Abstract
Objectives: Dermal and mucosal healing are mechanistically similar. However, scarring and closure rates are dramatically improved in mucosal healing, possibly due to differences in apoptosis. Apoptosis, nature's preprogrammed form of cell death, occurs via two major pathways, extrinsic and intrinsic, which intersect at caspase3 (Casp3) cleavage and activation. The purpose of this experiment was to identify the predominant pathways of apoptosis in mucosal and dermal wound healing.
Approach: Wounds (1 mm biopsy punch) were made in the dorsal skin (n=3) or tongue (n=3) of female Balb/C mice aged 6 weeks. Wounds were harvested at 6 h, 24 h, day 3 (D3), D5, D7, and D10. RNA was isolated and analyzed using real time reverse transcriptase–polymerase chain reaction. Expression levels for genes in the intrinsic and extrinsic apoptotic pathways were compared in dermal and mucosal wounds.
Results: Compared to mucosal healing, dermal wounds exhibited significantly higher expression of Casp3 (at D5; p<0.05), Casp7 (at D5; p<0.05), Trp53 (at 24 h and D5; p<0.05), Tnfrsf1b (at 24 h; p<0.05), FasR (at 24 h, D5, and D7; p<0.05), and Casp8 (at 24 h; p<0.05) and significantly lower gene expression of Tradd (at 24 h; p<0.05).
Innovation: Our observations indicate differential execution of apoptosis in oral wound healing compared to skin.
Conclusion: Expression patterns of key regulators of apoptosis in wound healing indicate that apoptosis occurs predominantly through the intrinsic pathway in the healing mucosa, but predominantly through the extrinsic pathway in the healing skin. The identification of differences in the apoptotic pathways in skin and mucosal wounds may allow the development of therapeutics to improve skin healing.

Luisa Ann DiPietro, DDS, PhD
Introduction
Wound healing is a complex process that requires succinct yet overlapping phases of hemostasis, inflammation, proliferation, and remodeling. Our lab and others have extensively examined the differences between mucosal and dermal healing. The differences range from macroscopic differences in wound closure rates and scarring outcomes to the microscopic differences in inflammatory cell infiltrates and rates of re-epithelialization, and differential prohealing and proangiogenesis protein production.1–4 Mucosal healing has several key features that mimic regeneration. Mucosal wounds are faster to re-epithelialize, have a decreased inflammatory response, and have a blunted angiogenic response with concomitant reduction in vascular endothelial growth factor (VEGF) gene and protein expression.4 Altogether, the phases of wound healing after mucosal injury are shortened in duration and generally have reduced gene and protein expression changes in comparison to skin wound healing.5
Apoptosis is an important mechanism for cellular elimination during wound healing and maintains tissue homeostasis in normal, uninjured tissue. In a recent study, the overall gene expression of mucosal and skin wounds was compared via microarray analysis.5 Among the multiple differences that were noted, the data suggested that wound healing in these two tissues might exhibit differential signatures of apoptosis related genes, such as tumor necrosis factor alpha (TNF-α) and several downstream signaling factors.5
Apoptosis can be induced via two main pathways, termed intrinsic and extrinsic. The intrinsic pathway is related to DNA damage from ultraviolet (UV) light, chemotherapy, ischemia, and oxidative stress. The extrinsic pathway requires extracellular input, specifically, activation of the intracellular portion of the death receptor (DR) by binding of a death ligand. Apoptosis in general is associated with an intracellular caspase cleavage cascade. Caspases (CASP) involved in apoptosis can be broken down into 3 broad categories: the initiators of apoptosis (Casp2, Casp8, and Casp9), executioners of apoptosis (Casp3 and Casp7), and inflammation-related (Casp1, Casp4, Casp5, and Casp12).6–8
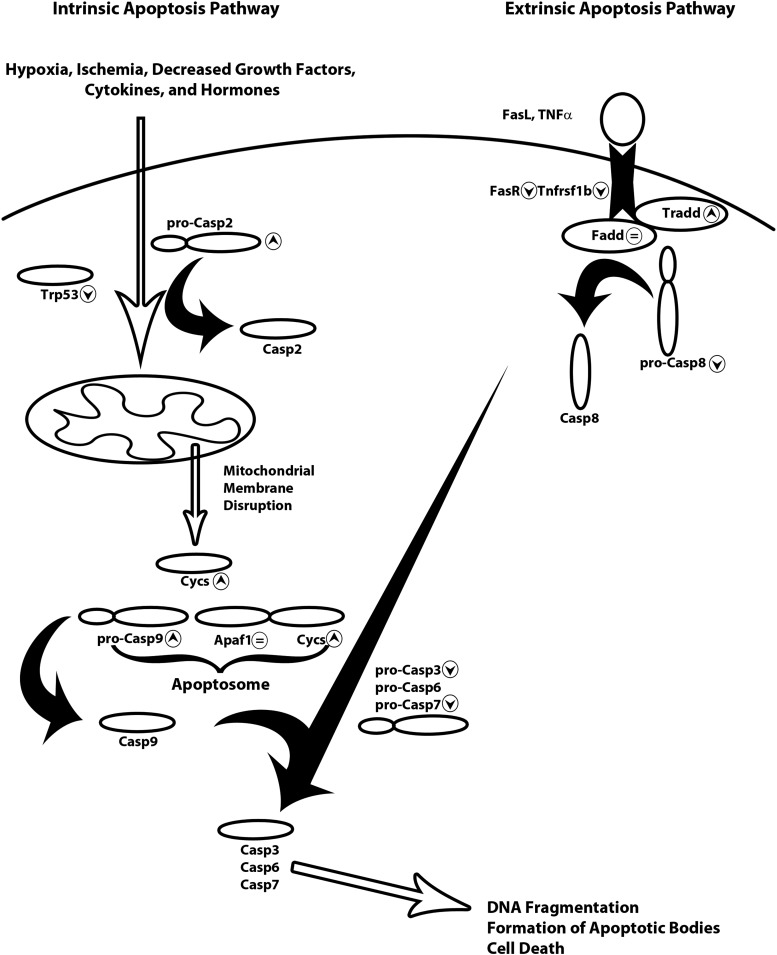
In the intrinsic pathway Casp2, the balance of Bcl-2 and Bax, and the levels of p53 (Trp53) determine cytochrome-c (Cycs) release from mitochondria after mitochondrial membrane disruption. Cycs then forms the apoptosome with apoptotic peptidase activating factor 1 (Apaf1), which cleaves and activates Casp9. The resulting caspase cleavage cascade ends with Casp3 cleavage and activation. Casp3 cleavage and activation represents the point of convergence for the intrinsic and extrinsic pathways and is the final step in initiation of cell death through further DNA fragmentation, and cleavage of cytoskeletal proteins (Fig. 1).
