Abstract
Hepatitis B vaccine has been administered in children and adults routinely to reduce the incidence of the disease. Even though, hepatitis B vaccine is considered as highly safe, some adverse reactions have been reported. A literature search was carried out in PubMed, accessed via the National Library of Medicine PubMed interface, searching used the following keywords: Hepatitis B vaccine and complications from 1980 to 2014. A total of 1147 articles were obtained out of which articles, which discuss the complications occurring orally or occurring elsewhere in the body, which have the potential to manifest orally after hepatitis B vaccination were selected. A total of 82 articles were identified which included 58 case series or case reports, 15 review articles, 4 cross sectional studies, 3 prospective cohort studies, one retrospective cohort study and a case control study. After reviewing the literature, we observed that complications seen after Hepatitis B vaccination are sudden infant death syndrome, multiple sclerosis, chronic fatigue syndrome, idiopathic thrombocytopenic purpura, vasculititis optic neuritis, anaphylaxis, systemic lupus erytymatosus, lichen planus and neuro-muscular disorder. Of these complications, some are manifested orally or have the potential to manifest orally. Although, most of the complications are self-limiting, some are very serious conditions, which require hospitalization with immediate medical attention.
Keywords: Complications, Hepatitis B vaccine, Oral, Vaccination
Introduction
According to World Health Organization (WHO) estimate, two billion people (one-third of the world population) have serologic evidence of past or present hepatitis B virus (HBV) infection and 360 million are chronic carriers and at risk of liver disease.[1,2] Approximately, 620,000 deaths occur every year from acute and chronic squealae secondary to hepatitis B and 4.5 million new cases of hepatitis B are reported each year worldwide.[3] Chronic hepatitis B has been identified as one of the most common causes of liver failure and hepatocellular carcinoma.[4]
Hepatitis B virus is spread by blood-to-blood contact, unprotected sexual contact with multiple partners, viral exposure during medical procedures such as dialysis and surgeries, accidental exposure such as needle stick injuries and vertical transmission from mother to child are the common routes of infection with both HBV. HBV is carried in the blood, and various body fluids, such as saliva, menstrual and vaginal discharges, seminal fluid, serous exudates, and various body fluids contaminated with blood.
Previous studies had evaluated the presence and transmission of HBV through saliva and gingival crevicular fluid, which emphasizes the risk of transmission of these viruses to dentists and dental health care workers.[5,6] Vice versa, dentists can infect their patients by HBV if adequate infection control policies are not applied.[7] As evidence, there are 9 reports of infected dentists and oral surgeons who transmitted the virus to their patients during dental procedures during 1974 and 1982.[8] It has also been seen that HBV virus can persist in the environment and last for 1 day.[9]
Method of Collection of Data
A literature search was done in PubMed, accessed via the National Library of Medicine PubMed interface (http://www.ncbi.nlm.nih.gov/pubmed), using the following keywords: Hepatitis B vaccine and complications from 1980 to 2014. A total of 1147 articles were obtained. These articles were obtained, and from their bibliographies, pertinent secondary references were also identified and acquired. We also used the “Related Articles” feature of PubMed to identify further references of interest within the primary search. Articles that discuss the complications occurring orally or occurring elsewhere in the body that have the potential to manifest orally after hepatitis B vaccination were selected. Based on these criteria, 82 articles were identified and included in this review. These articles included 58 case series or case reports, 15 review articles, 4 cross-sectional studies, 3 prospective cohort studies, one retrospective cohort study and case-control study.
Hepatitis B vaccine
Development of hepatitis B vaccine in 1982 has been a landmark progress in the prevention of this dreadful disease. Vaccination is the most effective measure to reduce the incidence of hepatitis B. In 1991, the WHO recommended the integration of universal hepatitis B vaccination by 1997 to prevent and control on a global scale HBV infection and its long-term, serious sequel.[10] Hepatitis B vaccine is administered in children in around 150 countries and has been routinely included in infant or adolescent immunization.
Several hundred million doses of hepatitis B vaccine have been administered worldwide with an excellent record of safety and efficacy. The site of injection and mode of administration are critical factors in achieving an optimal response. The vaccine should be given intramuscularly into the deltoid region in children (≥1 year of age) and adults or into the anterolateral thigh in newborns and infants (<1 year of age).[3] The result of effective implementation of hepatitis B vaccination has resulted in the reduced incidence of acute hepatitis infection, carrier state and also hepatitis related mortality.[3,11] A significant reduction of the incidence was reported after vaccination in highly endemic areas such as Taiwan, Hawai, etc.[12,13]
Even though hepatitis B vaccine is considered highly safe, rarely there have been contradicting case reports highlighting the adverse effect of this vaccine on certain individuals. Although, most of the dental practitioners are aware of the risks posed by hepatitis B in dental practice, not many are aware of the complications following hepatitis B vaccination. The aim of this paper is to discuss the possible complications associated with hepatitis B vaccinations with a special emphasis on the complications that is seen or has the potential to manifest orally.
