days) after the initiation of HAART. The results for lymphocyte subsets are presented in Table 1. The average percentage of CD4+CD45RA+CD62L+ and CD8+CD45RA+CD62L+naive T cells was increased in two HAART groups after initial HAART, but no significant differences were observed between any two groups or at any time point in the two groups of patients (p>0.05). HAART increased the average percentage of CD4+CD45RO+ and CD8+CD45RO+ memory T lymphocytes in IRIS and non-IRIS groups. The significant increase was observed at week 24 after HAART compared to baseline before HAART and healthy controls. No significant differences were observed between the IRIS and non-IRIS groups at any time point (p>0.05). The percentage of CD4+CD38+ and CD8+CD38+ was significantly higher in both groups of patients compared to healthy controls (p<0.05), but HAART significantly decreased the percentage of CD4+CD38+ and CD8+CD38+ at week 24 (p<0.05) in both groups of patients. No significant differences were observed between the IRS and non-IRS groups at any time point after HAART (p>0.05).
Table 1.
Comparison of Lymphocyte Subsetsin Patients With and Without Immune Reconstitution Inflammatory Syndrome and Healthy Controls
| Group | Week 0 (baseline) (%) | Week 12 (%) | Week 24 (%) | Onset of IRIS (%) | Healthy control group (%) |
|---|---|---|---|---|---|
| CD4+CD45RA+CD62L+ | |||||
| IRIS | 40.2±11.3 | 40.9±12.7 | 42.5±13.1 | 41.3±10.7 | 45.8±9.8 |
| Non-IRIS | 38.7±13.2 | 39.2±10.6 | 40.4±11.8 | NA | |
| CD8+CD45RA+CD62L+ | |||||
| IRIS | 16.5±5.7 | 17.3±5.4 | 18.4±4.8 | 18.5±5.3 | 40.9±12.3 |
| Non-IRIS | 17.3±6.1 | 16.7±6.9 | 19.3±6.5 | NA | |
| CD4+CD45RO+ | |||||
| IRIS | 65.2±9.7 | 71.9±9.5 | 72.9±11.2# | 71.6±11.7 | 53.3±9.3 |
| Non-IRIS | 67.4±10.3 | 70.8±11.9 | 71.6±12.4# | NA | |
| CD8+CD45RO+ | |||||
| IRIS | 32.3±7.9 | 40.4±9.2 | 44.4±13.1# | 39.8±10.3 | 34.1±8.9 |
| Non-IRIS | 34.2±9.4 | 41.1±10.4 | 43.1±12.6# | NA | |
| CD4+CD38+ | |||||
| IRIS | 73.5±11.3◊ | 67.1±12.4 | 61.3±10.7# | 66.3±10.3 | 50.2±12.1 |
| Non-IRIS | 71.4±12.5◊ | 64.2±11.3 | 60.7±9.2# | NA | |
| CD8+CD38+ | |||||
| IRIS | 70.1±18.6◊ | 62.7±9.4 | 53.2±11.7# | 63.4±11.9 | 41.2±13.2 |
| Non-IRIS | 68.3±19.2◊ | 61.2±13.2 | 52.4±10.1# | NA | |
| CD4+CD25+Foxp3+ | |||||
| IRIS | 2.3±1.1*,◊ | 2.0±1.3* | 2.2±1.2* | 1.8±0.8* | 5.4±1.6 |
| Non-IRIS | 2.6±1.4◊ | 2.4±0.9 | 3.0±1.9 | NA | |
p<0.05, compared between the two groups; #p<0.05, compared with baseline; ◊p<0.05, compared with the control group.
HAART, highly active antiretroviral therapy; IRIS, immune reconstitution inflammatory syndrome; percentage of lymphocytes in each group represented the percentage of CD4+ cells or CD8+ cells in their gating.
As shown in Table 1 and Fig. 1, the percentage of CD4+CD25+Foxp3+ regulatory T cells was significantly lower in both groups of patients compared to healthy controls (p<0.05). HAART had no significant effect on the percentage of CD4+CD25+Foxp3+ regulatory T cells (p>0.05). However, AIDS patients who developed IRIS after HAART showed a significantly lower percentage of CD4+CD25+Foxp3+ regulatory T cells at weeks 0, 12, and 24 and at the onset of IRIS (p<0.05).
