Abstract
Early antiretroviral therapy (ART) initiation reduces the risk of disease progression and HIV transmission, but data on time from HIV care entry to ART initiation are lacking. Using data from the Medical Monitoring Project (MMP), a population-based probability sample of HIV-infected adults receiving medical care in the United States, we assessed time from care entry to ART initiation among persons diagnosed May 2004–April 2009 and used multivariable Cox proportional-hazards models to identify factors associated with time to ART initiation. Among 1094 MMP participants, 83.9% reported initiating ART, with median time to ART initiation of 10 months. In multivariable models, blacks compared to whites [hazard ratio (HR) 0.82; 95% confidence interval (CI) 0.70–0.98], persons without continuous health insurance (HR 0.82; CI 0.70–0.97), heterosexual women and men who have sex with men compared to heterosexual men (HR 0.66; CI 0.51–0.85 and HR 0.71; CI 0.60–0.84, respectively), and persons without AIDS at care entry (HR 0.37; CI 0.31–0.43) had significantly longer times to ART initiation. Overall, time to ART initiation was suboptimal by current standards and significant disparities were noted among certain subgroups. Efforts to encourage prompt ART initiation should address delays among those without health insurance and among certain sociodemographic subgroups.
Introduction
Early initiation of antiretroviral therapy (ART) significantly reduces the risk of HIV-associated morbidity and mortality, as well as the risk of HIV transmission. HIV treatment guidelines, updated in March 2012, recommend initiating ART in all HIV-infected individuals regardless of CD4+ T-lymphocyte cell (CD4) count.1 These modifications were based on studies that demonstrated the benefits of early ART initiation and increased adverse outcomes associated with delayed ART initiation.2–6 To increase the proportion of HIV-infected persons who benefit from early or immediate ART initiation and reduce overall exposure to uncontrolled viremia, interventions need to be targeted at three distinct time periods: (1) Time from infection to diagnosis; (2) Time from diagnosis to care entry; and (3) Time from care entry to ART initiation. Although many studies have been conducted to better understand early diagnosis and early linkage to care, there are relatively few studies assessing time from care entry to ART initiation.
Previous studies have assessed time to ART initiation in specific HIV-diagnosed subpopulations (e.g., Medicaid recipients in New Jersey, residents of San Francisco, and injection drug users in Baltimore, Maryland)7–9 that might not be representative of all HIV-diagnosed persons in the United States. Moreover, although data from large, geographically diverse cohort collaborations such as NA-ACCORD exist,10 additional analyses on time to ART initiation from studies designed to be nationally representative, such as the Medical Monitoring Project (MMP), might provide additional insights. Lastly, there is substantial heterogeneity in all previous studies in terms of different definitions of time to ART initiation, treatment eligibility, and description of subgroups.7–10
Given changes in the HIV treatment guidelines, as well as noted limitations of previous studies, we assessed time to ART initiation using data from MMP, a probability sample of US HIV-infected adults receiving medical care in 2009. Applying the current guidelines, we aimed to establish a baseline for ongoing surveillance. Moreover, we assessed factors associated with time to ART initiation to identify potential disparities in ART initiation.
Methods
Ethics statement
In accordance with the federal human subjects protection regulations at 45 Code of Federal Regulations 46.101c and 46.102d11 and with the Guidelines for Defining Public Health Research and Public Health Non-Research,12 MMP was determined by the Centers for Disease Control and Prevention (CDC) to be a nonresearch, public health surveillance activity. Participating states or territories and facilities obtained local institutional review board approval to conduct MMP if required locally. Informed consent was obtained from all interviewed participants.