Complications seen after hepatitis B vaccination
Serious disorders, which are alleged to be resulted or associated with hepatitis B vaccination are:
Sudden infant death syndrome
This allegation was made by Margolis et al. in 1999, though this doesn’t have much credibility.[14]
Chronic fatigue syndrome
Chronic fatigue syndrome (CFS) is described as a prolonged persistent or relapsing fatigue. The etiologies of CFS are similar to infection.[15] A retrospective Canadian study found many patients who reported with CFS had undergone a hepatitis B vaccination.[16]
Multiple sclerosis
Multiple sclerosis is a chronic inflammatory demyelinating disorder of the central nervous system. Between 1996 and 1997 concerns were raised that hepatitis B immunization may be linked to new cases or flare-ups of multiple sclerosis or other demyelinating diseases, following a report of primary demyelinating events within 8–10 weeks of immunization against hepatitis B using a recombinant vaccine at a hospital in Paris.[16]
Thrombocytopenic purpura
Thrombocytopenic purpura occurring after hepatitis B vaccination was first reported in 1994 by Poullin and Gabriel.[17]
Vasculitis
Vasculitis is a disorder that destroys blood vessels by inflammation where both arteries and veins are affected. Some patients have developed vasculitis after hepatitis B vaccination. Vasculitis after hepatitis B vaccination was first reported by Allen et al. in 1993.[18] Clinical manifestations, include polyarthritis, pain in the cervical column, maculopapular rash, Raynauds phenomenon and fever.[19] In another case report, two women developed large artery vasculitis shortly after receiving hepatitis B vaccine, which resulted in renal failure.[20]
Rheumatic arthritis
Rheumatic arthritis is a chronic inflammatory disease that principally affects the joints. Rheumatic arthritis, followed by hepatitis B vaccination was first reported by Vautier and Carty[21] Maillefert et al. have reported of six women who developed rheumatic arthritis following hepatitis B vaccination. They also reported the occurrence of arthritis, arthralgia and myalgia after the vaccination.[19]
Optic neuritis
Optic neuritis is a multi-etiological condition consisting of the inflammation of the optic nerve that may cause a complete or partial loss of vision. A case of optic neuritis after hepatitis B vaccination was reported by Albitar et al.[22]
Vaccine related anaphylaxis
Anaphylaxis after hepatitis B vaccination was first reported by Lear et al. in 1995.[23]
Minor adverse reactions seen after hepatitis B vaccination include minor symptoms at the site of injection, malaise, headache, nausea, rash, influenza such as symptoms, dizziness, arthralgia, lichen planus (LP), lupus erythematosus, urticaria, parasthesia and drowsiness and neuromuscular disorders.[24,25]
Oral manifestations of hepatitis B vaccination
The complications after hepatitis B vaccination, which may be observed intra-orally are discussed below.
Lichen planus
Lichen planus is a chronic inflammatory mucocutaneous disease, which affects the stratified squamous epithelium exclusively and frequently involves the oral and genital mucosa, skin, nails and scalp. The etiology of LP is unknown. Predisposing factors for this condition are anxiety, diabetes, autoimmune diseases, intestinal diseases, drugs, stress, hypertension, infection dental materials, neoplasms, and genetic predisposition.
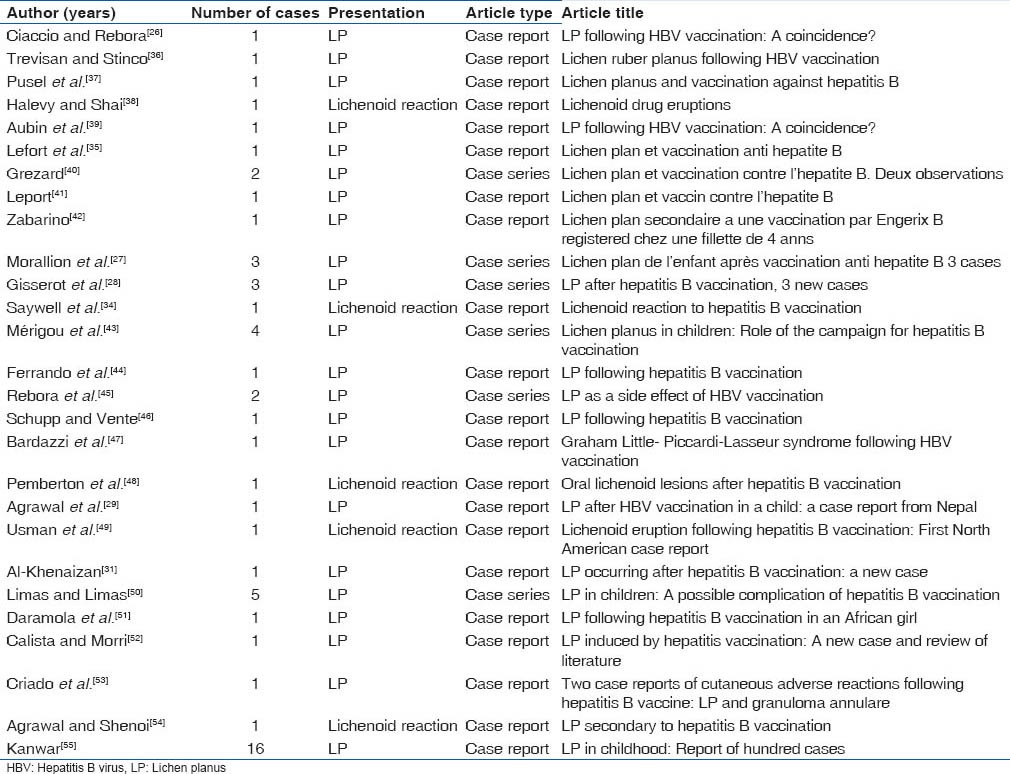
Incidence of LP after hepatitis B vaccination was first reported by Ciaccio et al. in 1990.[26] Fiftycases of LP have been reported after hepatitis B vaccination. Some cases are seen as early as 3 days after vaccination, and some are seen as late as 120 days after vaccination. Although, most of the LP cases had presented with skin involvement, some cases had mucosal involvement, including the oral mucosa.[27,28,29] The clinical signs and symptoms and management of LP manifested in the oral cavity are mentioned in Table 1.