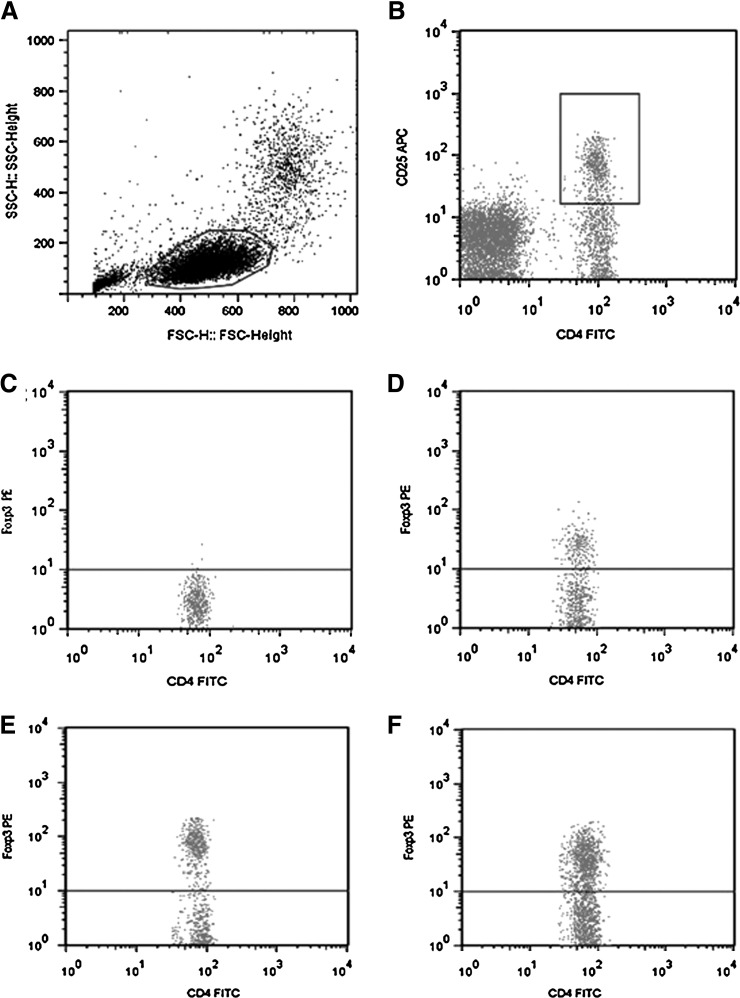
FIG. 1.
Flow cytometry of CD4+CD25+Foxp3+ regulatory T lymphocytes. (A) Delineation of lymphocytes with forward scatter (FSC) and side scatter (SSC) and gating. (B) Selection of FITC-CD4 and APC-CD25 double-positive cells and gating. (C) Define PE-Foxp3-positive cells by reference to IgG2a-PE isotype control. (D) Representative image of PE-Foxp3-positive cells in the immune reconstitution inflammatory syndrome (IRIS) group. (E) Representative image of PE-Foxp3-positive cells in the non-IRIS group. (F) Representative image of PE-Foxp3-positive cells in the healthy control group.
Comparison of cytokine levels
IL-2 and IFN-γ of Th1 cytokines, IL-4, IL-10 of Th2 cytokines, and IL-7 were measured by ELISA (Table 2). The levels of IL-2 and IFN-γ were significantly lower in AIDS patients in the IRIS group or non-IRIS group before HAART than in healthy controls (p<0.05). HAART significantly increased IL-2 and IFN-γ levels in these two groups of patients with AIDS at week 24 after initial HAART compared to baseline levels before HAART (p<0.05). Significant differences in IL-2 and IFN-γ levels were observed between patients in the IRIS and non-IRIS groups at baseline and at week 4 after HAART (p<0.05). The IL-2 and IFN-γ levels were significantly higher at the onset of IRIS compared to baseline (p<0.05).
Table 2.