Study design
MMP is a national HIV surveillance system designed to produce representative estimates of behavioral and clinical characteristics of HIV-infected adults receiving medical care in the United States.13–15 MMP is a complex-sample, cross-sectional survey. For the 2009 data collection cycle, US states and territories were sampled first, followed by facilities providing HIV care, and then by HIV-infected adults (persons aged 18 years and older) who had at least one medical care visit during January–April 2009 at participating facilities. Data were collected through face-to-face interviews and medical record abstractions from June 2009 through May 2010. All sampled states and territories participated in MMP: California (including the separately funded jurisdictions of Los Angeles County and San Francisco), Delaware, Florida, Georgia, Illinois (including Chicago), Indiana, Michigan, Mississippi, New Jersey, New York (including New York City), North Carolina, Oregon, Pennsylvania (including Philadelphia), Puerto Rico, Texas (including Houston), Virginia, and Washington. Of 603 sampled facilities, 461 participated in MMP (facility response rate, 76%). Most of the HIV care facilities sampled were private practices (60%), followed by hospital-based facilities (30%) and community health centers (19%). The remainder facilities were clinical research facilities (10%), state or local health department clinics (5%), community-based service organizations (4%), and Veterans Administration facilities (4%). A facility could belong to multiple categories. Of 9338 sampled persons, 4217 completed the interview and had their medical records abstracted (patient-level response rate, 51%). Data were weighted on the basis of known probabilities of selection at state or territory, facility, and patient levels. In addition, to adjust for potential non-response bias, data were weighted by using predictors of patient-level response, including facility size, race/ethnicity, time since HIV diagnosis, and age group.16 After weighting for probability of selection and nonresponse, the 4217 participants in the 2009 MMP data collection cycle were estimated to represent a population of 421,186 [95% confidence interval (CI) 378,187–464,186] HIV-infected adults receiving medical care in the United States during January–April 2009.16
Analysis
To minimize recall bias, we restricted our analysis to persons diagnosed close to the time of interview, specifically, persons diagnosed in the 5 years prior to April 30, 2009. Our outcome was time from self-reported date of HIV care entry to self-reported date of first ART initiation. Persons who did not initiate ART (n=173) were censored at the date of interview. Predictors and covariates of interest comprised variables classified into three broad groups: sociodemographic, clinical, and facility characteristics. Sociodemographic variables include age, race/ethnicity, sexual risk category, education level, English as primary language, income at or below poverty level, and continuous health insurance. Clinical variables include year of care initiation and diagnosis of AIDS at care entry. Facility variables include being seen in a Ryan White funded facility, being seen in a university-affiliated facility and facility patient volume (actual number of patients receiving care at facility within the first four months of 2009). Sexual risk category was classified into three groups: men who have sex with men (MSM) inclusive of men who have sex with men and women, men who have sex exclusively with women (MSW), and women who have sex with men (WSM). Persons who reported other sexual behaviors not otherwise captured within the three groups above were excluded from analysis and comprised less than 1% of the study population. Persons classified as lacking continuous health insurance include those who reported a lapse in or no health insurance in the 12 months prior to interview. As data on date of AIDS diagnosis were not available, we estimated date of AIDS diagnosis by using age at AIDS diagnosis in years and date of birth obtained from the MMP jurisdictions' HIV case registry data. If the calculated date at AIDS diagnosis was the same as the date of HIV care entry or earlier, a person was classified to have AIDS at the time of care entry. If no diagnosis of AIDS was reported, or if the calculated date of AIDS diagnosis was later than the date of HIV care entry, a person was classified as not having AIDS at the time of care entry.
We generated Kaplan-Meier survival curves for the entire retrospective cohort and stratified by race/ethnicity and by sexual risk categories. Median time to ART initiation was calculated using Kaplan-Meier product-limit estimates.17 We evaluated all predictor variables for adherence to the proportional hazards assumption using log-log curves, an extended Cox approach with time-dependent variables and a correlation analysis of the Schoenfeld residuals with ranked follow-up time.18 We then constructed a multivariable Cox proportional hazards model to evaluate the effect of potential risk factors on time to ART initiation. All variables were systematically assessed for inclusion in the final model using a backward elimination approach. This approach has been well described and recommended because it is valuable in helping to methodically identify the best model starting with the largest meaningful model and then assesses interaction, confounding and precision.19 In our crude analysis using product limit survival curves and log-log survival curves, we found that the effect of sexual risk category on ART initiation appeared to change after 36 months. Thus we evaluated sexual risk category as a time-dependent variable and calculated separate hazard ratios estimates for two different time blocks: ≤36 and >36 months. However, in crude and adjusted models, the hazard ratio estimates were not significantly different in the two time periods and in the interest of parsimony, the time-dependent covariate for sexual risk category was excluded from our final model. All analyses were conducted using SUDAAN v.11 (RTI International, Research Triangle Park, NC) and SAS 9.3 (SAS Institute, Cary, NC) survey procedures.20 Data were evaluated at the 0.05 significance level.
Results
Our analysis included a total of 1094 participants, representing an estimated 111,910 (CI 95,775–128,045) HIV-infected adults diagnosed May 2004–April 2009 and receiving medical care in the United States in 2009. Of those, 83.9% reported initiating antiretroviral therapy. Of the 16.1% of persons who did not initiate ART prior to interview, 82.9% reported their doctor advised treatment delay, 9.3% reported they did not need medications because they felt healthy or believed their HIV laboratory results were good, 1.3% delayed treatment because of medication side effects, and the remaining 6.5% delayed for personal and other reasons.