Table 1.
Clinical features and management of lichen planus after hepatitis B vaccination

In a multicenter case study done by gruppo italiano studi epidemiologici in dermatologia on 577 newly diagnosed LP cases, it was seen that hepatitis B surface antigen (HBsAg) patients of any age and sex had double the risk of developing LP compared with HBsAg negative patients.[30] Al-Khenaizan et al. made three conclusions regarding the appearance of LP after hepatitis B vaccination.[31]
Association is a rare event
Lichen planus can occur irrespective of the type of vaccine
Latent period for the appearance of eruption ranges from a few days to 3 months after any of the three doses recommended.
Lichen planus is probably caused by a T-cell mediated immunological reaction to an induced antigenic change in the skin or mucosa in predisposed patients. A key early event in LP is the genetically induced increased production of TH1 cytokines.[32] HBsAg plays a central role in LP secondary to hepatitis B vaccination. It is assumed that LP is caused by a sensitizing protein S, which has epitopes common to keratinocytes and to the protein component of different vaccines. Hence, it is likely that the immune system recognizes an epitope on keratinocytes that is similar or identical to protein S of the virus and thus stimulates cytotoxic T lymphocytes to induce apoptosis of disturbed keratinocytes.[33] LP after hepatitis B vaccination was managed with oral steroids and oral histamines.[29]
Lichenoid reaction
Saywell et al. reported a case where a 16-year-old Caucasian male developed pruritic eruption 8 weeks after vaccination.[34] Macules were seen over limbs and trunk with no mucosal involvement. Skin biopsy confirmed it to be a lichenoid reaction. Lichenoid like reaction was also reported by Lefort et al.[35] Protein S fraction of HBsAg is believed to play a role in the pathogenesis of lichenoid reaction. Cases reported with either LP or lichenoid reaction after hepatitis B vaccinations are given in Table 2.[26,27,28,29,31,35,36,37,38,39,40,1,42,43,44,45,46,47,48,49,50,51,52,53,54,55]
Table 2.
LP or lichenoid reaction following hepatitis B vaccination
Idiopathic thrombocytopenic purpura
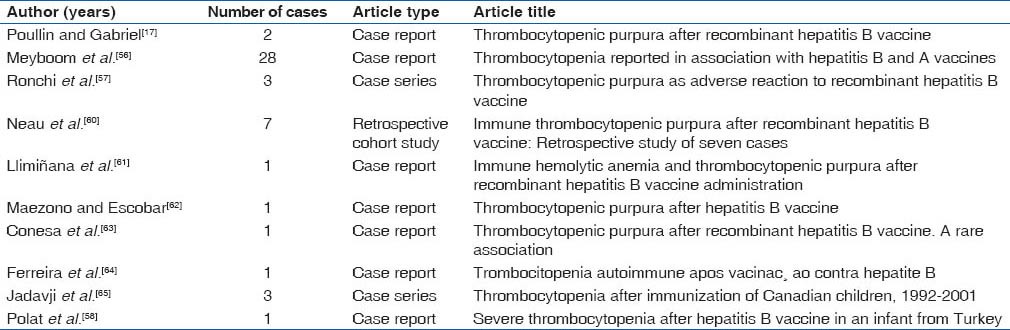
Incidence of idiopathic thrombocytopenic purpura after hepatitis B vaccination was first reported by Poullin and Gabriel in 1994 and then by Meyboom et al. in 1999.[17,56] Forty eight cases of thrombocytopenic purpura after hepatitis B vaccination were published in the literature; all have been cured with some cases showing signs of recurrence. Thrombocytopenia was seen after every dosage of vaccination and it seems to start after 11 days to 3 months of vaccination.
Patients may present with petechiae, ecchymosis and splenomegaly. Intraorally, some patients manifested with wet petechiae.[57,58] Hepatitis B vaccine contains yeast, aluminum, thimerosal and HBsAg epitopes, which may trigger an autoimmune reaction resulting in idiopathic thrombocytopenic purpura.[59] Most of the cases do not require any treatment, while some patients responded with corticosteroid therapy. The complete reversibility of these cases reveals the benign nature of this complication.[58] Cases of idiopathic thrombocytopenic purpura reported after hepatitis B vaccination have been listed in Table 3.[17,56,56,57,58,60,61,62,63,64,65]
Table 3.