Figure 1.
Diagram of the intrinsic and extrinsic apoptosis signaling pathways. Intrinsic apoptosis (the left side of the figure) is usually the result of hypoxia, ischemia, or UV damage. These induce cell stress which is propagated through Casp2 and Trp53 disruption of the balance of the mitochondrial membrane. Cycs is released after mitochondrial membrane disruption and recruited to form the apoptosome with Apaf1 and pro-Casp9. Pro-Casp9 is cleaved and activates the caspase cascade resulting in cleavage and activation of Casp3, Casp6, and Casp7. The downstream effect of activation of the caspase cascade is DNA fragmentation, formation of apoptotic bodies, and cell death. The extrinsic apoptosis signaling pathway (the right side of the figure) requires binding of the death ligand (FasL or TNF-α) to its respective receptor (FasR or Tnfrsf1b). Binding of the death ligand to the death signals the recruitment of Tradd, Fadd, and pro-Casp8. Pro-Casp8 is cleaved by the complex and begins the caspase cleavage cascade resulting in the cleavage and activation of Casp3, Casp6, and Casp7. Similar to the intrinsic pathway, the result of the caspase cleavage cascade is DNA fragmentation, formation of apoptotic bodies, and cell death. ▲, significantly increased in oral mucosa versus skin; ▼, significantly decreased in oral mucosa versus skin; =, no significant difference in oral mucosa versus skin gene expression. Cycs, cytochrome C; Casp, caspase; TNF-α, tumor necrosis factor alpha; UV, ultraviolet
The extrinsic apoptotic signaling pathway involves transmembrane DRs of the TNF receptor gene superfamily, FasR, TnfR1, and DRs 3, 4, and 5. Upon binding of the DR ligand, Fas ligand (FasL) or TNF-α, the TnfR-associated death domain (Tradd) is activated and recruits21 Fas-associated death domain (Fadd) to the intracellular portion of the DR. This begins the intracellular signaling cascade of recruitment and cleavage of pro-Casp8 and ultimately cleavage and activation of Casp3 (Fig. 1).
The purpose of this study was to determine if differential apoptotic responses occur in oral and skin wound healing. Equally-sized wounds from the oral mucosa and the dorsal skin were compared at five different time points (6 h, 24 h, day 3 [D3], D5, and D7) over the course of wound healing for changes in gene expression of key factors in the apoptosis pathways. We hypothesized that apoptosis would be initiated through different pathways in the oral wounds compared to skin.
Clinical Problem Addressed
In the skin, fibrosis, or scarring can vary from normal to hypertrophic scars, keloids, or painful contractures. Effective antifibrotic or antiscarring treatments are currently limited. Oral wound healing, like fetal wound healing, closely resembles optimal healing with very rare occurrences of keloids or hypertrophic scars. Further examination of the mechanisms involved in cellular clearance may direct the development of therapeutic tools to improve the healing process and in turn, patient scarring outcomes.
Materials and Methods
Animals and wound models
All animal procedures were approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee. The standard skin and tongue wounding protocols were described previously.5 Briefly, female 6-week-old Balb/c mice (Harlan, Inc. Indianapolis, IN) were anesthetized with intraperitoneal injection of 100 mg/kg ketamine and 0.05 mg/kg xylazine. For mice with dorsal wounds (n=3, per time point, six wounds per mouse) the dorsal skin was shaved and six excisional dermal wounds were placed using a 1 mm punch biopsy (Acu-Punch; Acuderm, Inc., Ft. Lauderdale, FL) on opposing sides of the midline starting at the scapula level and continuing caudally. For mice with mucosal wounds (n=3, per time point, 1 wound per mouse), a 1 mm biopsy punch (Acu-punch; Acuderm) was used to make wounds lateral to and equal distance from the midline of the tongue.
Tissue harvesting and fixation
All mice were euthanized via CO2 inhalation combined with cervical dislocation. Dorsal skin wounds were excised by first cutting a 2 cm×2 cm square encompassing all dorsal wounds (6 total) followed by 2 mm biopsy punch (Acu-punch; Acuderm) of the original wound sites. Oral wound tissue was harvested by excision of the tongue as close to the base as possible. The tongue was then bisected laterally, followed by 2 mm biopsy punch at the site of the original wound on each half of the bisected tongue. Uninjured tissue was harvested in a similar manner from 2 mm biopsy punches taken at the beginning of the experiment in euthanized mice. The wounds and surrounding tissues were collected, and placed in 0.5 mL RNAlater (Sigma, St. Louis, MO) for RNA isolation and stored at −20°C before analysis, or snap frozen in OCT compound (Sakura Finetechnical, Tokyo, Japan) for cryotome sectioning and immunofluorescence, and stored at−80°C before analysis. For RNA analysis and isolation, wounds were harvested at 6 h, 24 h, D3, D5, and D7 (n=3 mice per time point, per tissue type) postinjury for RNA isolation. The small size of the mouse oral cavity and tongue only allow for 1 mm wounds, making it the standard protocol for mucosal injury in mice. Although these small wounds heal quickly, significant site-specific patterns of healing have been identified in this model.1,4,5,9,10
Real time reverse-transcriptase polymerase chain reaction
Total RNA was isolated from 3 wounds per time point per group using TriZol (Invitrogen, Carlsbad, CA) according to the instructions of the manufacturer. The concentration of RNA was determined with Nanodrop 1000 (Thermo Scientific, Wilmington, DE), and 1 μg of RNA was used from each sample for the remainder of the reverse transcriptase–polymerase chain reaction (RT-PCR) protocol. RNA was treated with DNase I (Invitrogen), and reverse transcription performed with Retroscript kit (Ambion/Life Technologies, Grand Island, NY) according to the manufacturer's instructions. The cDNA was amplified on an ABI Step One Plus Real Time PCR System (Applied Biosystems, Life Technologies, Foster City, CA) in 96-well plate reactions with 3 reference gene wells and 3 target gene wells per sample. Primer sequences used for target genes analyzed are listed in Table 1. To quantify relative differences in mRNA expression, the comparative CT method (ΔΔCT) was used to determine relative quantity.11 All target genes were normalized to Gapdh expression in uninjured tongue tissue. Gene expression patterns of 18S RNA, β-actin, and ribosomal protein large, p0 (Rplp0) were also examined and Gapdh was determined to be the most stable reference gene. To compare expression over the time, and to assess differences between the uninjured tongue and skin, gene expression was normalized to the reference uninjured tongue as a single baseline. Results were analyzed with two-way analysis of variance to analyze the time and tissue effects comparing skin and tongue at each time point to each other and to uninjured tissue followed by a Bonferroni's post-test with α=0.05.