The mean age was 39.4 (CI 38.6–40.2) years. Most persons (74%) were male; 30% were non-Hispanic white, 44% were non-Hispanic black, and 21% were Hispanic or Latino (Table 1). More than half (52%) had greater than high school education, and 37% reported a lapse in or no health insurance within the 12 months prior to interview. A majority (75%) of participants received care at a facility that received Ryan White HIV/AIDS Program funding, and 46% received care at a university-affiliated facility. The median patient volume at facilities where participants received care was 407 patients.
Table 1.
Baseline Characteristics and Hazard Ratios for Time to Antiretroviral Therapy (ART) Initiation from Time to HIV Care Entry Among HIV-Infected Adults Who Were Diagnosed May 2004–April 2009 and Were Receiving Medical Care in the United States in 2009—Medical Monitoring Project
| All persons | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Sample | Weighted % (95% CI) | No. initiated ART | Person months | ART initiation ratea | Median months to ART initiationb | Crude hazard ratio | Adjusted hazard ratio |
| Total cohort | 1094 | - | 920 | 8698 | 10.6 | 10 | – | – |
| Age in years | ||||||||
| 18–29 | 250 | 22.2 (19.1, 25.3) | 191 | 2406 | 7.9 | 18 | 0.65 (0.50, 0.83) | – |
| 30–39 | 297 | 28.0 (25.0, 31.0) | 255 | 2684 | 9.5 | 10 | 0.83 (0.66, 1.03) | – |
| 40–49 | 344 | 31.9 (29.2, 34.7) | 296 | 2520 | 11.7 | 7 | 0.92 (0.73, 1.16) | – |
| 50+ | 201 | 17.9 (15.7, 20.2) | 176 | 1088 | 16.2 | 5 | Ref | – |
| Race/ethnicity | ||||||||
| Non-Hispanic white | 304 | 29.7 (22.9, 36.5) | 274 | 2579 | 10.6 | 7 | Ref | Ref |
| Non-Hispanic black | 489 | 44.1 (34.7, 53.5) | 391 | 3929 | 10.0 | 15 | 0.88 (0.76, 1.02) | 0.82 (0.70, 0.98) |
| Hispanic/Latino | 246 | 21.0 (15.2, 26.7) | 210 | 1601 | 13.1 | 8 | 0.98 (0.83, 1.15) | 0.99 (0.83, 1.18) |
| Other | 55 | 5.3 (3.9, 6.6) | 45 | 589 | 7.6 | 11 | 0.88 (0.69, 1.14) | 0.89 (0.70, 1.14) |
| Sexual risk category | ||||||||
| MSM | 511 | 49.5 (44.1, 54.9) | 435 | 4866 | 8.9 | 10 | 0.65 (0.56, 0.75) | 0.71 (0.60. 0.84) |
| MSW | 266 | 24.2 (20.3, 28.1) | 229 | 1099 | 20.8 | 3 | Ref | Ref |
| WSM | 290 | 26.4 (22.3, 30.4) | 233 | 2567 | 9.1 | 11 | 0.60 (0.47, 0.76) | 0.66 (0.51, 0.85) |
| Education | ||||||||
| >High school | 549 | 52.4 (46.5, 58.2) | 460 | 5079 | 9.1 | 11 | 0.83 (0.71, 0.96) | – |