Idiopathic thrombocytopenic purpura after hepatitis B vaccination
Systemic lupus erythematosus
Systemic lupus erythematosus is an autoimmune disease of unknown etiology. The administration of hepatitis B vaccine has been found to be associated with the onset of systemic lupus erythematosus.[66]
Tudela et al. reported a 43-year-old woman with a manifestation of systemic lupus erythematosus, 2 weeks after administration of hepatitis B vaccine.[67] The anti-nuclear antibodies and anti DNA antibodies were positive. No oral manifestation was reported in this case. In another case report by Guiserix et al., a 26 year old female was reported with systemic lupus eythematosus 1 week after receiving hepatitis B vaccination.[68] Although the patient manifested with cutaneous and mucocutaneous eruptions, no intra oral change was reported. Another case of systemic lupus erythematosus was reported by Maillefert et al. in 1999.[19]
It is believed that, thimerosal or aluminum hydroxide or Hb surface protein present in the vaccine may induce an immune reaction, which leads to systemic lupus erythematosus.[68] Tudela et al.,[67] recommended treatment of lupus erythematosus with prednisone and cyclophosphamide.
Neuromuscular disorders
Certain neuromuscular disorders have been reported after hepatitis B vaccination. Case reports include sensory nerve neuropathy, vestibulocochlear neuropathy, and precipitation of hereditary motor sensor neuropathy.[69] Maillefert et al. reported a case where a patient manifested with mental nerve neuropathy following hepatitis B vaccination. This patient also manifested with pain in the cervical column and arthralgia.[70]
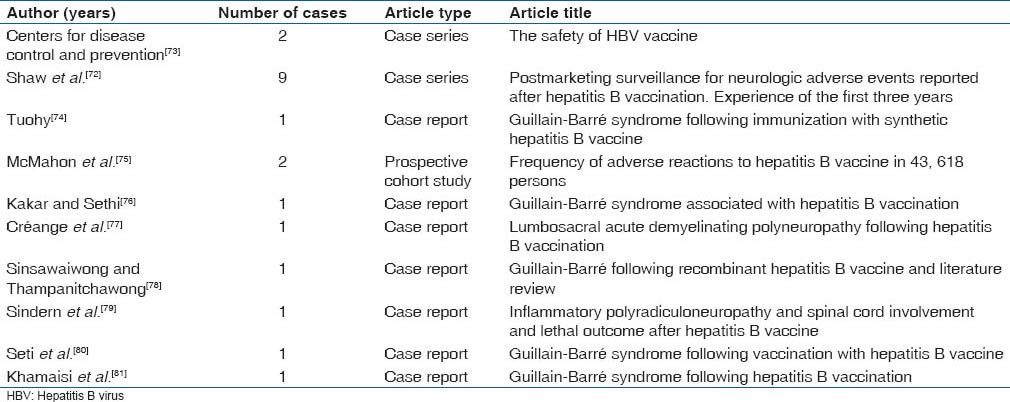
Guillain-Barre syndrome is an acute polyneuropathy affecting the peripheral nervous system which is possibly caused by an auto immune response to foreign agents. The disorder is characterized by a symmetrical weakness that affects the lower limbs first and progresses in an ascending fashion. Lower cranial nerves may be affected leading to oropharyngeal dysphagia and respiratory difficulties. Patient may also have pain, trouble speaking and bilateral weakness of facial muscles.[71] Some cases of Bell's palsy have also been reported after hepatitis B vaccination.[72] Twenty cases of polyradiculo-neuropathy and Guillain-Barre syndrome have been reported in literature after hepatitis B vaccination. These are listed in Table 4.[72,73,74,75,76,77,78,79,80,81] Apart from these disorders, hepatitis B vaccination has also been associated with myasthenia gravis, polyarteritis nodosa and myopathy.[69] Patients with Guillain-Barre syndrome most often require hospitalization.
Table 4.
Guillain-Barre syndrome reported after hepatitis B vaccination
Incidence of Bell's palsy has been reported after hepatitis B vaccination.[72,82,83] Bell's palsy is a sudden onset of unilateral temporary paralysis of facial muscles, resulting from seventh cranial nerve dysfunction. The etiology and pathogenesis of Bell's palsy remains unclear. There is a concern that reactivation of latent herpes simplex virus-associated infections of the geniculate ganglia of facial nerves may be one of the causes of Bell's palsy. Auto-immune mechanism has also been proposed for the cause of Bell's palsy. It has been hypothesized that an immunomediated segmental demyelination may be involved.[82]
Conclusion
Hepatitis B vaccination has reduced the incidence of hepatitis B. Over a thousand million doses of hepatitis B vaccine have been administered already and rarely a few adverse effects were reported after vaccination. But we have to remember that all medical procedures including vaccination have a risk of side effects.
As some complications after hepatitis B vaccinations are manifested intra orally, it is necessary for dentists to know about these adverse reactions. According to our knowledge, this is the first attempt ever made to review the complications appearing orally after hepatitis B vaccination. Dentists need to keep in mind the possibility of hepatitis B vaccination as a possible etiology if a patient presents with LP, systemic lupus erythematosus, idiopathic thrombocytopenic purpura and neuro muscular disorders. A randomized control clinical trial with a large sample size should be undertaken in the future to substantiate the findings in these case reports.
