Abstract
Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and the proliferating antigen Ki67 have been widely studied in several tumors. However, their role as indicator in non-small cell lung cancer (NSCLC) remains unknown. Here, we investigated the expression of PTEN and Ki67 in NSCLC tissues and paired normal lung tissues to identify whether these proteins are associated with lung cancer development and survival. Immunohistochemistry for PTEN and Ki67 was performed on 67 lung cancer tissues and 41 paired adjacent normal lung tissues to detect the expression of these two proteins. The expression of PTEN in NSCLC tissues (32.8%) was significantly lower than that in normal tissues (82.9%, P < 0.05). In contrast, the expression of Ki67 in NSCLC tissues (76.1%) was significantly higher than that in normal tissues (27.3%, P < 0.05). Expression of both PTEN and Ki67 were strongly associated with tumor histology, clinical stage, lymph node metastasis, differentiation and 4-year postoperative survival rate (P < 0.05). However, PTEN expression was negatively correlated with Ki67 expression (r = -0.279, P < 0.05). In conclusion, low PTEN expression and Ki67 overexpression are associated with malignant invasion and lymph node metastasis of NSCLC. These proteins may serve as diagnostic and prognostic biomarkers of NSCLC.
Keywords: non-small cell lung cancer (NSCLC), Ki67, phosphatase and tensin homolog deleted on chromosome 10 (PTEN), immunohistochemistry, lymph node, prognosis
INTRODUCTION
Non-small cell lung cancer (NSCLC) is a slow-developing cancer with a complex pathogenesis; its progression involves several stages as well as activation of many oncogenes and inactivation of tumor suppressor genes[1]. Alterations in the expression of extracellular growth factors and signaling molecules commonly occur during NSCLC development. These proteins are involved in various cellular functions, including proliferation and cell cycle progression. Changes in their expressions can lead to undue proliferation of tumor cells or escape from cell cycle checkpoints and apoptosis[2].
One such factor, phosphatase and tensin homolog deleted on chromosome 10 (PTEN), is involved in receptor tyrosine kinase (RTK) signaling due to its phosphorylase functions[3],[4]. PTEN is a negative control factor in the phosphoinositide-3 kinase (PI3K)/serine-threonine kinase (AKT) pathway. This protein plays a role as tumor suppressor to inhibit the malignant transformation of cells as well as the invasion, metastasis and growth of tumors. Mutation in PTEN eliminates its antitumor effects and promotes tumorigenesis[5]–[9]. Previous studies have demonstrated the importance of other RTKs in lung tumors (particularly epidermal growth factor receptor (EGFR)); alterations in PTEN may, therefore, play a role in the pathogenesis of NSCLC[10]. Another protein with demonstrated roles in tumorigenesis is Ki67, a nuclear antigen related to cell proliferation as increased Ki67 expression is closely correlated with the proliferation and invasiveness of tumors[11]. Although the exact functions of Ki67 are unclear, there is accumulating evidence that Ki67 plays a critical role in cell cycle progression[2],[12]–[14].
In this study, immunohistochemistry was performed to detect the expression of PTEN and Ki67 in NSCLC tissues and paired adjacent normal lung tissues. Additionally, the expression was compared with clinicopathologic features of NSCLC.
PATIENTS AND METHODS
Patients
Surgical tissues were collected from 67 patients with lung cancer who were treated at Wuxi People's Hospital Affiliated to Nanjing Medical University from June 2007 to May 2008. Diagnosis was confirmed as NSCLC by postoperative pathology. The patients with lung cancer included 33 males and 34 females with age ranging from 39 to 80 years. Pathological staging was performed for all cases in accordance with the diagnostic criteria of the Union for International Cancer Control (UICC) and there were 20 cases in stage I, 29 cases in stage II and 18 cases in stage III. Twenty-eight cases were squamous cell carcinoma, 31 cases were adenocarcinoma and 8 cases were large-cell carcinoma. In addition, 25 cases were poorly differentiated tumors, while 42 cases were highly or moderately differentiated tumors. Lymph node metastasis was evident in 29 cases and absent in 38 cases. Paired normal tissues were collected from adjacent tissues (> 5 cm away from tumors) of 41 cases. No patients had received radiotherapy or chemotherapy before surgery.