| High school | 316 | 27.8 (23.4, 32.1) | 262 | 2321 | 11.3 | 8 | 0.83 (0.65, 1.05) | – |
| <High school | 229 | 19.9 (16.8, 22.9) | 198 | 1298 | 15.3 | 8 | Ref | – |
| English as primary language | ||||||||
| Yes | 926 | 86.1 (80.8, 91.3) | 776 | 7881 | 9.8 | 10 | 0.74 (0.66, 0.91) | – |
| No | 168 | 13.9 (8.7, 19.2) | 144 | 817 | 17.6 | 10 | Ref | – |
| Poverty level | ||||||||
| At or below | 502 | 44.6 (39.6, 49.6) | 416 | 3767 | 11.0 | 12 | 0.95 (0.82, 1.09) | – |
| Above | 534 | 55.4 (50.4, 60.4) | 455 | 4604 | 9.9 | 8 | Ref | – |
| Health insurance | ||||||||
| Lapse/ none | 401 | 37.2 (32.4, 41.9) | 313 | 3529 | 8.9 | 11 | 0.78 (0.66, 0.92) | 0.82 (0.70, 0.97) |
| Continuous | 689 | 62.8 (58.1, 67.6) | 603 | 5158 | 11.7 | 9 | Ref | Ref |
| AIDS at care entry | ||||||||
| No | 619 | 67.3 (63.2, 71.3) | 474 | 8156 | 5.8 | 17 | 0.36 (0.30, 0.42) | 0.37 (0.31, 0.43) |
| Yes | 324 | 32.7 (28.7, 36.8) | 318 | 542 | 58.7 | 1 | Ref | Ref |
| Year of care entry | ||||||||
| 2004 | 106 | 11.0 (9.1, 12.9) | 96 | 1551 | 6.2 | 10 | 0.89 (0.66, 1.20) | – |
| 2005 | 164 | 18.1 (15.5, 20.7) | 148 | 2039 | 7.3 | 11 | 0.92 (0.71, 1.18) | – |
| 2006 | 172 | 17.9 (15.8, 20.0) | 149 | 1821 | 8.2 | 9 | 0.92 (0.73, 1.16) | – |
| 2007 | 181 | 19.5 (16.7, 22.2) | 154 | 1682 | 9.2 | 15 | 0.92 (0.70, 1.23) | – |
| 2008 | 232 | 24.6 (21.2, 27.9) | 183 | 1320 | 13.9 | 10 | 0.97 (0.73, 1.28) | – |
| 2009 | 81 | 8.9 (7.0, 10.8) | 58 | 285 | 20.4 | 5 | Ref | – |
| Facility patient volumec,d | 1094 | 406.8 (283.0, 530.5) | - | - | - | - | 0.98 (0.94, 1.02) | – |
| Received care in RWP-funded facility | ||||||||
| Yes | 782 | 74.5 (68.5, 80.4) | 654 | 5938 | 11.0 | 10 | 0.96 (0.77, 1.21) | – |
| No | 222 | 25.5 (19.6, 31.5) | 192 | 1976 | 9.7 | 10 | Ref | – |
| Received care in university-affiliated facility | ||||||||
| Yes | 528 | 46.0 (37.9, 54.0) | 447 | 4581 | 9.8 | 12 | 1.01 (0.86, 1.17) | - |
| No | 552 | 54.0 (46.0, 62.1) | 459 | 4109 | 11.2 | 7 | Ref | - |
Per 100 person-months.
Median months to ART initiation, based on Kaplan-Meier estimates of months to ART initiation.
Hazard ratios in bold statistically significant at the p<0.05 level.
Effect estimate calculated for a unit change of 500 patients.
Median and 95% CI reported for continuous variable.