The Results of Th1, Th2, and IL-7 of Highly Active Antiretroviral Therapy in Two Groups (pg/ml)
| Group | Baseline (%) | Week 4 (%) | Week 12 (%) | Week 24 (%) | At onset of IRIS (%) | Healthy controls (%) |
|---|---|---|---|---|---|---|
| IL-2 | ||||||
| IRIS | 8.5±2.25b,d | 15.72±4.91b,d | 12.97±3.68 | 13.76±3.92a | 16.71±4.22a,c | 15.64±4.25 |
| Non-IRIS | 10.69±2.99d | 12.87±3.07 | 13.05±3.65 | 14.02±3.81a | NA | |
| IFN-γ | ||||||
| IRIS | 18.28±5.32b,d | 32.24±8.24b,d | 22.04±7.31 | 23.98±7.32a | 35.91±9.08a,c | 32.21±7.54 |
| Non-IRIS | 21.03±6.38d | 21.26±5.99 | 23.66±6.05 | 25.43±9.65a | NA | |
| IL-4 | ||||||
| IRIS | 15.10±4.09d | 11.50±1.45 | 11.07±2.16 | 10.76±1.69a | 11.33±2.04a | 7.24±1.51 |
| Non-IRIS | 14.50±3.39d | 12.44±1.27 | 11.55±1.97 | 9.76±1.21a | NA | |
| IL-10 | ||||||
| IRIS | 20.38±5.95d | 16.19±3.95b,d | 17.45±4.49 | 14.23±4.67a | 15.21±5.54a,c | 9.66±2.96 |
| Non-IRIS | 22.21±6.61d | 21.56±5.77 | 18.61±5.17 | 14.45±5.44a | NA | |
| IL-7 | ||||||
| IRIS | 3.96±1.86b,d | 3.70±1.61b,d | 2.95±1.67b | 2.05±1.14a,b | 3.51±1.52a,c | 1.50±0.49 |
| Non-IRIS | 2.83±1.17d | 2.42±1.26 | 2.10±0.84 | 1.73±0.76a | NA | |
Compared with baseline groups, p<0.05.
Compared IRIS group with non-IRIS group at baseline, 4 weeks, 12 weeks, and 24 weeks, p<0.05.
Compared value at the onset of IRIS with its value at 4 weeks in the non-IRIS group, p<0.05.
Compared with normal value, p<0.05.
However, IL-4, IL-10, and IL-7 levels were significantly elevated in AIDS patients in both the IRIS and non-IRIS groups compared to the healthy controls (p<0 .05), while HAART significantly increased their levels in these two groups of patients at week 24 after HAART compared to baseline before HAART (p<0.05). Significant differences in IL-7 levels were observed between the IRIS and non-IRIS groups at all time points (p<0.05). In contrast, no significant differences were observed in IL-4 and IL-10 levels at almost all time points except for the IL-10 level at week 4. IL-7 and IL-10 levels at the onset of IRIS in IRIS patients were significantly higher than in patients without IRIS at week 4 after HAART (p<0.05).
Discussion
HAART is currently the most important method of controlling and treating AIDS.1,8 However, a portion of patients who receive HAART develop IRIS, which impedes the recovery of patients from AIDS and is even life-threatening. Understanding the pathogenesis of IRIS in AIDS patients receiving HAART is not only important for preventing the development of IRIS, but also for the successful recovery of patients from AIDS. This study found that (1) AIDS patients with a low peripheral CD4+CD25+Fox3+ percentage, low plasma IL-2, low IFN-γ, and high IL-7 levels are prone to develop IRIS after HAART. (2) The recovery of CD4+CD25+Fox3+ percentage and IL-7 levels was significantly slower in IRIS patients compared to AIDS patients who did not develop IRIS 24 weeks after HAART. (3) The percentage of CD4+CD45RO+, CD8+CD45RO+, CD4+CD38+, and CD8+CD38+ subsets, and the levels of plasma IL-2, IFN-γ, IL-4, and IL-7 were significantly normalized after HAART in both IRIS and non-IRIS patients without significant differences between the two groups. This study suggests that the imbalance of Th1/Th2 cytokines and the slow normalization of the CD4+CD25+Fox3+ subset and IL-7 level may be crucial for the pathogenesis of IRIS in AIDS patients who received HAART.