Follow-up
The patients were followed up by telephone or letter, and all had complete follow-up information on survival. Survival time was defined as the time interval from the date of surgery until the date of last follow-up (June 2012) or death.
Detection of PTEN and Ki67expression
Tissues were fixed in 10% formaldehyde and embedded in paraffin by using conventional methods. Serial tissue sections were cut at 4 µmol/L thickness and placed on microscope slides for immunohistochemistry. We used a mouse monoclonal antibody for PTEN and a mouse monoclonal antibody for Ki-67 (both from Zhongshan Golden Bridge Biotechnology, Beijing, China). As immunohistochemistry with antibodies against PTEN and Ki67 was performed using the streptomycin avidin-peroxidase (SP) method, sections were deparaffinized and rehydrated. The slides were then heated in a microwave for 10 minutes in a 10-µmol/L citrate buffer solution at pH 6.0 and then cooled to room temperature. After quenching endogenous peroxidase activity with 0.3% H2O2 (in absolute methanol) for 30 minutes, the sections were incubated for 2 hours at room temperature with 5% bovine serum albumin. Duplicate sections were then incubated overnight at 4 °C with the specific primary antibodies against PTEN and Ki-67, respectively. Sections were then treated successively with secondary antibodies and streptavidin (Zhongshan Golden Bridge Biotechnology). The 3, 3′-diaminobenzidine (DAB) substrate was performed to develop staining color, and then sections were counterstained with hematoxylin prior to dehydration and mounting. Sections of breast cancer tissue containing the above-mentioned antigens were used as positive control. Phosphate buffered saline (PBS) was used in place of primary antibody as negative control. Positive staining appeared in cells as yellowish-brown puncta of PTEN and Ki67. Stained tissues were scored for the proportion of positive cells out of the total number of cells. Fewer than 5% positive cells were considered as negative; 5%-20% positive cells were considered as weakly positive (+); > 20% positive cells were considered as strongly positive (++). Therefore, “positive expression” referred to both + and ++.
Statistical analysis
Analyses were performed by using SPSS l6.0 statistical analysis software, including χ2 test and Spearman rank correlation analysis. Quantitative variables were compared by using Student's t-test, and categorical variables were compared by using χ2 test. Kaplan-Meier survival curve and Log-rank tests were used to compare the survival rate between groups as designed. Two-sided alpha level was set at 0.05, with P < 0.05 considered statistically significant.
RESULTS
PTEN and Ki67 expression in NSCLC and paired normal lung tissues
We examined the expression of PTEN and Ki67 in 67 NSCLC specimes and 41 adjacent paired normal lung tissue specimens. PTEN was detected in both NSCLC and normal tissues (Fig. 1), but the positive expression rate of PTEN in NSCLC tissues was 32.8%, which was significantly lower than that of normal lung tissues (82.9%) (P < 0.05). In contrast, Ki67 was detected significantly more often in NSCLC tissues (76.1%) than in normal lung (27.3%) (P < 0.05). Furthermore, we analyzed whether the expression of PTEN correlated with the expression of Ki67 in NSCLC. The result showed that their expression in NSCLC was negatively correlated (r = -0.239, P = 0.022) (Table 1).
Fig. 1. PTEN and Ki67 expression by immunohistochemistry in non-small cell lung cancer (NSCLC) tissues.