AIDS, acquired immune deficiency syndrome; ART, antiretroviral therapy; CI, confidence interval; MSM, men who have sex with men; MSW, men who have sex with women; RWP, Ryan White HIV/AIDS Program; WSM, women who have sex with men.
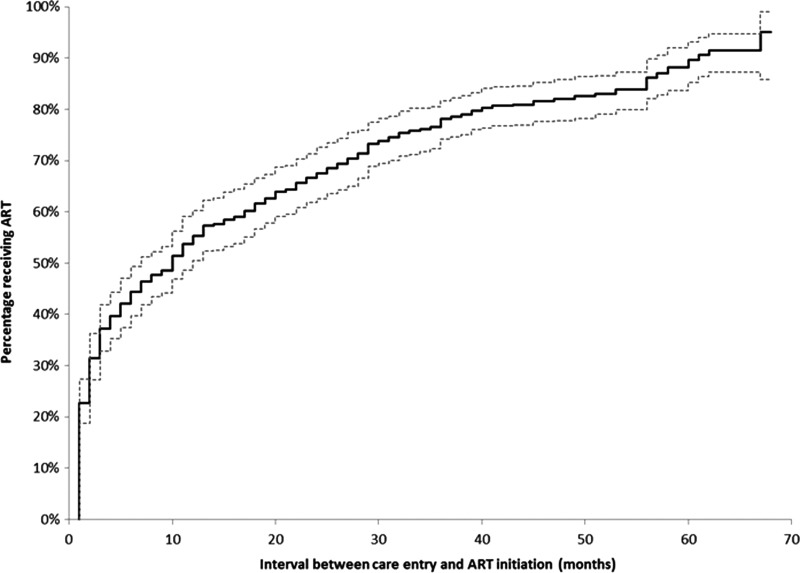
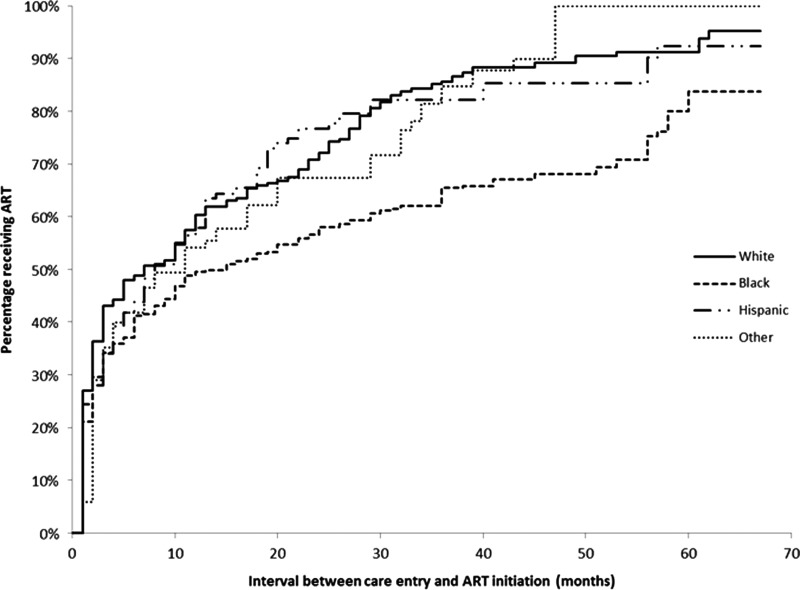
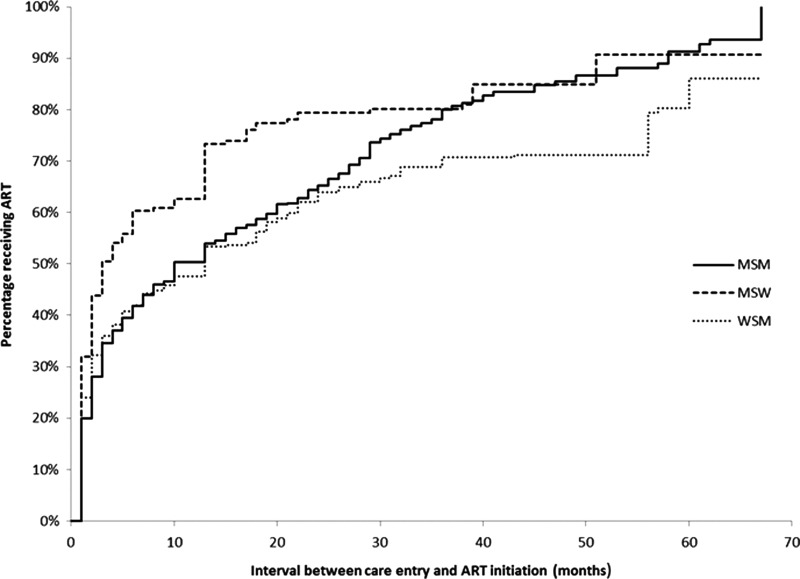
The median time to ART initiation was 10 months (Fig. 1). Using Kaplan-Meier curves stratified by race/ethnicity, non-Hispanic blacks appear to initiate ART less promptly than all other race groups (Fig. 2). While non-Hispanic whites had a median time to ART initiation of 7 months, non-Hispanic blacks initiated ART at a median of 15 months (Table 1). WSM and MSM had median ART initiation times of 11 months and 10 months, respectively, compared to MSW who had a median time to ART initiation of 3 months (Fig. 3). Persons living at or below poverty level and those who reported a lapse in or no health insurance had longer times to ART initiation (12 vs. 8 months and 11 vs. 9 months, respectively). Time to ART initiation did not vary significantly by year of care entry. The short median time to ART initiation (5 months) among those who entered care in 2009 might be due to the relatively small size of this group, as data on this group were limited to persons who initiated care within the first 4 months of 2009.
FIG. 1.
Kaplan-Meier estimates and 95% confidence limits (dotted lines) for time to antiretroviral therapy (ART) initiation from time to HIV care entry among HIV-infected adults who were diagnosed May 2004–April 2009 and were receiving medical care in the United States in 2009—Medical Monitoring Project.
FIG. 2.
Kaplan-Meier estimates for time to antiretroviral therapy (ART) initiation from time to HIV care entry among HIV-infected adults who were diagnosed May 2004–April 2009 and were receiving medical care in the United States in 2009, stratified by race/ethnicity—Medical Monitoring Project.
FIG. 3.
Kaplan-Meier estimates for time to antiretroviral therapy (ART) initiation from time to HIV care entry among HIV-infected adults who were diagnosed May 2004–April 2009 and were receiving medical care in the United States in 2009, stratified by sexual risk category; showing heterosexual women (WSM) compared to heterosexual men (MSW) and men who have sex with men (MSM)—Medical Monitoring Project.