Doctors should be careful in advising hepatitis B vaccination for a patient who is already manifesting an autoimmune disease. Informed consent should be obtained from the patient before hepatitis B vaccination. Most of the dental practitioners and assistants who themselves undergo hepatitis B vaccination should also be aware of these complications. Although, most of the complications are self-limiting, some are very serious conditions, which require hospitalization with immediate medical attention. Benefits provided by the hepatitis B vaccination far outweigh its adverse reactions because hepatitis B is a serious disease, which can even lead to chronic liver failure and hepatocellular carcinoma.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.World Health Organization. Geneva, Switzerland: World Health Organization; 2000. [Last accessed on 2013 Dec 11]. Hepatitis B.(Fact sheet no. 204) Available from: http://www.who.int/mediacentre/factsheets/fs204/en/index.html . [Google Scholar]
- 2.World Health Organization. Hepatitis B vaccines. Wkly Epidemiol Rec. 2004;79:255–63. [PubMed] [Google Scholar]
- 3.Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: A historical overview. Vaccine. 2008;26:6266–73. doi: 10.1016/j.vaccine.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 4.Lehman EM, Wilson ML. Epidemiology of hepatitis viruses among hepatocellular carcinoma cases and healthy people in Egypt: A systematic review and meta-analysis. Int J Cancer. 2009;124:690–7. doi: 10.1002/ijc.23937. [DOI] [PubMed] [Google Scholar]
- 5.Mahboobi N, Porter SR, Karayiannis P, Alavian SM. Oral fluid and hepatitis A, B and C: A literature review. J Oral Pathol Med. 2012;41:505–16. doi: 10.1111/j.1600-0714.2011.01123.x. [DOI] [PubMed] [Google Scholar]
- 6.Mahboobi N, Porter SR, Karayiannis P, Alavian SM. Dental Treatment as a risk factor for hepatitis B and C viral infection. A review of the recent literature. J Gastrointestin Liver Dis. 2013;22:79–86. [PubMed] [Google Scholar]
- 7.Mahboobi N, Agha-Hosseini F, Mahboobi N, Safari S, Lavanchy D, Alavian SM. Hepatitis B virus infection in dentistry: A forgotten topic. J Viral Hepat. 2010;17:307–16. doi: 10.1111/j.1365-2893.2010.01284.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahtone J, Goodman RA. Hepatitis B and dental personnel: Transmission to patients and prevention issues. J Am Dent Assoc. 1983;106:219–22. doi: 10.14219/jada.archive.1983.0416. [DOI] [PubMed] [Google Scholar]
- 9.Gillcrist JA. Hepatitis viruses A, B, C, D, E and G: Implications for dental personnel. J Am Dent Assoc. 1999;130:509–20. doi: 10.14219/jada.archive.1999.0245. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. WHO expanded programme on immunisation. Global advisory group. Weekly Epidemiol Rec. 1992;3:6–11. [Google Scholar]
- 11.Coursaget P, Leboulleux D, Soumare M, le Cann P, Yvonnet B, Chiron JP, et al. Twelve-year follow-up study of hepatitis B immunization of Senegalese infants. J Hepatol. 1994;21:250–4. doi: 10.1016/s0168-8278(05)80404-0. [DOI] [PubMed] [Google Scholar]
- 12.Antwerp: EUROHEP.NET; 2004. [Last accessed on Nov 2013 10]. EUROHEP.NET. Data on surveillance and prevention of hepatitis A and B in 22 countries, 1990–2001. Available from: http://www.eurohep.net . [Google Scholar]
- 13.Perz JF, Elm JL, Jr, Fiore AE, Huggler JI, Kuhnert WL, Effler PV. Near elimination of hepatitis B virus infections among Hawaii elementary school children after universal infant hepatitis B vaccination. Pediatrics. 2006;118:1403–8. doi: 10.1542/peds.2006-0724. [DOI] [PubMed] [Google Scholar]
- 14.Margolis HS. Testimony before the US house of representatives committee on government reform subcommittee on criminal justice, drug policy and human resources. 1999. [Last accessed on 2013 Nov 26]. Available from: http://www.cdc.gov/ncidod/diseases/hepatitis .