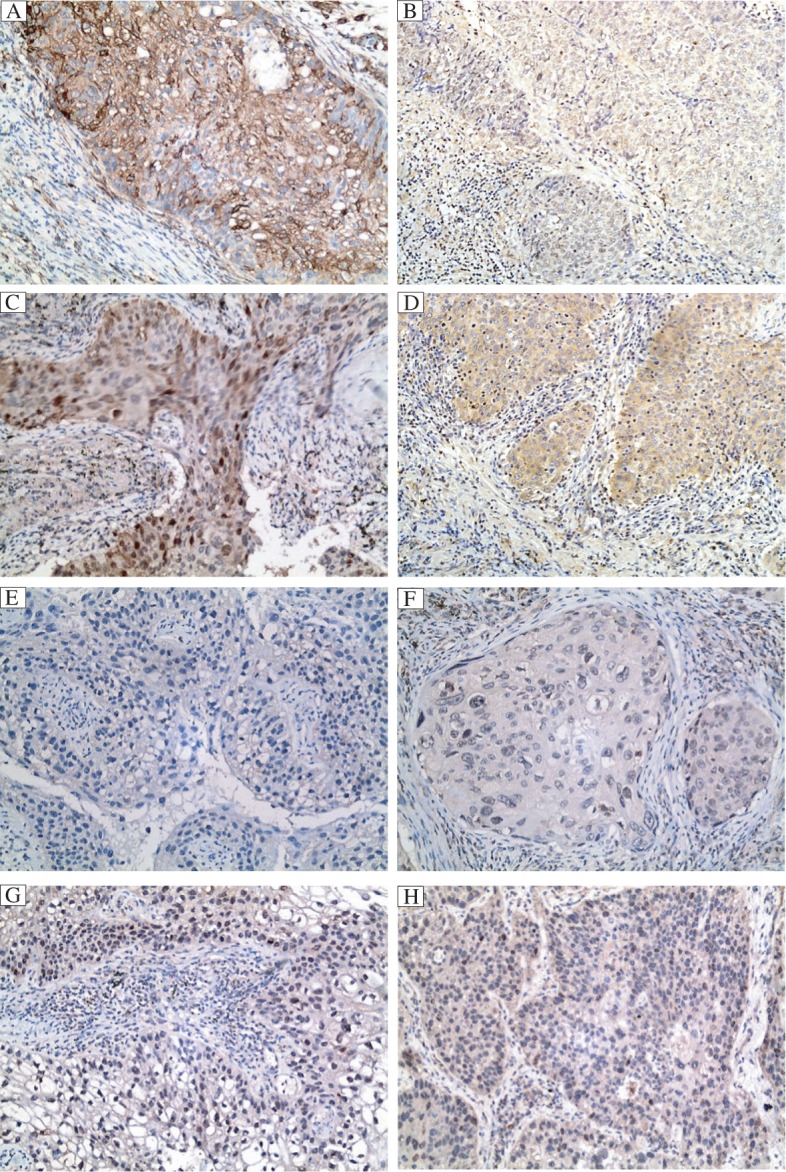
PTEN (brown) is expressed in the cytoplasm of NSCLC cells, A: adenocarcinoma; B: squamous carcinoma. Ki67 (brown) is expressed in the nuclei of NSCLC cells, C: adenocarcinoma; D: squamous carcinoma. PTEN is negatively expressed in adenocarcinoma (E) and squamous carcinoma (F). Ki67 is negatively expressed in adenocarcinoma (G) and squamous carcinoma (H).
Table 1. Correlation between PTEN and Ki67 expression in NSCLC.
| PTEN expression | Ki67 expression |
Total | R value | P-value | |
| + | - | ||||
| Positive | 13 | 9 | 22 | ||
| Negative | 38 | 7 | 45 | - 0.279 | 0.022 |
| Total | 51 | 16 | 67 | ||
Association between PTEN and Ki67 expression and clinicopathologic features
To identify whether the expression of the two proteins in lung tumors was associated with clinical features of the disease, we assessed expression patterns in terms of clinicopathologic features (Table 2). No statistically significant differences were observed in the expressions of PTEN and Ki67 when the data were stratified for gender and age. However, expressions of PTEN and Ki67 correlated with the degree of differentiation, presence or absence of lymph node metastasis, clinical stage and tumor histological category, respectively. Interestingly, inverse correlations were found: PTEN expression gradually increased with increased malignant differentiation while Ki67 expression gradually decreased. The expression of PTEN was significantly negatively correlated with lymph node metastasis, but Ki67 expression was positively correlated with lymph node metastasis (P < 0.05). Moreover, we showed that positive expression of PTEN and Ki67 in adenocarcinoma was higher than that in squamous cell carcinoma (P < 0.05). The expression of either PTEN or Ki67 did not correlate with tumor location and size (P > 0.05).