Table 1 shows the unadjusted and adjusted hazard ratios for demographic, clinical, and facility characteristics on ART initiation. All hazard ratios (or instantaneous risk of initiating ART) above 1.0 indicate a shorter time to ART initiation and those less than 1.0 denote a longer time to ART initiation. In bivariate models, younger persons, those with greater than high school education, participants whose primary language was English, persons lacking continuous health insurance, and those without a diagnosis of AIDS at care entry were less likely to initiate ART promptly. Compared to MSW, WSM and MSM had a 40% and 35% reduction in the instantaneous risk of initiating ART, respectively. The instantaneous risk of initiating ART did not differ significantly by poverty level, year of care initiation, and facility characteristics in bivariate models. In adjusted models, non-Hispanic blacks had significantly longer times to ART initiation than non-Hispanic whites. Compared to MSW, MSM and WSM were less likely to initiate ART promptly. Persons lacking continuous health insurance and those without an AIDS diagnosis at care entry also initiated ART less promptly.
Discussion
Reducing time from care entry to ART initiation may improve clinical outcomes and reduce HIV transmission. In this analysis, median time from care entry to ART initiation was 10 months among persons diagnosed with HIV in 2004–2009 in a national, diverse, probability sample of HIV-infected adults receiving care in the United States. After adjusting for stage of disease at care entry, we identified disparities in time to ART initiation. Blacks compared to whites, MSM and WSM compared to MSW, and persons lacking continuous insurance had significantly longer times to ART initiation. However, we found no temporal trends in time to ART initiation among person who entered care in 2004–2009 nor any differences by facility characteristics.
Previous studies have produced varying estimates of time to ART initiation. In an early study median time to ART initiation was 14.1 months among HIV-infected Medicaid recipients in New Jersey initiating ART in 1996–1998.7 In a prospective study of HIV-infected drug users in Baltimore, Maryland followed from 1996 to 2008, median time to ART initiation was approximately 15 months among persons who became treatment eligible in 2003–2007.9 More recent data from San Francisco suggest that time to ART initiation has decreased with the median time to ART initiation among treatment eligible persons decreasing from 8 months in 2007 to 3 months in 2011.8 In addition, data from the NA-ACCORD show that the cumulative incidence of 6-month ART initiation increased from 51% in 2001 to 72% in 2009.10 Our finding of median time to ART initiation of 10 months is within range of these previous estimates. Given temporal changes in time to ART initiation due to improvements in ART, changes in guidelines, and evolving provider practices, further analyses of future years of MMP data as well as data from other sources will be critical to assess improvement in this key indicator of HIV care.
Although disparities in any ART use have been noted in numerous studies, fewer studies have addressed the issue of disparities in time to ART initiation. Our results are consistent with most studies that have examined predictors of ART use and time to ART initiation.7–10,21,22 Racial and gender disparities in time to ART initiation have been consistently noted, with non-Hispanic blacks having longer times to ART initiation than non-Hispanic whites, and women have been shown to have delayed ART initiation compared to men. However, our analysis is the only one to find that MSM have delayed ART initiation compared to MSW. This finding needs to be explored further in future analyses. Lastly, we found lack of continuous insurance to be associated with delayed ART initiation, an association that had not been rigorously assessed.7,9 Efforts to improve time to ART initiation would benefit from addressing noted disparities.
Time to ART initiation is dependent on clinical eligibility as well as patient-, provider-, and facility-level factors. The recommended CD4 threshold for initiating ART was increased from <350 cells/mm3 in December 2007 to <500 cells/mm3 in December 2009, then to recommending ART regardless of CD4 count in March 2012. Treatment eligibility has been a key driver of time to ART initiation, but even among treatment-eligible patients, substantial delays to ART initiation exist.7–10 Many patient-level factors such as age, gender, race/ethnicity, substance use, homelessness, lack of insurance and psychological factors including perceived capacity to tolerate psychological distress and avoidance of physical discomfort7–10,21–31 have been shown affect ART initiation. However, provider-level factors have been relatively understudied. In a recent national survey of HIV care providers, provider-level reasons for delaying ART initiation included concerns about patients' acceptance of ART (e.g., lack of readiness, refusal to start, and denial, fear, or lack of knowledge about ART), concerns about patients' ability to adhere to ART (e.g., substance use, mental health, appointment non-adherence, unstable social situation), and concerns about structural barriers (e.g., cost, lack of insurance) (Beer et. al., unpublished observation). Gargliado et al. in a survey of US providers from the American Academy of HIV Medicine also found unstable housing and drug use were perceived providers as large barriers to ART initiation.32 Lastly, there are almost no data on facility-level barriers to ART initiation or time to ART initiation. In this analysis, we did not find any association between time to ART initiation and facility characteristics (facility patient volume, Ryan White HIV/AIDS Program funding status, and university affiliation). However, further analysis of facility level characteristics, including facility size and Ryan White HIV/AIDS Program funding status, is warranted to better understand how they might contribute to delayed ART initiation.