Table 1.
Reverse transcriptase–polymerase chain reaction primer sequences
| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| Gapdh | TCA CCA CCA TGG AGA AGG C | GCT AAG CAG TTG GTG GTG CA |
| Tnfrsf1b | ACT CCA AGC ATC CTT ACA TCG | TTC ACC AGT CCT AAC ATC AGC |
| FasR | AAG TCC CAG AAA TCG CCT ATG | GGT ATG GTT TCA CGA CTG GAG |
| Tradd | ACG AAC TCA CTA GTC TAG CAG AG | AAT ACC CCA ACA GCC ACC |
| Fadd | GCA AGA GTG AGA ATA TGT CCC C | TCA TGG TGT GAT CAA GTC CAC |
| Casp8 | AAC TTC CTA GAC TGC AAC CG | TCT CAA TTC CAA CTC GCT CAC |
| Casp3 | GAC TGA TGA GGA GAT GGC TTG | TGC AAA GGG ACT GGA TGA AC |
| Casp7 | CCC ACT TAT CTG TAC CGC ATG | GGT TTT GGA AGC ACT TGA AGA G |
| Trp53 | ATG TTC CGG GAG CTG AAT G | CCC CAC TTT CTT GAC CAT TG |
| Apaf1 | GAT GTG GAG GTG ATC GTG AAG | TAC TGG ATG GTG CTG TGA TG |
| Cycs | AAG GGA GGC AAG CAT AAG AC | ATT CTC CAA ATA CTC CAT CAG GG |
| Casp9 | TGT GTC AAG TTT GCC TAC CC | CCA CTT TTC TTG TCC CTC CAG |
| Casp2 | CAA GTC TCC CTT TCT CGG TG | AGT GTG CCT GGT AAA ACT CAG |
Immunofluorescence
For immunofluorescence, tissue (n=2 per group) was sectioned with a cryotome (Leica 3050CS; Buffalo Grove, IL) at a 8 μm thickness and placed on UltraStick glass slides (Gold Seal, Portsmouth, NH). Tissue sections were fixed with ice cold acetone for 5 min and then washed 2×5 min with Tris-buffered saline (TBS) 0.025% Triton X-100, followed by 1× TBS wash 3×5 min. The slides were then blocked with normal goat serum (10% normal goat serum in 0.1% BSA 1× PBS) for 2 h. The primary antibody, rabbit antiactive Casp3 (1:100; Abcam, Cambridge, MA) diluted with 1% BSA in PBS was applied in a humidified chamber overnight at 4°C. Slides were rinsed 3×5 min with TBS. Secondary antibody, Alexafluor 488 conjugated goat anti rabbit (1:1,000; Invitrogen Molecular Probes, Grand Island, NY) with 0.1 μg/mL Hoescht nuclear stain (Immunochemistry, Bloomington, MN) diluted with 1% BSA in PBS was applied in a humidified chamber at room temperature for 2 h in the dark. Slides were rinsed 3×5 min with 1× TBS and 0.5% Tween 20. Slides were then mounted in aqueous mounting media (VectaMount AQ; Vector Laboratories, Burlingame, CA) followed by a coverslip and sealed with nail polish. All slides were visualized on a Carl Zeiss fluorescence microscope using AxioVision LE (Thornwood, NY).