Table 2. Correlation between expressions of PTEN and Ki67 in NSCLC with clinicopathologic features.
| N | PTEN |
Ki67 |
||||||||
| + | - | Positive expression (%) | P-value | + | - | Positive expression (%) | P-value | |||
| Age (years) | ||||||||||
| ≤60 | 30 | 11 | 19 | 36.67 | 0.548 | 21 | 9 | 70.00 | 0.290 | |
| > 60 | 37 | 11 | 26 | 29.73 | 30 | 7 | 81.08 | |||
| Sex | ||||||||||
| Male | 33 | 10 | 23 | 30.30 | 0.664 | 26 | 7 | 78.79 | 0.614 | |
| Female | 34 | 12 | 22 | 35.29 | 25 | 9 | 73.53 | |||
| Tumor position | ||||||||||
| Periphery type | 40 | 10 | 30 | 25.00 | 0.096 | 31 | 9 | 77.50 | 0.747 | |
| Central type | 27 | 12 | 15 | 44.44 | 20 | 7 | 74.07 | |||
| Tumor size (cm) | ||||||||||
| ≤3 | 35 | 13 | 22 | 37.14 | 0.432 | 24 | 11 | 68.57 | 0.130 | |
| > 3 | 32 | 9 | 23 | 28.13 | 27 | 5 | 84.38 | |||
| Tumor category | ||||||||||
| Squamous cancer | 28 | 5 | 23 | 18.71 | 19 | 9 | 67.9 | |||
| Adenocarcinoma | 31 | 12 | 19 | 38.70 | 0.014 | 25 | 6 | 80.65 | 0.037 | |
| Large cell carcinoma | 8 | 3 | 5 | 37.50 | 5 | 3 | 62.50 | |||
| Lymph metastasis | ||||||||||
| Yes | 29 | 5 | 24 | 17.24 | 0.018 | 31 | 9 | 77.5 | 0.023 | |
| No | 38 | 17 | 21 | 44.74 | 8 | 9 | 47.0 | |||
| TNM Stage | ||||||||||
| I,II | 49 | 22 | 27 | 44.90 | 0.038 | 30 | 19 | 61.22 | 0.043 | |
| III | 18 | 6 | 12 | 33.33 | 14 | 4 | 77.8 | |||
| Differentiation degree | ||||||||||
| Low | 25 | 5 | 20 | 20.00 | 0.007 | 23 | 2 | 92.00 | 0.020 | |
| High/moderate | 42 | 19 | 23 | 45.24 | 28 | 14 | 66.67 | |||
Relationship between Ki67 or PTEN expression in NSCLC tissues and postoperative survival
Follow-up data from all patients indicated that there were 30 living patients on the cut-off date of follow-up, while 4 patients experienced recurrence during follow-up and 33 patients died. Kaplan-Meier survival curve analysis was performed (Fig. 2). Furthermore, Kaplan-Meier plots of survival showed that patients with negative Ki67 expression had a median overall survival time of 31.1 months, whereas patients with positive Ki67 expression had an overall survival of 17.9 months (Log-rank, P < 0.05). For PTEN expression, the overall survival was 19.3 months for patients with negative expression and 24.6 months for those with positive expression (Log-rank, P < 0.05; Table 3).
Fig. 2. Kaplan-Meier survival curves of NSCLC patients stratified by positive or negative expression of PTEN (A) or Ki67 (B).
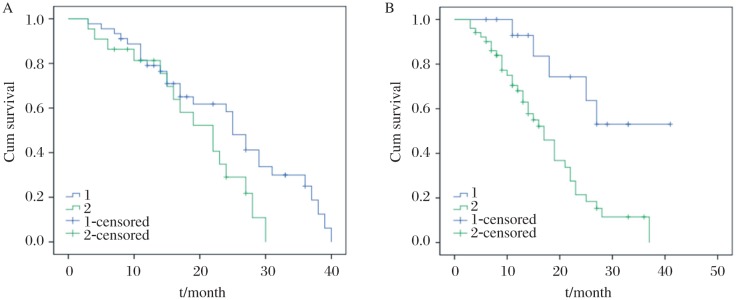
Signficiant difference was noted between patients with positive or negative expression of PTEN or Ki67 (P < 0.05).
Table 3. The relationship between PTEN and Ki67 expression and prognosis of NSCLC patients.
| Protein | Total (n) | Deaths (n) | Median overall survival (Months) | Adjusted HR (95% CI)a | Adjusted P a |
| PTEN | |||||
| + | 22 | 9 | 24.6 | ref | |
| - | 45 | 24 | 19.3 | 2.85 (1.32-4.21) | < 0.001 |
| Ki67 | |||||
| + | 51 | 27 | 17.9 | ref | |
| - | 16 | 6 | 31.1 | 0.55 (0.32-0.88) | 0.021 |
HR: hazard ratio; CI: confidence interval; ref: reference aCox regression with adjustment for age, sex and stage.