- 15.Steele L, Dobbins JG, Fukuda K, Reyes M, Randall B, Koppelman M, et al. The epidemiology of chronic fatigue in San Francisco. Am J Med. 1998;105:83S–90. doi: 10.1016/s0002-9343(98)00158-2. [DOI] [PubMed] [Google Scholar]
- 16.Tourbah A, Gout O, Liblau R, Lyon-Caen O, Bougniot C, Iba-Zizen MT, et al. Encephalitis after hepatitis B vaccination: Recurrent disseminated encephalitis or MS? Neurology. 1999;53:396–401. doi: 10.1212/wnl.53.2.396. [DOI] [PubMed] [Google Scholar]
- 17.Poullin P, Gabriel B. Thrombocytopenic purpura after recombinant hepatitis B vaccine. Lancet. 1994;344:1293. doi: 10.1016/s0140-6736(94)90777-3. [DOI] [PubMed] [Google Scholar]
- 18.Allen MB, Cockwell P, Page RL. Pulmonary and cutaneous vasculitis following hepatitis B vaccination. Thorax. 1993;48:580–1. doi: 10.1136/thx.48.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maillefert JF, Sibilia J, Toussirot E, Vignon E, Eschard JP, Lorcerie B, et al. Rheumatic disorders developed after hepatitis B vaccination. Rheumatology. 1999;38:978–83. doi: 10.1093/rheumatology/38.10.978. [DOI] [PubMed] [Google Scholar]
- 20.Zaas A, Scheel P, Venbrux A, Hellmann DB. Large artery vasculitis following recombinant hepatitis B vaccination: 2 cases. J Rheumatol. 2001;28:1116–20. [PubMed] [Google Scholar]
- 21.Vautier G, Carty JE. Acute sero-positive rheumatoid arthritis occurring after hepatitis vaccination. Br J Rheumatol. 1994;33:991. doi: 10.1093/rheumatology/33.10.991-a. [DOI] [PubMed] [Google Scholar]
- 22.Albitar S, Bourgeon B, Genin R, Fen-Chong M, N’Guyen P, Serveaux MO, et al. Bilateral retrobulbar optic neuritis with hepatitis B vaccination. Nephrol Dial Transplant. 1997;12:2169–70. doi: 10.1093/ndt/12.10.2169. [DOI] [PubMed] [Google Scholar]
- 23.Lear JT, English JS. Anaphylaxis after hepatitis B vaccination. Lancet. 1995;345:1249. doi: 10.1016/s0140-6736(95)92039-0. [DOI] [PubMed] [Google Scholar]
- 24.Report of the working group on the possible relationship between hepatitis B vaccination and the chronic fatigue syndrome. Can Med Assoc J. 1993;149:314–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Zuckerman JN. Protective efficacy, immunotherapeutic potential, and safety of hepatitis B vaccines. J Med Virol. 2006;78:169–77. doi: 10.1002/jmv.20524. [DOI] [PubMed] [Google Scholar]
- 26.Ciaccio M, Rebora A. Lichen planus following HBV vaccination: A coincidence? Br J Dermatol. 1990;122:424. doi: 10.1111/j.1365-2133.1990.tb08294.x. [DOI] [PubMed] [Google Scholar]
- 27.Moraillon I, Merle F, Laglenne S, Vignon MD, Prigent F, Bourrillon A, et al. Lichen plan de l’enfant après vaccination anti-hepatite B. 3 cas [abstract] Ann Dermatol Venerol. 1996;123(suppl 1):S63. in French. [Google Scholar]
- 28.Gisserot O, Carsuzaa F, Marlier S, Morand JJ, Marrot E. Lichen planus after hepatitis B vaccination 3 new cases. Presse Med. 1997;26:760. [PubMed] [Google Scholar]
- 29.Agrawal S, Garg VK, Joshi A, Agarwalla A, Sah SP. Lichen planus after HBV vaccination in a child: A case report from Nepal. J Dermatol. 2000;27:618–20. doi: 10.1111/j.1346-8138.2000.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 30.Lichen planus and liver diseases: A multicentre case. control study. Gruppo Italiano Studi Epidemiologici in Dermatologia (GISED) BMJ. 1990;300:227–30. doi: 10.1136/bmj.300.6719.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Khenaizan S. Lichen planus occurring after hepatitis B vaccination: A new case. J Am Acad Dermatol. 2001;45:614–5. doi: 10.1067/mjd.2001.114590. [DOI] [PubMed] [Google Scholar]
- 32.Carrozzo M, Uboldi de Capei M, Dametto E, Fasano ME, Arduino P, Broccoletti R, et al. Tumor necrosis factor-alpha and interferon-gamma polymorphisms contribute to susceptibility to oral lichen planus. J Invest Dermatol. 2004;122:87–94. doi: 10.1046/j.0022-202X.2003.22108.x. [DOI] [PubMed] [Google Scholar]
- 33.Drago F, Rebora A. Cutaneous immunologic reactions to hepatitis B virus vaccine. Ann Intern Med. 2002;136:780. doi: 10.7326/0003-4819-136-10-200205210-00019. [DOI] [PubMed] [Google Scholar]
- 34.Saywell CA, Wittal RA, Kossard S. Lichenoid reaction to hepatitis B vaccination. Australas J Dermatol. 1997;38:152–4. doi: 10.1111/j.1440-0960.1997.tb01134.x. [DOI] [PubMed] [Google Scholar]
- 35.Lefort A, Dachary D, Vergier B, Boiron G. Lichen planus and vaccination against hepatitis B. Ann Dermatol Venereol. 1995;122:701–3. [PubMed] [Google Scholar]
- 36.Trevisan G, Stinco G. Lichen ruber planus following HBV vaccination. Acta Derm Venereol. 1993;73:73. doi: 10.2340/000155557373. [DOI] [PubMed] [Google Scholar]