Results
Apoptosis in skin and tongue wounds
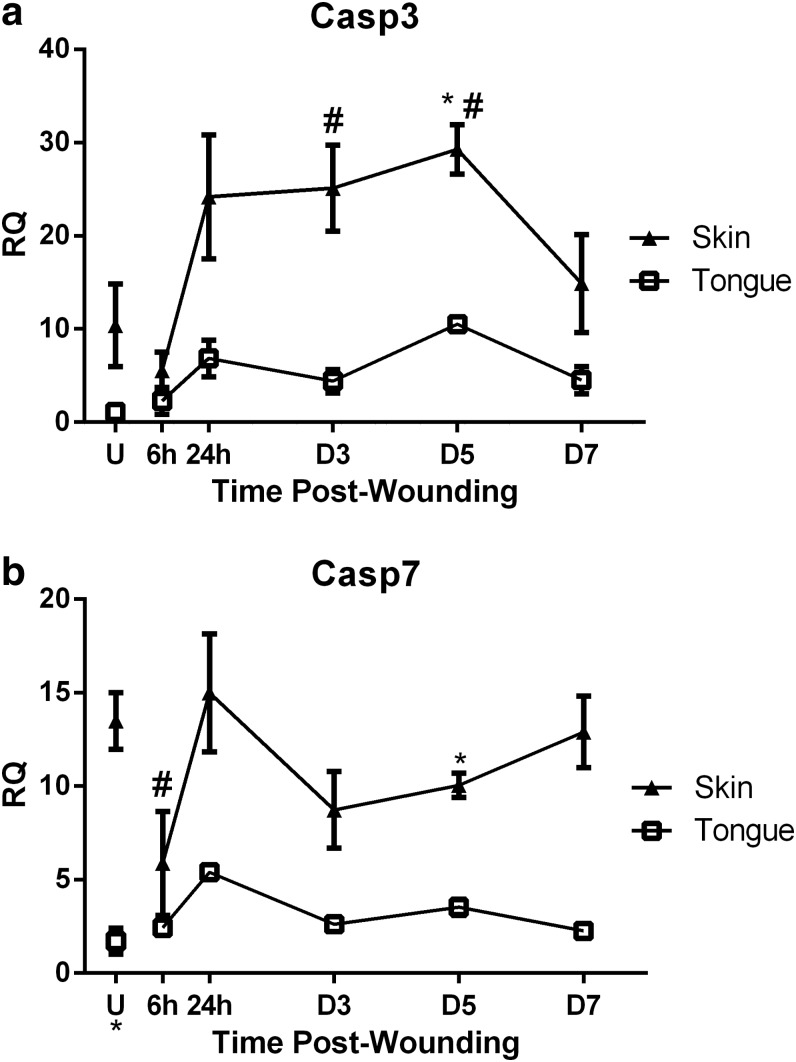
To identify the relative amounts of apoptosis occurring over the course of wound healing, the levels of gene expression of Casp3 and Casp7, the executioner caspases involved in the final steps of both intrinsic and extrinsic apoptosis pathways, were examined. Casp3 expression was higher in uninjured skin and over the time course of wound healing in skin, significantly at D5 (Fig. 2a) as compared to tongue wound healing. Casp3 expression in skin wounds showed a significant increase compared to uninjured skin at D3 and D5 (Fig. 2a). When compared to tongue, Casp7 expression was significantly higher in uninjured skin (Fig. 2b). Over the time course of wound healing, skin wounds exhibited significantly increased levels of Casp7 at D5 (Fig. 2b), when compared to tongue wounds.
Figure 2.
Apoptosis markers in skin and tongue. Real time RT-PCR of (a) Casp3 and (b) Casp7 were performed on RNA isolated from uninjured tissue (U) and wound samples at 6 h, 24 h, D3, D5, and D7 postinjury. To determine RQ of mRNA levels during wound healing, all samples were normalized to Gapdh expression in uninjured tongue tissue. The results are shown as the mean±SEM; n=3. Data were analyzed by two-way ANOVA and Bonferroni's posttest (*p<0.05 for skin vs. tongue wounds, #p<0.05 for skin vs. uninjured skin). ANOVA, analysis of variance; RT-PCR, reverse transcriptase–polymerase chain reaction; RQ, relative quantity.
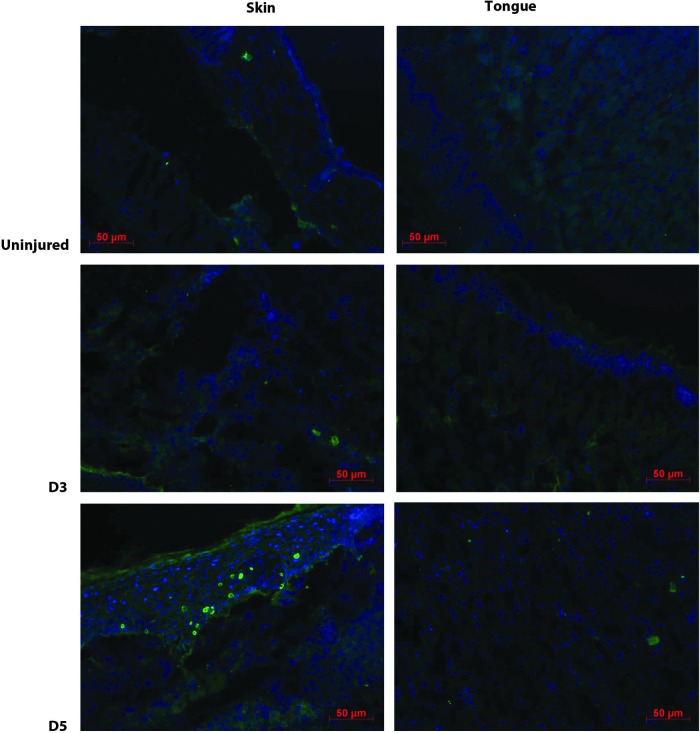
CASP3 protein expression was also qualitatively examined by immunofluorescence staining of active (cleaved) CASP3 in uninjured, D3, and D5 skin and tongue tissue (Fig. 3). These time points were identified as having significant differences in Casp3 gene expression. Qualitatively, active CASP3 protein was seen in both uninjured skin and skin wounds; minimal expression was seen in tongue. These results support the concept that, as compared to skin, both normal tongue tissue and tongue wounds exhibit significantly less active, cleaved CASP3.
Figure 3.