DISCUSSION
PTEN-encoded phosphatases can dephosphorylate focal adhesion kinase by blocking the PI3K/protein kinase B pathway and downstream signaling, thereby regulating cell cycle, proliferation, infiltration and migration of cells[15]–[17]. Alterations in its activity, therefore, can effect changes in regulation of these cellular process and lead to tumorigenesis[18],[19]. In addition, the mutation rate of PTEN was found to be 43% in prostate cancer[4]. Here, we demonstrated that loss of PTEN expression was commonly found in lung cancer tissues, indicating that the deletion or mutation of PTEN may promote lung cancer development and/or progression. PTEN loss or reduced expression may lead to altered epithelium and excessive proliferation of lung tissues, thus forming tumors[19]. The expression of PTEN was less common in lung squamous carcinoma than other histological categories of lung tumor, such as adenocarcinoma or large cell cancer. Moreover, it also varied among differently differentiated lung tumors. In fact, poorer differentiation leads to lower expression rate of PTEN, indicating that loss of PTEN expression correlated with tumor histology and malignant differentiation degree. In addition, with advanced stage, PTEN expression level gradually declined, more patients developed lymph node metastases and 4-year survival rate gradually decreased. Therefore, PTEN expression may be useful as a prognostic biomarker, as the loss of expression indicates more advanced disease.
The proliferation marker Ki67 has been shown to be upregulated in many tumors[20]. Indeed, it is an indicator of poorer prognosis for some cancers[21],[22]. Therefore, this study extended previous work by assessing the relationship between Ki67 and malignant biological characteristics of NSCLC. Perhaps unsurprisingly, Ki67 expression was significantly more frequent in NSCLC than in normal adjacent lung tissues. These results indicated that Ki67 indirectly reflects undue proliferation of malignant tumors. Furthermore, Ki67 expression was also associated with lymph node metastasis, tumor histology and more advanced tumors. The 4-year survival of patients with Ki67-positive tumors was significantly lower than those with Ki67-negative tumors. This finding suggested that Ki67 expression in NSCLC is closely related to disease prognosis. The expression of Ki67 also varied among differently differentiated lung tumors. On the contrary, Ki67 expression in poorly differentiated tumor was significantly higher than in moderately or highly differentiated tumor, indicating that Ki67 expression closely correlated with tumor differentiation degree.
In conclusion, this study demonstrated that low PTEN expression and high Ki67 expression are associated with high proliferation activity, low differentiation of tumor tissues and high possibility of early invasion and metastasis. Additionally, the expression levels of both proteins are closely related to postoperative 4-year survival of NSCLC patients. Combined detection of PTEN and Ki67 can aid in determining malignancy degree and the prognosis of patients with NSCLC.
References
- 1.Lee S, Choi EJ, Jin C, Kim DH. Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA amplification contributes to cisplatin resistance in an ovarian cancer cell line. Gynecologic Oncology. 2005;97:26–34. doi: 10.1016/j.ygyno.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 2.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Neto JC, Ikoma MM, Carvalho KC, Vassallo J, De Brot M, Gobbi H, et al. MGMT and PTEN as potential prognostic markers in breast cancer. Exp Mol Pathol. 2012;92:20–6. doi: 10.1016/j.yexmp.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimoto M, Cutz JC, Nuin PA, Joshua AM, Bayani J, Evans AJ, et al. Interphase FISH analysis of PTEN in histologic of sections shows genomic deletions in 68% of primary prostate cancer and 23% high-grade prosraticintra-epithelial neoplasias. Cancer Genet Cytogenet. 2006;169:128–37. doi: 10.1016/j.cancergencyto.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Gomes CP, Andrade LA. PTEN and p53 expression in primary ovarian carcinomas: immunohistochemical study and discussion of pathogenetic mechanisms. Int J Gynecol Cancer. 2006;16:254–8. doi: 10.1111/j.1525-1438.2006.00306.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu SC, Sauter ER, Clapper ML, Feldman RS, Levin L, Chen SY, et al. Markers of cell proliferation in normal epithelia and dysplastic leukoplakias of the oral cavity. Cancer Epidemiol Biomarkers Prev. 1998;7:597–603. [PubMed] [Google Scholar]