CD4+CD25+Foxp3+ regulatory T cells (Treg) play crucial roles in maintaining the balance of the immune system and the homeostatic regulation of T cells. Lack of Treg could lead to an imbalance of immune regulation and autoimmune diseases.9 Reduction in the proportion of peripheral Treg cells has been observed in HIV/AIDS.10 IRIS is the exaggerated inflammatory response of the immune system to antigens in patients receiving HAART.11 In this study, the percentage of Treg was significantly lower in IRIS patients than in patients without IRIS before and after HAART as well as at the onset of IRIS. Therefore, the decrease in regulatory T cells may result in a decline in T cell suppression and uncontrolled inflammation in IRIS. The study by Cianchetta-Sivori et al.12 observed no obvious variations in CD4+CD25+Foxp3+ regulatory T cells in IRIS patients after 4 months of HAART.13
Similar to the study by Cianchetta-Sivori et al.,12 the Treg percentage did not change in IRIS patients 24 weeks after HAART in our study. In addition, the study by Antonelli et al. did not observe any differences in the numbers or proportion of Treg cells between AIDS patients with and without IRIS.14 In this study, the percentage of Treg cells was significantly lower in IRIS patients than in AIDS patients who did not develop IRIS after HAART at weeks 0, 12, and 24. This may be associated with the different types of pathogens in IRIS patients.
In the normal human body, Th1/Th2 cytokines maintain homeostasis, and the imbalance of Th1/Th2 can lead to immune disorders and diseases. In HIV/AIDS patients, the levels of Th1 cytokines such as IL-2 and IFN-γ decrease, Th2 cytokines such as IL-4 and IL-10 increase, and this imbalance can be corrected by HAART.13 Consistent with a previous study,13 the plasma IFN-γ and IL-2 levels in this study were lower while the IL-4 and IL-10 levels were higher before HAART in AIDS patients with or without IRIS than in healthy controls. After HAART, IFN-γ and IL-2 levels were upregulated, while IL-4 and IL-10 levels were downregulated with a significant difference at week 24, suggesting a normalization of Th1/Th2 balance. Moreover, this study found that Th1-type cytokines were significantly higher in IRIS patients at the onset of IRIS and at week 4 of HAART than in AIDS patients who did not develop IRIS. In this study, the average number of days before IRIS occurred was 28 days after the initiation of HAART.
This finding suggests that IRIS occurred at a peak of IL-2 and IFN-γ during immune reconstruction. However, Th2 type cytokines IL-10 were significantly lower in IRIS patients than in non-IRIS patients at the onset of IRIS and at week 4 of HAART. Previous studies also observed that the incidence of IRIS was followed by augmentation of the levels of Th1 cytokines such as IFN-γ and IL-2 and insufficient production of IL-10.15 Considering that Treg levels were significantly decreased in IRIS patients and the inhibitory effect of Treg on Th1 cells was stronger than on Th2 cells, we hypothesized that the low number of Tregs cannot produce enough IL-10 to regulate other Th1-type proinflammatory cytokines such as IL-2 and IFN-γ effectively, which may be one of the pathological mechanisms of IRIS.
Recent studies demonstrated that IL-7 plays an important role in the survival of naive T cells, the genesis of memory T cells, and the pathogenesis of AIDS.16 The plasma IL-7 level was significantly elevated in patients with HIV infection.17 Although many studies have demonstrated that IL-7 is important for CD4+ T cell homeostasis during HIV/SIV infection,18,19 its exact role remains to be fully elucidated. With the progression of HAART, plasma IL-7 levels usually gradually decrease to near normal.15,16 Consistent with previous studies,15–17 plasma IL-7 levels were higher before HAART in AIDS patients who developed or did not develop IRIS 24 weeks after treatment compared to healthy controls. Interestingly, although the IL-7 level was significantly downregulated in IRIS patients 24 weeks after HAART, the IL-7 levels were significantly higher in IRIS patients than in AIDS patients who did not develop IRIS 0, 12, and 24 weeks after HAART. Thus, the slow normalization of IL-7 levels is another feature of IRIS. These observations suggest a critical role of IL-7 in the pathogenesis of IRIS after HAART.