Active CASP3 protein expression. Immunofluorescence for cleaved (active) CASP3 was performed on uninjured, D3, and D5 postinjury tissues (n=2). The images were not quantified, merely observed to detect the presence of active CASP3 protein. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Intrinsic apoptosis pathway
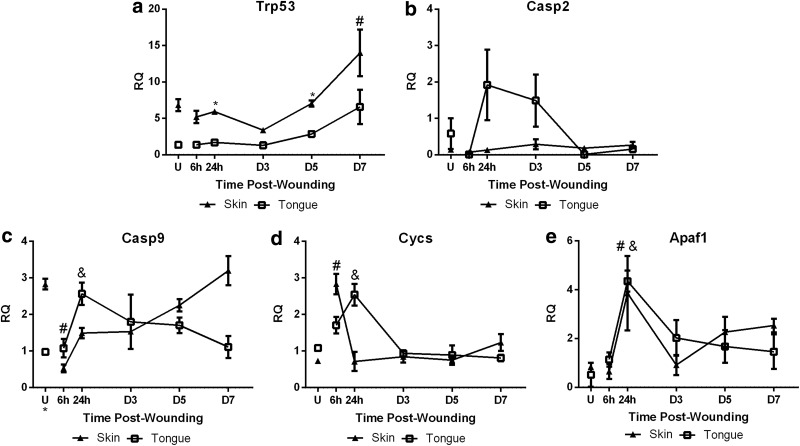
To determine if differential involvement of the intrinsic apoptosis pathway occurs in wound healing of the oral mucosa and the dermis, gene expression of Trp53, Casp2, Casp9, Cycs, and Apaf1 was examined in skin and oral wounds. Significantly lower gene expression of Trp53 was seen in oral wounds at 24 h and D5 (Fig. 4a). Compared to uninjured skin, levels of Trp53 showed a significant increase in skin wounds through D7 (Fig. 4a). Casp2 expression was initially higher in uninjured tongue compared to skin, followed by a peak at D3 in oral wound healing. In contrast, skin wounds demonstrated little change in Casp2 expression (Fig. 4b). Casp9 expression was significantly higher in uninjured skin compared to tongue (Fig. 4c). In tongue wounds, the expression of Casp9 significantly increased at 24 h (Fig. 4c) followed by a decrease to baseline levels by D7. However, skin wounds exhibited a significant decrease in Casp9 expression at 6 h postwounding (Fig. 4c) and then increased back to baseline levels by D7. Cycs expression was not significantly different for uninjured tissues. In skin wounds, Cycs levels increased at 6 h after injury (Fig. 4d), while a significant increase in tongue wound healing did not occur until 24 h (Fig. 4d) compared to uninjured tongue tissue. Apaf1 expression in uninjured tissues was not significantly different. Both skin and tongue wounds showed significantly increased Apaf1 at 24 h after injury (Fig. 4e); Apaf1 levels remained elevated through D7. Overall, the pattern of expression of the signaling factors of the apoptosis pathway suggested that intrinsic apoptosis may play a more significant role in oral wound healing compared to skin wound healing.
Figure 4.
Intrinsic pathway markers. Real time RT-PCR of (a) Trp53, (b) Casp2, (c) Casp9, (d) Cycs, and (e) Apaf1 were performed on RNA isolated from uninjured tissue (U) and wound samples at 6 h, 24 h, D3, D5, and D7 postinjury. To determine relative changes in mRNA levels during wound healing, all samples were normalized to Gapdh expression in uninjured tongue tissue. The results are shown as the mean±SEM; n=3. Data were analyzed by two-way ANOVA and Bonferroni's posttest (*p<0.05 for skin vs. tongue wounds, # p<0.05 for skin vs. uninjured skin, &p<0.05 for tongue vs. uninjured tongue).
Extrinsic apoptosis pathway
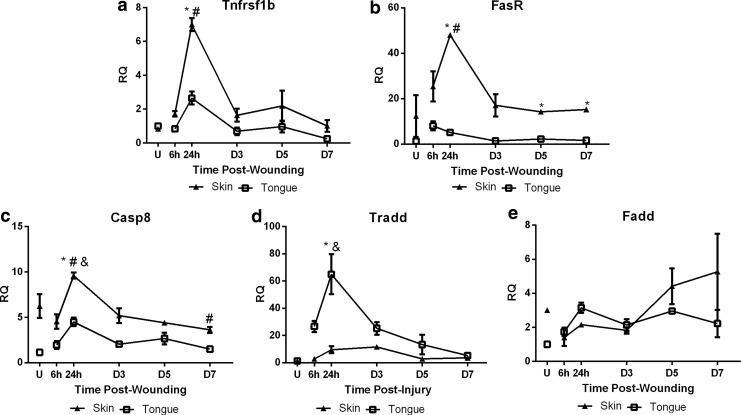
To assess differences in the contribution of the extrinsic pathway to apoptosis in skin and oral wound healing, we examined the relative gene expression of Tnfrsf1b, FasR, Casp8, Tradd, and Fadd. Tnfrsf1b expression was similar in uninjured skin and tongue; however, a significant increase in expression was observed in skin wounds at 24 h (Fig. 5a) followed by a return to baseline levels. FasR expression was higher in uninjured skin, and significantly increased by 24 h postinjury in skin wounds (Fig. 5b). No corresponding increase in FasR was seen in oral wounds, and, in fact, FasR expression was significantly lower in oral wounds at 24 h, D3, and D5 (Fig. 5b) compared to skin wounds. Casp8 expression was higher in uninjured skin, and significantly increased by 24 h postinjury in both skin and tongue wound healing compared to uninjured tissues (Fig. 5c),with skin showing greater levels than tongue at 24 h (Fig. 5c). Casp8 expression also decreased significantly by D7 in skin wound healing compared to uninjured skin (Fig. 5c). Tradd expression was not significantly different in uninjured tissues; however, by 24 h a significant increase in expression was seen in oral but not skin wounds (Fig. 5d) compared to uninjured tongue and 24 h skin wounds. No corresponding peaks in skin wound healing were observed over the course of wound healing. Fadd expression was higher in uninjured skin (Fig. 5e), and increased over the time course of the experiment, trending to higher than baseline levels (Fig. 5e). When Fadd expression in healing wounds was examined, no significant differences were seen (Fig. 5e). Overall, the expression of genes involved in the extrinsic pathway tended to be significantly increased in skin wound healing as compared to oral wound healing, suggesting more involvement of the extrinsic apoptosis pathway in skin wound healing.
Figure 5.
Extrinsic pathway markers. Real time RT-PCR of (a) Tnfrsf1b, (b) FasR, (c) Casp8, (d) Tradd, and (e) Fadd were performed on RNA isolated from uninjured tissue (U) and wound samples at 6 h, 24 h, D3, D5, and D7 postinjury. To determine relative changes in mRNA levels during wound healing, all samples were normalized to Gapdh expression in uninjured tongue tissue. The results are shown as the mean±SEM; n=3. Data were analyzed by two-way ANOVA and Bonferroni's post-test (*p<0.05 for skin vs. tongue wounds, #p<0.05 for skin vs. uninjured skin, &p<0.05 for tongue vs. uninjured tongue).