- 37.Pusel B, Will F, Grosshans E. Lichen plan et vaccination contre l’hepatite B. Nouv Dermatol. 1993;12:709–10. in French. [Google Scholar]
- 38.Halevy S, Shai A. Lichenoid drug eruptions. J Am Acad Dermatol. 1993;29:249–55. doi: 10.1016/0190-9622(93)70176-t. [DOI] [PubMed] [Google Scholar]
- 39.Aubin F, Angonin R, Humbert P, Agache P. Lichen planus following hepatitis B vaccination. Arch Dermatol. 1994;130:1329–30. doi: 10.1001/archderm.130.10.1329. [DOI] [PubMed] [Google Scholar]
- 40.Grezard P, Philippot V, Perrot H. Lichen plan et vaccination contre l’hepatite B. Deux observations. Nouv Dermatol. 1995;14:444–5. in French. [Google Scholar]
- 41.Leport Y. Lichen plan et vaccin contre l’hepatite B. Nouv Dermatol. 1995;14:594. in French. [Google Scholar]
- 42.Zabarino P. Lichen plan secondaire a une vaccination par Engerix B registered chez une fillette de 4 anns. Nouv Dermatol. 1996;15:487. in French. [Google Scholar]
- 43.Mérigou D, Léauté-Labrèze C, Louvet S, Bioulac-Sage P, Taïeb A. Lichen planus in children: Role of the campaign for hepatitis B vaccination. Ann Dermatol Venereol. 1998;125:399–403. [PubMed] [Google Scholar]
- 44.Ferrando MF, Doutre MS, Beylot-Barry M, Durand I, Beylot C. Lichen planus following hepatitis B vaccination. Br J Dermatol. 1998;139:350. doi: 10.1046/j.1365-2133.1998.02386.x. [DOI] [PubMed] [Google Scholar]
- 45.Rebora A, Rongioletti F, Drago F, Parodi Lichen planus as a side effect of HBV vaccination. Dermatology. 1999;198:1–2. doi: 10.1159/000018054. [DOI] [PubMed] [Google Scholar]
- 46.Schupp P, Vente C. Lichen planus following hepatitis B vaccination. Int J Dermatol. 1999;38:799–800. [PubMed] [Google Scholar]
- 47.Bardazzi F, Landi C, Orlandi C, Neri I, Varotti C. Graham Little-Piccardi-Lasseur syndrome following HBV vaccination. Acta Derm Venereol. 1999;79:93. doi: 10.1080/000155599750011886. [DOI] [PubMed] [Google Scholar]
- 48.Pemberton MN, Sloan P, Thakker NS. Oral lichenoid lesions after hepatitis B vaccination. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:717–9. doi: 10.1067/moe.2000.104541. [DOI] [PubMed] [Google Scholar]
- 49.Usman A, Kimyai-Asadi A, Stiller MJ, Alam M. Lichenoid eruption following hepatitis B vaccination: First North American case report. Pediatr Dermatol. 2001;18:123–6. doi: 10.1046/j.1525-1470.2001.018002123.x. [DOI] [PubMed] [Google Scholar]
- 50.Limas C, Limas CJ. Lichen planus in children: A possible complication of hepatitis B vaccines. Pediatr Dermatol. 2002;19:204–9. doi: 10.1046/j.1525-1470.2002.00079.x. [DOI] [PubMed] [Google Scholar]
- 51.Daramola OO, Ogunbiyi AO, George AO. Lichen planus following hepatitis B vaccination in an African girl. Trop Doct. 2002;32:117–8. doi: 10.1177/004947550203200227. [DOI] [PubMed] [Google Scholar]
- 52.Calista D, Morri M. Lichen planus induced by hepatitis B vaccination: A new case and review of the literature. Int J Dermatol. 2004;43:562–4. doi: 10.1111/j.1365-4632.2004.01740.x. [DOI] [PubMed] [Google Scholar]
- 53.Criado PR, de Oliveira Ramos R, Vasconcellos C, Jardim Criado RF, Valente NY. Two case reports of cutaneous adverse reactions following hepatitis B vaccine: Lichen planus and granuloma annulare. J Eur Acad Dermatol Venereol. 2004;18:603–6. doi: 10.1111/j.1468-3083.2004.00989.x. [DOI] [PubMed] [Google Scholar]
- 54.Agrawal A, Shenoi SD. Lichen planus secondary to hepatitis B vaccination. Indian J Dermatol Venereol Leprol. 2004;70:234–5. [PubMed] [Google Scholar]
- 55.Kanwar AJ, De D. Lichen planus in childhood: Report of hundred cases. Int J Dermatol. 2004;43:562–4. [Google Scholar]
- 56.Meyboom RH, Fucik H, Edwards IR. Thrombocytopenia reported in association with hepatitis B and A vaccines. Lancet. 1995;345:1638. doi: 10.1016/s0140-6736(95)90143-4. [DOI] [PubMed] [Google Scholar]
- 57.Ronchi F, Cecchi P, Falcioni F, Marsciani A, Minak G, Muratori G, et al. Thrombocytopenic purpura as adverse reaction to recombinant hepatitis B vaccine. Arch Dis Child. 1998;78:273–4. doi: 10.1136/adc.78.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polat A, Akca H, Dagdeviren E. Severe thrombocytopenia after hepatitis B vaccine in an infant from Turkey. Vaccine. 2008;26:6495–6. doi: 10.1016/j.vaccine.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 59.Geier MR, Geier DA, Zahalsky AC. A review of hepatitis B vaccination. Expert Opin Drug Saf. 2003;2:113–22. doi: 10.1517/14740338.2.2.113. [DOI] [PubMed] [Google Scholar]