This analysis has certain limitations. First, we acknowledge that CD4 count at care initiation and throughout care was a critical factor to ascertain treatment eligibility prior to 2012. However, using a combination of MMP and the jurisdictions' state HIV case registry data, we were only able to ascertain AIDS at care entry as a proxy variable to adjust for treatment eligibility based on disease severity in this analysis. Thus, we assessed time from care entry to ART initiation for all persons without adjusting for treatment eligibility according to CD4 cell count criteria. Therefore, our analysis describes practice rather than compliance with applicable guidelines. If estimates of time to ART initiation had been restricted to treatment-eligible persons, they would likely have been lower. However, given current treatment recommendations to initiate ART in all HIV-diagnosed persons regardless of CD4 cell count, our analysis sets a baseline time to ART initiation for future MMP analyses given that MMP is an ongoing surveillance system in which data are collected annually. Second, the date of care entry and first ART use were self-reported and thus may be subject to recall bias. However, to minimize recall errors, only persons diagnosed within a 5-year period prior to the interview (from May 2004 to April 2009) were asked questions regarding care entry and ART initiation dates. Also, MMP samples only HIV-infected adults receiving care, as such, our results cannot be generalized to all HIV-infected persons in the United States. Although provider-level data were not measured, a large proportion of persons who did not initiate ART prior to interview reported their doctor advised treatment delay. Though we found no associations between measured facility factors and ART initiation, we suspect that other unmeasured facility level factors including administrative processes and medication cost might be associated with delays. Both provider and facility-level factors merit further exploration.
We provide national estimates of time to ART initiation among HIV-infected adults receiving care in the United States, to assess current trends in HIV treatment initiation practices and provide a baseline measure for evaluating the impact of new treatment guidelines. Improving overall time to ART initiation and addressing disparities in time to ART initiation will be an important step in improving treatment outcomes among HIV-infected persons.
Acknowledgments
This research was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an agreement between the US Department of Energy and CDC. Funding for the Medical Monitoring Project is provided by a cooperative agreement (PS09-937) from the Centers for Disease Control and Prevention.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author Disclosure Statement
None of the authors have any relevant financial relationships that would present a conflict of interest with the material reported in this article.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-Infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf (Last accessed July29, 2014)
- 2.Walensky RP, Paltiel AD, Losina E, et al. . Test and treat DC: Forecasting the impact of a comprehensive HIV strategy in Washington DC. Clin Infect Dis 2010;51:392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Early initiation of ART produces better clinical outcomes. Clin Infect Dis 2009;48:iii. [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, et al. . Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geng EH, Hare CB, Kahn JO, et al. . The effect of a “Universal Antiretroviral Therapy” recommendation on HIV RNA levels among HIV-infected patients entering care with a CD4 count greater than 500/mul in a public health setting. Clin Infect Dis 2012;55:1690–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianella S, von Wyl V, Fischer M, et al. . Effect of early antiretroviral therapy during primary HIV-1 infection on cell-associated HIV-1 DNA and plasma HIV-1 RNA. Antivir Ther 2011;16:535–545 [DOI] [PubMed] [Google Scholar]
- 7.Crystal S, Sambamoorthi U, Moynihan PJ, McSpiritt E. Initiation and continuation of newer antiretroviral treatments among medicaid recipients with AIDS. J Gen Intern Med 2001;16:850–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu LC, Truong HM, Vittinghoff E, Zhi Q, Scheer S, Schwarcz S. Trends in early initiation of antiretroviral therapy and characteristics of persons with HIV initiating therapy in San Francisco, 2007–2011. J Infect Dis 2014;209:1310–1314 [DOI] [PubMed] [Google Scholar]