- 7.lgarashi N, Takahashi M, Ohkubo H, Omata K, lida R, Fujimoto S. Predictive value of Ki67, p53 protein, and DNA content in the diagnosis of gastric carcinoma. Cancer. 1999;86:1449–54. doi: 10.1002/(sici)1097-0142(19991015)86:8<1449::aid-cncr10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Leonardo E, Volante M, Barbareschi M, Cavazza A, Dei Tos AP, Bussolati G, et al. Cell membrane reactivity of MIB-1 antibody to Ki67 in human tumors: fact or artifact. Appl Immunohistochem Mol Morphol. 2007;15:220–3. doi: 10.1097/01.pai.0000213122.66096.f0. [DOI] [PubMed] [Google Scholar]
- 9.Tian XX, Zhang YG, Du J, Fang WG, Ng HK, Zheng J. Effects of cotransfection of antisense-EGFR and wild-type PTEN cDNA on human glioblastoma cells. Neuropathology. 2006;26:178–87. doi: 10.1111/j.1440-1789.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 10.Ayoola A, Barochia A, Belani K, Belani CP. Primary and acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer: an update. Cancer Invest. 2012;30:433–6. doi: 10.3109/07357907.2012.666691. [DOI] [PubMed] [Google Scholar]
- 11.Antonarakis ES, Keizman D, Zhang Z, Gurel B, Lotan TL, Hicks JL, et al. An immunohistochemical signature comprising PTEN, MYC, and Ki67 predicts progression in prostate cancer patients receiving adjuvant docetaxel after prostatectomy. Cancer. 2012;118:6063–71. doi: 10.1002/cncr.27689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinross KM, Montgomery KG, Kleinschmidt M, Waring P, Ivetac I, Tikoo A, et al. An activating Pik3ca mutation coupled with Pten loss is sufficient to initiate ovarian tumorigenesis in mice. J Clin Invest. 2012;122:553–7. doi: 10.1172/JCI59309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Moles MA, Ruiz-Avila I, Gil-Montoya JA, Esteban F, Bravo M. Analysis of Ki-67 expression in oral squamous cell carcinoma: why Ki-67 is not a prognostic indicator. Oral Oncol. 2010;46:525–30. doi: 10.1016/j.oraloncology.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Dowsett M, Nielsen TO, A'hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: recommendations from the international Ki67 in breast cancer working group. J Nat Cancer Inst. 2011;103:1656–64. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, Griffith C, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11:951–8. [PubMed] [Google Scholar]
- 16.Van Rijn SJ, Grinwis GC, Penning LC, Meij BP. Expression of Ki-67, PCNA, and p27kip1 in canine pituitary corticotroph adenomas. Domest Anim Endocrin. 2010;38:244–52. doi: 10.1016/j.domaniend.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Wang X, Zhang J, Sun G, Luo H, Kang C, et al. MicroRNAs involved in the EGFR/PTEN/AKT pathway in gliomas. J Neuro-oncology. 2012;106:217–24. doi: 10.1007/s11060-011-0679-1. [DOI] [PubMed] [Google Scholar]
- 18.Ettl T, Baader K, Stiegler C, Agaimy A, Zenk J, Kühnel T, et al. Loss of PTEN is associated with elevated EGFR and HER2 expression and worse prognosis in salivary gland cancer. Brit J Cancer. 2012;106:719–26. doi: 10.1038/bjc.2011.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallardo A, Lerma E, Escuin D, Tibau A, Muñoz J, Ojeda B, et al. Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas. Brit J Cancer. 2012;106:1367–73. doi: 10.1038/bjc.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown DC, Gatter KC. Ki67 protein: the immaculate deception? Histopathol. 2002;40:2–11. doi: 10.1046/j.1365-2559.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y, Wang L, Zhang JP, Yang JY, Zhao ZM, Zhang XY. Expression of p53, c-erbB-2 and Ki67 in intestinal metaplasia and gastric carcinoma. World J Gastroenterol. 2010;16:339–44. doi: 10.3748/wjg.v16.i3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patil DT, Chou PM. Sialoblastoma: Utility of Ki-67 and p53 as a prognostic tool and review of literature. Pediatr Dev Pathol. 2010;13:32–8. doi: 10.2350/09-05-0650-OA.1. [DOI] [PubMed] [Google Scholar]