Numerous studies have demonstrated that HAART significantly increases the CD4+ T cell percentage and decreases the CD8+ T cell count.4 Recently, the number of central memory cells (CD4+CD45RA−) and activated CD8+ T cells (CD8+CD38+) is believed to be a better indicator of immune restoration in patients who received HAART.20 In this study, no significant changes in the average percentage of CD4+CD45RA+CD62L+ and CD8+CD45RA+CD62L+ naive T cell were observed in two groups of patients after HAART. Previous studies reported that the active naive T cells normalize slowly after several months and increase remarkably in the following years.21,22 In this study, the observation time was only 24 weeks. The short observation duration may be the main reason why this study did not observe significant increases in CD4+CD45RA+CD62L+ and CD8+CD45RA+CD62L+ naive T cells. The naive T cells could be transformed in to memory T cells under the influence of antigens after the initiation of HAART.23
In this study, the proportion of CD4+CD45RO+ and CD8+CD45RO+ memory T cells increased significantly in AIDS patients with or without IRIS after HAART treatment for 24 weeks. Thus, memory T cells made up the majority of the increased CD4+ T cells during the early stages of normalization post-HAART.2,24 It was believed that the pathogenesis of IRIS may be related to the rapid increase of CD4+ T memory cells.25 But in this study, although the percentage of CD4+ memory T cells and CD8+ memory T cells increased gradually during HAART, the percentage of memory T cells showed no significant differences between IRIS patients and AIDS patients who did not develop IRIS 24 weeks after HAART. The changes in the percentage of CD4+CD38+ and CD8+CD38+ activated T cells showed the same trend as CD4+CD45RO+ and CD8+CD45RO+ cells. These findings suggest that CD4+CD45RA+CD62L+ and CD8+CD45RA+CD62L+ naive T cells, CD4+CD45RO+ and CD8+CD45RO+ memory T cells, and CD4+CD38+ and CD8+CD38+ activated T cells may not be key players in the pathogenesis of IRIS.
In conclusion, the present study indicated that AIDS patients with a low CD+CD25+Fox3+ percentage, low IL-2 and IFN-γ levels, and high IL-7 levels are prone to develop IRIS after a HAART regimen and that a slow normalization of CD+CD25+Fox3+ and IL-7 levels is a feature of IRIS patients. An imbalance of Th1/Th2 cytokines, a consistently low CD+CD25+Fox3+ percentage, and a high IL-7 level may be a crucial for the pathogenesis of IRIS in AIDS patients who received HAART.
Acknowledgments
This study was supported by the program for the 12th Five-Year Plan of China (Grant 2012ZX10001003).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Li H, Zheng Y, Chen Z, et al. : Evaluation for two-year highly active antiretroviral therapy in Chinese HIV-1 infection patients. Zhonghua Yi Xue Za Zhi 2007;87:2973–2976 [PubMed] [Google Scholar]
- 2.Zhou HY, Zheng YH, He Y, et al. : Evaluation of a 6-year highly active antiretroviral therapy in Chinese HIV-1 infected patients. Intervirology 2010;53:240–246 [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Zheng Y, Zhou H, et al. : Study on the effects of G2 arrest and apoptosis in Jurkat cells by HIV-1 Vpr. Chin J Microbiol Immunol 2009;20:1025–1030 [Google Scholar]
- 4.Zhou G. and Zheng Y: Antiretroviral therapy and immune reconstitution inflammatory syndrome. Chin J Infect Control 2009;8:368–372 [Google Scholar]
- 5.Glencross DK, Janossy G, Coetzee LM, et al. : CD8/CD38 activation yields important clinical information of effective antiretroviral therapy: Findings from the first year of the CIPRA-SA cohort. Cytometry B Clin Cytom 2008;74(Suppl 1):S131–140 [DOI] [PubMed] [Google Scholar]
- 6.International Network for the Study of HIV-associated IRIS (INSHI). www.inshi.umn.edu/definitions/General_IRIS/home.html