Discussion
Oral mucosal wound healing has previously been shown to exhibit reduced scar formation, a faster rate of re-epithelialization, lower levels of inflammation, and lower levels of angiogenesis compared to wound repair in the skin.1,4 However, very little attention has been given to the mechanisms that regulate cell death in wounds of these two tissues. Cell death and the mechanism of cell elimination may play an important role in the scarring outcome via paracrine signaling or immune modulation. Recent studies have suggested that apoptotic cells secrete factors that can modulate immune cell phenotypes to affect myofibroblast differentiation, fibroblast and myofibroblast proliferation, and apoptosis resistance.12 Since increased levels of myofibroblasts, increased fibroblast and myofibroblast proliferation, and increased apoptosis resistance are known to influence scarring and fibrosis, apoptotic cells may play an important role in determining the final result of wound healing. The current study demonstrates that the dominant mechanisms of apoptosis differ for wounds of the oral mucosa and skin. Given the differential scar formation in these two anatomic sites, these results suggest possible connections for apoptotic mechanisms and scarring outcomes.
The mechanism of apoptosis is known to derive from the local environment of preapoptotic cells. The intrinsic apoptotic pathway is generally initiated by ischemia, DNA damage, and a reduction in the levels of growth factors, cytokines, or hormones. In oral wound healing the predominance of the intrinsic apoptosis pathway is early, generally peaking at 24 h. This early peak may have to do with lower levels of pro-survival growth factors in oral wounds, such as VEGF, EGF, and TGF-β1. Previous studies have shown lower levels of these key antiapoptotic growth factors in oral mucosal wounds.1,10 The lower levels of important antiapoptotic growth factors in oral wound healing may be responsible for triggering the intrinsic apoptosis pathway by reducing pro-survival signaling. In contrast, the extrinsic apoptosis pathway requires extracellular input to initiate cell death. Skin wound healing is characterized by robust growth factor production, and hyperproliferation, effectively preventing the initiation of the intrinsic apoptosis pathway. In this situation, activation of the extrinsic apoptosis signaling pathway may be required to induce cell death.
Several other characteristics of oral wound healing have been suggested to play a role in the increased healing rate: faster re-epithelialization, increase proliferation of oral keratinocytes, decreased immune response, increased oxygen availability in the oral cavity, the moist wound environment, temperature, saliva flow, and local microflora. Previous studies have determined that the saliva-based, moist-wound environment plays a role in oral healing;13,14 however, the presence of saliva seems to be more important in larger wounds. Smaller mucosal wounds heal at similar rates independent of salivary influence.15 Saliva contains growth factors, including EGF and VEGF, both of which have been suggested to important to oral wound healing.16,17 The tissue levels of these growth factors, however, is low when compared to skin wound healing. Correspondingly, skin that is transplanted into the oral cavity and shows a healing response that more closely resembled that of skin rather than that of oral mucosa.18,19 Together these studies suggest that environmental factors have a somewhat limited role in healing of oral mucosa. Intrinsic differences between oral mucosa and skin tissue seem likely to play an important part in defining the improved healing of oral wounds.
Our observations indicate that there are distinct and often significant differences in the gene expression of key mediators of both the intrinsic and extrinsic apoptosis pathways in oral wound healing compared to skin wound healing. Overall, our results show that the gene expression of the mediators of both intrinsic and extrinsic apoptosis pathways generally maintain low levels over the course of oral wound healing and return to baseline levels faster (Figs. 2–5) than skin wounds. This observation leads to the conclusion that apoptosis in oral wounds occurs via rapid and concise mechanisms. Although changes in gene expression levels do not necessarily translate to protein expression or function, our findings show that cleaved Casp3 protein levels follow similar trends to that of gene expression (Fig. 3). Our work does not address the protein levels and activation status of the remaining elements of the apoptotic cascade. Further studies will be necessary to quantitatively determine how translational regulation, post-translational modification, and release of intracellular stores influence the many other elements of the apoptotic pathways.
The data here suggest that overall, expression of genes related to the intrinsic pathway are generally higher in oral wound healing compared to skin wound healing (Fig. 1). The timing of the peak of the gene expression related to intrinsic apoptosis in oral wound healing was most commonly seen at 24 h (Fig. 4b–e). This peak may correspond with the particular events occurring at that time. Specifically, inflammatory cells in an oral wound peak at around 24 h and the peak in intrinsic apoptosis may be related to the resolution of inflammation and the elimination of inflammatory cells present in the wound bed.
In contrast to the intrinsic pathway, our studies suggest that mediators of the extrinsic pathway are significantly increased in skin versus oral wound healing. Here again the timing of the peak may be related to the other events occurring in the wound. For example, the peak gene expression of Tnfrsf1b and Casp 8 occurs at 24 h for both oral and skin wound healing (Fig. 5a, c, respectively), although the levels are significantly higher in skin wounds. Apoptosis occurring at 24 h may again be related to the elimination of inflammatory cells and the resolution of inflammation. Interestingly, FasR gene expression also peaks at 24 h in skin wound healing, but there is no corresponding peak in oral wound healing (Fig. 5b). This phenomenon may be due to a more significant role of Fas-mediated apoptosis in skin wound healing, both in general and at 24 h. Also of interest, Tradd gene expression peaks in oral wound healing at 24 h, but there is no corresponding peak in skin wound healing (Fig. 5d). Tradd may be the rate limiting mediator in extrinsic apoptosis in skin wound healing, while it may be in excess in oral wound healing. The significant differences later on in skin wound healing (FasR at D5 and D7, Fig. 5b) could correspond to apoptosis occurring during vessel regression. As opposed to oral mucosa, the robust angiogenesis seen in skin wounds requires a pruning of the overabundant new vessels. As normal skin wound healing progresses into the remodeling phase, large numbers of endothelial cells undergo apoptosis as unnecessary vessels regress.