- 9.Mehta SH, Kirk GD, Astemborski J, Galai N, Celentano DD. Temporal trends in highly active antiretroviral therapy initiation among injection drug users in Baltimore, Maryland, 1996–2008. Clin Infect Dis 2010;50:1664–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanna DB, Buchacz K, Gebo KA, et al. . Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001–2009. Clin Infect Dis 2013;56:1174–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DHHS. Protection of Human Subjects, US Federal Code Title 45 Part 46. Code of Federal Regulations, 2009 [PubMed]
- 12.CDC. Distinguishing Public Health Research and Public Health Nonresearch. In: Services HaH, ed, 2010 [Google Scholar]
- 13.Blair JM, McNaghten AD, Frazier EL, Skarbinski J, Huang P, Heffelfinger JD. Clinical and behavioral characteristics of adults receiving medical care for HIV infection—Medical Monitoring Project, United States, 2007. MMWR Surveill Summ 2011;60:1–20 [PubMed] [Google Scholar]
- 14.Frankel MR, McNaghten A, Shapiro MF, et al. . A probability sample for monitoring the HIV-infected population in care in the U.S. and in selected states. Open AIDS J 2012;6:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNaghten AD, Wolfe MI, Onorato I, et al. . Improving the representativeness of behavioral and clinical surveillance for persons with HIV in the United States: The rationale for developing a population-based approach. PLoS One 2007;2:e550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blair JM, Fagan JL, Frazier EL, et al. . Behavioral and clinical characteristics of persons receiving medical care for HIV infection—Medical Monitoring Project, United States, 2009. MMWR Surveill Summ 2014;63:1–22 [PubMed] [Google Scholar]
- 17.Kleinbaum DG, Klein M. Survival Analysis: Springer, 1996 [Google Scholar]
- 18.Kleinbaum DG, Klein M. Evaluating the proportional hazards assumption. Survival Analysis: Springer; 2012; pp. 161–200 [Google Scholar]
- 19.Kleinbaum DG. Epidemiologic methods: The “art” in the state of the art. J Clin Epidemiol 2002;55:1196–1200 [DOI] [PubMed] [Google Scholar]
- 20.SAS: Survey Sampling and Analysis Procedures. https://support.sas.com/documentation/cdl/en/statug/63033/HTML/default/viewer.htm#statug_introsamp_sect001.htm (Last accessed June6, 2014)
- 21.Shapiro MF, Morton SC, McCaffrey DF, et al. . Variations in the care of HIV-infected adults in the United States: Results from the HIV Cost and Services Utilization Study. JAMA 1999;281:2305–2315 [DOI] [PubMed] [Google Scholar]
- 22.Cunningham WE, Markson LE, Andersen RM, et al. . Prevalence and predictors of highly active antiretroviral therapy use in patients with HIV infection in the united states. HCSUS Consortium. HIV Cost and Services Utilization. J Acquir Immune Defic Syndr 2000;25:115–123 [DOI] [PubMed] [Google Scholar]
- 23.Hall HI, Byers RH, Ling Q, Espinoza L. Racial/ethnic and age disparities in HIV prevalence and disease progression among men who have sex with men in the United States. Am J Public Health 2007;97:1060–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hightow-Weidman LB, Smith JC, Valera E, Matthews DD, Lyons P. Keeping them in “STYLE”: Finding, linking, and retaining young HIV-positive Black and Latino men who have sex with men in care. AIDS Patient Care STDS 2011;25:37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christopoulos KA, Das M, Colfax GN. Linkage and retention in HIV care among men who have sex with men in the United States. Clin Infect Dis 2011;52:S214–S222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beer L, Fagan JL, Garland P, et al. . Medication-related barriers to entering HIV care. AIDS Patient Care STDS 2012;26:214–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghani AC, Donnelly CA, Anderson RM. Patterns of antiretroviral use in the United States of America: Analysis of three observational databases. HIV Med 2003;4:24–32 [DOI] [PubMed] [Google Scholar]
- 28.Hall HI, Frazier EL, Rhodes P, et al. . Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med 2013;173:1337–1344 [DOI] [PubMed] [Google Scholar]
- 29.Dombrowski JC, Kitahata MM, Van Rompaey SE, et al. . High levels of antiretroviral use and viral suppression among persons in HIV care in the United States, 2010. J Acquir Immune Defic Syndr 2013;63:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Celentano DD, Galai N, Sethi AK, et al. . Time to initiating highly active antiretroviral therapy among HIV-infected injection drug users. AIDS 2001;15:1707–1715 [DOI] [PubMed] [Google Scholar]
- 31.Magidson JF, Seitz-Brown CJ, Listhaus A, Lindberg B, Anderson KE, Daughters SB. Distress tolerance and use of antiretroviral therapy among HIV-infected individuals in substance abuse treatment. AIDS Patient Care STDS 2013;27:518–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gagliardo C, Murray M, Saiman L, Neu N. Initiation of antiretroviral therapy in youth with HIV: A U.S.-based provider survey. AIDS Patient Care STDS 2013;27:498–502 [DOI] [PMC free article] [PubMed] [Google Scholar]