- 7.He Y, Li J, Zheng Y, et al. : A randomized case-control study of dynamic changes in peripheral blood Th17/Treg cell balance and interleukin-17 levels in highly active antiretroviral-treated HIV type 1/AIDS patients. AIDS Res Hum Retroviruses 2012;28:339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y. and Zheng Y: Clinical analysis and curative effect of opportunity infection in 100 AIDS patients. Chin J Infect Control 2007;6:311–315 [Google Scholar]
- 9.Ochs HD, Ziegler SF, and Torgerson TR: FOXP3 acts as a rheostat of the immune response. Immunol Rev 2005;203:156–164 [DOI] [PubMed] [Google Scholar]
- 10.Apoil PA, Puissant B, Roubinet F, et al. : FOXP3 mRNA levels are decreased in peripheral blood CD4+lymphocytes from HIV-positive patients. J Acquir Immune Defic Syndr 2005;39:381–385 [DOI] [PubMed] [Google Scholar]
- 11.Seddiki N. and Kelleher AD: Regulatory T cells in HIV infection: Who's suppressing what? Curr HIV/AIDS Rep 2008;5:20–26 [DOI] [PubMed] [Google Scholar]
- 12.Cianchetta-Sivori M, Raso S, Fernandez-Guerrero M, et al. : Do CD8(+)CD25(+) cells predict immune reconstitution syndrome in HIV-positive patients who begin HAART? AIDS 2007;21:2347–2349 [DOI] [PubMed] [Google Scholar]
- 13.Lim A, D'Orsogna L, Price P, et al. : Imbalanced effector and regulatory cytokine responses may underlie mycobacterial immune restoration disease. AIDS Res Ther 2008;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonelli LR, Mahnke Y, Hodge JN, et al. : Elevated frequencies of highly activated CD4+T cells in HIV+patients developing immune reconstitution inflammatory syndrome. Blood 2010;116:3818–3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seddiki N, Sasson SC, Santner-Nanan B, et al. : Proliferation of weakly suppressive regulatory CD4+T cells is associated with over-active CD4+T-cell responses in HIV-positive patients with mycobacterial immune restoration disease. Eur J Immunol 2009:39:391–403 [DOI] [PubMed] [Google Scholar]
- 16.Murdoch DM, Venter WD, Van Rie A, et al. : Immune reconstitution inflammatory syndrome (IRIS): Review of common infectious manifestations and treatment options. AIDS Res Ther 2007;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rallón NI, López M, Lozano S, et al. : Longitudinal assessment of interleukin 7 plasma levels in HIV-infected patients in the absence of and under antiretroviral therapy. J Acquir Immune Defic Syndr 2011;58:436–441 [DOI] [PubMed] [Google Scholar]
- 18.Fry TJ. and Mackall CL: Interleukin-7 and immunorestoration in HIV: Beyond the thymus. J Hematother Stem Cell Res 2002;11:803–807 [DOI] [PubMed] [Google Scholar]
- 19.Fry TJ, Moniuszko M, Creekmore S, et al. : IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood 2003;101:2294–2299 [DOI] [PubMed] [Google Scholar]
- 20.French MA: HIV/AIDS: Immune reconstitution inflammatory syndrome: A reappraisal. Clin Infect Dis 2009;48:101–107 [DOI] [PubMed] [Google Scholar]
- 21.Zhou HY, Zheng YH, Zhang CY, et al. : A one-year clinical trial using didanosine, stavudine and nevirapine for highly active antiretroviral therapy. Chinese Med J 2005;118;609–611 [PubMed] [Google Scholar]
- 22.Saag MS: The impact of highly active antiretroviral therapy on HIV-specific immune function. AIDS 2001;15(Suppl 2):S4–10 [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y, Chen J, Lei J, et al. : Immunological changes in HIV-1 infected patients treated with highly active antiretroviral therapy. Clin J Dermatol 2006;39:281–284 [Google Scholar]
- 24.Dhasmana DJ, Dheda K, Ravn P, et al. : Immune reconstitution inflammatory syndrome in HIV-infected patients receiving antiretroviral therapy: Pathogenesis, clinical manifestations and management. Drugs 2008;68:191–208 [DOI] [PubMed] [Google Scholar]
- 25.Phillips P, Bonner S, Gataric N, et al. : Nontuberculous mycobacterial immune reconstitution syndrome in HIV-infected patients: Spectrum of disease and long-term follow-up. Clin Infect Dis 2005;41:1483–1497 [DOI] [PubMed] [Google Scholar]