Recent studies have suggested differences in the mechanism of apoptosis in fetal wound healing compared to adult wound healing. Cleavage of Casp7 and PARP were significantly increased in scarless fetal wound healing (embryonic day 15) compared to fetal wound that resulted in a scar (embryonic day 18).20 Similar to oral mucosal wound healing, regeneration or improved scarring outcomes have been identified in fetal wound healing; several observations suggest potential reasons for the similarities. First, similar to oral wound healing, the immune response in fetal wound healing is significantly lower than adult wound healing.21 Second, the extracellular matrix in fetal and oral wounds have lower collagen I to collagen III ratios compared to normal adult skin wound healing.22–30 Third, the presence and persistence of myofibroblasts during fetal and oral healing are lower compared to adult skin wound healing.31,32 On the same thread, the growth factors that stimulate myofibroblast differentiation have been identified as differentially regulated, with predominance of transforming growth factor (TGF)-β3 in fetal wound healing and TGF-β1 in adult wound healing.33 TGF-β1 protein levels are significantly lower in oral wound healing compared to tongue,10 in vitro, oral fibroblasts exhibit a decreased fibrotic response to the same levels of TGF-β1 compared to dermal fibroblasts.34 Lastly, similar to oral wound healing, the angiogenic response in fetal wound healing is significantly lower than adult wound healing.1 Given the numerous similarities between fetal and oral wound healing, the finding of differences in the levels and mechanisms of apoptosis in both oral and fetal wounds20 suggests that apoptosis may play a significant role in the determination of scarring outcomes.
In summary, our results indicate that intrinsic apoptosis may be the predominant mechanism of induction of apoptosis in oral wound healing, while extrinsic apoptosis may play a more significant role in skin wound healing. The differences in the pathways for apoptosis induction may provide potential targets for modifying skin wound healing outcomes to resemble the regeneration seen in oral and fetal wound healing.
Innovation
Apoptosis maintains normal tissue homeostasis, but in wound healing the process of apoptosis is not completely understood. Fetal and oral wound healing are examples of wound repair that result in regeneration. Further examination of the mechanisms involved in tissue repair in fetal and oral wound healing may generate therapeutic targets to improve skin wound healing. Our observations indicate differential execution of apoptosis in oral wound healing compared to skin. Oral wound healing is characterized by increased gene expression of mediators in the intrinsic apoptosis pathway, while skin wound healing has increased gene expression of mediators in the extrinsic pathway.
Key Findings.
• Execution of apoptosis in oral wound healing occurs at lower levels over the entire course of wound healing in oral wounds compared to skin.
• Intrinsic apoptosis is the predominant mechanism of apoptosis in oral wound healing.
• Extrinsic apoptosis is the predominant mechanism of apoptosis in skin wound healing.
Abbreviations and Acronyms
- ANOVA
analysis of variance
- Apaf1
apoptotic peptidase activating factor 1
- Bax
BCL2-associated X protein
- Bcl-2
B-cell CLL/lymphoma 2
- Casp
caspase
- Cycs
cytochrome C
- DR
death receptors
- Fadd
Fas associated death domain
- FasR
TNF receptor superfamily member 6
- RQ
relative quantity
- RT-PCR
reverse transcriptase–polymerase chain reaction
- TGF
transforming growth factor
- Tnfrsf1b
tumor necrosis factor receptor gene super family 1b
- TNF-α
tumor necrosis factor alpha
- Tradd
TNFRSF1A-associated death domain
- Trp53
transformation related protein 53
- UV
ultraviolet
- VEGF
vascular endothelial growth factor
Acknowledgments and Funding Sources
The authors wish to thank Ms. Angelica Lagunas, College of Dentistry, University of Illinois at Chicago, for her help in the preliminary studies that led to the experiments discussed in this manuscript. The work described in this article was funded in part by NIGMS R01 GM50875 (LAD) and NIDCR T32 DE018381(AJ LD).
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the author(s) listed. No ghostwriters were used to write this article.
About the Authors
Dr. Luisa A. DiPietro is a Professor and the Director of the Center for Wound Healing and Tissue Regeneration at the University of Illinois at Chicago (UIC) College of Dentistry. She currently directs the UIC Multidisciplinary Oral Sciences Training Program (MOST); this NIH-supported program provides research training for graduate students (PhD), postdoctoral fellows, and dual degree (DMD-PhD) students, including Ms. Ariel R. L. Johnson, who is currently a 4th year PhD candidate working on her dissertation research. Research in the DiPietro laboratory focuses on how wounds heal, with the ultimate goal of developing therapies that will allow humans to regenerate perfect tissue after an injury. Ms. Marybeth M. Francis, also a MOST trainee (DMD-PhD candidate), worked closely with Ms. Johnson on this research project.
References
- 1.Szpaderska A, Walsh CG, Steinberg MJ, and DiPietro LA: Distinct patterns of angiogenesis in oral and skin wounds. J Dent Res 2005; 84:309. [DOI] [PubMed] [Google Scholar]
- 2.Warburton G, Nares S, Angelov N, Brahim J, Dionne R, and Wahl S: Transcriptional events in a clinical model of oral mucosal injury and repair. Wound Rep Reg 2005; 13:19. [DOI] [PubMed] [Google Scholar]
- 3.Stephens P, Davies KJ, al-Khateeb T, Shepherd JP, and Thomas DW: A comparison of the ability of intra-oral and extra-oral fibroblasts to stimulate extracellular matrix reorganization in a model of wound contraction. J Dent Res 1996; 75:1358. [DOI] [PubMed] [Google Scholar]
- 4.Szpaderska AM, Zuckerman JD, and DiPietro LA: Differential injury responses in oral mucosal and cutaneous wounds. J Dental Res 2003; 82:621. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Arbieva ZH, Guo S, Marucha PT, Mustoe TA, and DiPietro LA: Positional differences in the wound transcriptome of skin and oral mucosa. BMC Genomics 2010; 11:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen GM: Caspases the executioner of apoptosis. Biochem J 1997; 326:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rai NK: Apoptosis: a basic physiologic process in wound healing. Int J Lower Extremity Wounds 2005; 4:138. [DOI] [PubMed] [Google Scholar]
- 8.Yazdi AS, Guarda G, D'Ombrain MC, and Drexler SK: Inflammatory caspases in innate immunity and inflammation. J Innate Immun 2010; 2:228. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Gajendrareddy PK, and DiPietro LA: Differential expression of HIF-1alpha in skin and mucosal wounds. J Dent Res 2012; 91:871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrementi ME, Ferreira AM, Zender C, and DiPietro LA: Site-specific production of TGF-beta in oral mucosal and cutaneous wounds. Wound Repair Regen 2008; 16:80. [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ. and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25:402. [DOI] [PubMed] [Google Scholar]
- 12.Laplante P, Sirois I, Raymond MA, Kokta V, Béliveau A, Prat A, Pshezhetsky AV, and Hébert MJ: Caspase-3-mediated secretion of connective tissue growth factor by apoptotic ECs promotes fibrosis. Cell Death Differ 2010; 17:291. [DOI] [PubMed] [Google Scholar]
- 13.Bodner L, Dayan D, Oberman M, Hirshberg A, and Tal H: Healing of experimental wounds in sialadenectomized rat. J Clin Periodontol 1992; 19:345. [DOI] [PubMed] [Google Scholar]
- 14.Hutson JM, Niall M, Evans D, and Fowler R: Effect of salivary glands on wound contraction in mice. Nature 1979; 279:793. [DOI] [PubMed] [Google Scholar]
- 15.Bodner L, Kaffe I, Cohen Z, and Dayan D: Long-term effect of desalivation on extraction wound healing: a densitometric study in rats. Dentomaxillofac Radiol 1993; 22:195. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi S, Ohba Y, and Oka T: Effect of salivary epidermal growth factor on wound healing of tongue in mice. Am J Physiol 1991; 260 (4 Pt 1):E620. [DOI] [PubMed] [Google Scholar]
- 17.Taichman NS, Cruchley AT, Fletcher LM, Hagi-Pavli EP, Paleolog EM, Abrams WR, Booth V, Edwards RM, and Malamud D: Vascular endothelial growth factor in normal human salivary glands and saliva: a possible role in the maintenance of mucosal homeostasis. Lab Invest 1998; 78:869. [PubMed] [Google Scholar]
- 18.Magnano M, Bussi M, De Stefani A, Milan F, Lerda W, Ferrero V, and Gervasio F, Ragona R, Gabriele P, and Valente G: Prognostic factors for head and neck tumor recurrence. Acta Otolaryngol 1995; 115:833. [DOI] [PubMed] [Google Scholar]
- 19.Reilly JS, Behringer WH, and Trocki I: Intraoral keloid: complication of forehead flap. Otolaryngol Head Neck Surg (1979) 1980; 88:139. [DOI] [PubMed] [Google Scholar]
- 20.Carter R, Sykes V, and Lanning D: Scarless fetal mouse wound healing may initiate apoptosis through caspase 7 and cleavage of PARP. J Surg Res 2009; 156:74. [DOI] [PubMed] [Google Scholar]
- 21.Colwell AS, Longaker MT, and Lorenz HP: Fetal wound healing. Front Biosci 2003; 8:s1240. [DOI] [PubMed] [Google Scholar]
- 22.Burd D, Greco RM, Regauer S, Longaker MT, Siebert JW, and Garg HG: Hyaluronan and Wound Healing a new perspective Brit J Plas Surg 1991; 44:579. [DOI] [PubMed] [Google Scholar]
- 23.Occleston NL, Metcalfe AD, Boanas A, Burgoyne NJ, Nield K, O'Kane S, and Ferguson MW: Therapeutic improvement of scarring: mechanisms of scarless and scar-forming healing and approaches to the discovery of new treatments. Dermatol Res Pract 2010; 2010: pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolfe KJ. and Grobbelaar AO: A review of fetal scarless healing. ISRN Dermatol 2012; 2012:698034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawai T, Usui N, Sando K, Fukui Y, Kamata S, Okada A, Taniguchi N, Itano N, and Kimata K: Hyaluronic acid of wound fluid in adult and fetal rabbits. J Ped Surg 1997; 32:41. [DOI] [PubMed] [Google Scholar]
- 26.Cuttle L, Nataatmadja M, Fraser JF, Kempf M, Kimble RM, and Hayes MT: Collagen in the scarless fetal skin wound: detection with picrosirius-polarization. Wound Repair Regen 2005; 13:198. [DOI] [PubMed] [Google Scholar]
- 27.Hallock GG, Rice DC, Merkel JR, and DiPaolo BR: Analysis of collagen content in the fetal wound. Ann Plast Surg 1988; 21:310. [DOI] [PubMed] [Google Scholar]
- 28.Knight KR, Lepore DA, Horne RS, Ritz M, Hurley JV, Kumta S, and O'Brien BM: Collagen content of uninjured skin and scar tissue in foetal and adult sheep. Int J Exp Pathol 1993; 74:583. [PMC free article] [PubMed] [Google Scholar]
- 29.Lovvorn HN, Cheung DT, Nimni ME, Perelman N, Estes JM, and Adzick NS: Relative distribution and crosslinking of collagen distinguish fetal from adult sheep wound repair. J Pediatr Surg 1999; 34:218. [DOI] [PubMed] [Google Scholar]
- 30.Merkel JR, DiPaolo BR, Hallock GG, and Rice DC: Type I and type III collagen content of healing wounds in fetal and adult rats. Proc Soc Exp Biol Med 1988; 187:493. [DOI] [PubMed] [Google Scholar]
- 31.Cass D, Sylvester KG, Yang EY, Crombleholme TM, and Adzick NS: Myofibroblast persistence in fetal sheep wounds is associated with scar formation. J Ped Surg 1997; 22:1017. [DOI] [PubMed] [Google Scholar]
- 32.Estes JM, van de Berg JS, Adzick NS, MacGillivray TE, Desmoulière A, and Gabbiani G: Phenotypic and functional features of myofibroblasts in sheep fetal wounds. Differentiation 1994; 56:173. [DOI] [PubMed] [Google Scholar]
- 33.Rolfe KJ, Richardson J, Vigor C, Irvine LM, Grobbelaar AO, and Linge C: A role for TGF-beta1-induced cellular responses during wound healing of the non-scarring early human fetus? J Invest Dermatol 2007; 127:2656. [DOI] [PubMed] [Google Scholar]
- 34.Lee HG. and Eun HC: Differences between fibroblasts cultured from oral mucosa and normal skin: implication to wound healing. J Dermatol Sci 1999; 21:176. [DOI] [PubMed] [Google Scholar]