- 60.Neau D, Bonnet F, Michaud M, Perel Y, Longy-Boursier M, Ragnaud JM, et al. Immune thrombocytopenic purpura after recombinant hepatitis B vaccine: Retrospective study of seven cases. Scand J Infect Dis. 1998;30:115–8. doi: 10.1080/003655498750003465. [DOI] [PubMed] [Google Scholar]
- 61.Llimiñana C, Soler JA, Melo M, Roig I. Hemolytic anemia and thrombocytopenic purpura after the administration of the recombinant hepatitis B vaccine. Med Clin (Barc) 1999;113:39. [PubMed] [Google Scholar]
- 62.Maezono R, Escobar AM. Thrombocytopenic purpura after hepatitis B vaccine. J Pediatr (Rio J) 2000;76:395–8. doi: 10.2223/jped.81. article in Portuguese. [DOI] [PubMed] [Google Scholar]
- 63.Conesa V, Nuñez Mf, Navarro JF, Mompel A, Ruiz J, Gómez A. Thrombocytopenic purpura after recombinant hepatitis B vaccine. A rare association. Haematologica. 2001;86:E09. [PubMed] [Google Scholar]
- 64.Ferreira GC, Cruz N, Santos NF, Silva TJ, Fischer MG. Trombocitopenia autoimmune apos vacinac¸ ao contra hepatite B. Pediatria Atual. 2002;15:16–21. article in Portuguese. [Google Scholar]
- 65.Jadavji T, Scheifele D, Halperin S. Canadian Paediatric Society/Health Cananda Immunization Monitoring Program. Thrombocytopenia after immunization of Canadian children, 1992 to 2001. Pediatr Infect Dis J. 2003;22:119–22. doi: 10.1097/01.inf.0000048961.08486.d1. [DOI] [PubMed] [Google Scholar]
- 66.Ayvazian LF, Badger TL. Disseminated lupus erythematosus occurring among student nurses. N Engl J Med. 1948;239:565–70. doi: 10.1056/NEJM194810142391601. [DOI] [PubMed] [Google Scholar]
- 67.Tudela P, Martí S, Bonal J. Systemic lupus erythematosus and vaccination against hepatitis B. Nephron. 1992;62:236. doi: 10.1159/000187043. [DOI] [PubMed] [Google Scholar]
- 68.Guiserix J. Systemic lupus erythematosus following hepatitis B vaccine. Nephron. 1996;74:441. doi: 10.1159/000189357. [DOI] [PubMed] [Google Scholar]
- 69.Stubgen JP. Neuro muscular diseases associated with hepatitis B vaccination. J Neurol Sci. 2010;292:1–4. doi: 10.1016/j.jns.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 70.Maillefert JF, Farge P, Gazet-Maillefert MP, Tavernier C. Mental nerve neuropathy as a result of hepatitis B vaccination. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:663–4. doi: 10.1016/s1079-2104(97)90315-2. [DOI] [PubMed] [Google Scholar]
- 71.van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. 2008;7:939–50. doi: 10.1016/S1474-4422(08)70215-1. [DOI] [PubMed] [Google Scholar]
- 72.Shaw FE, Jr, Graham DJ, Guess HA, Milstien JB, Johnson JM, Schatz GC, et al. Postmarketing surveillance for neurologic adverse events reported after hepatitis B vaccination. Experience of the first three years. Am J Epidemiol. 1988;127:337–52. doi: 10.1093/oxfordjournals.aje.a114808. [DOI] [PubMed] [Google Scholar]
- 73.Centers for Disease Control and Prevention. The safety of hepatitis B virus vaccine. Morb Mortal Wkly Rep. 1983;32:134–6. [PubMed] [Google Scholar]
- 74.Tuohy PG. Guillain-Barré syndrome following immunization with synthetic hepatitis B vaccine. N Z Med J. 1989;102:114–5. [PubMed] [Google Scholar]
- 75.McMahon BJ, Helminiak C, Wainwright RB, Bulkow L, Trimble BA, Wainwright K. Frequency of adverse reactions to hepatitis B vaccine in 43,618 persons. Am J Med. 1992;92:254–6. doi: 10.1016/0002-9343(92)90073-k. [DOI] [PubMed] [Google Scholar]
- 76.Kakar A, Sethi PK. Guillain Barre syndrome associated with hepatitis B vaccination. Indian J Pediatr. 1997;64:710–2. doi: 10.1007/BF02726131. [DOI] [PubMed] [Google Scholar]
- 77.Créange A, Temam G, Lefaucheur JP. Lumbosacral acute demyelinating polyneuropathy following hepatitis B vaccination. Autoimmunity. 1999;30:143–6. doi: 10.3109/08916939908993848. [DOI] [PubMed] [Google Scholar]
- 78.Sinsawaiwong S, Thampanitchawong P. Guillain – Barre’ syndrome following recombinant hepatitis B vaccine and literature review. J Med Assoc Thai. 2000;83:1124–6. [PubMed] [Google Scholar]
- 79.Sindern E, Schröder JM, Krismann M, Malin JP. Inflammatory polyradiculoneuropathy with spinal cord involvement and lethal [correction of letal] outcome after hepatitis B vaccination. J Neurol Sci. 2001;186:81–5. doi: 10.1016/s0022-510x(01)00510-x. [DOI] [PubMed] [Google Scholar]
- 80.Seti NK, Reddi R, Anand I, Sethi PK. Gulliane Barre syndrome following vaccination with hepatitis B vaccine. J Assoc Physicians India. 2002;50:989. [PubMed] [Google Scholar]
- 81.Khamaisi M, Shoenfeld Y, Orbach H. Guillain-Barré syndrome following hepatitis B vaccination. Clin Exp Rheumatol. 2004;22:767–70. [PubMed] [Google Scholar]
- 82.Alp H, Tan H, Orbak Z. Bell's palsy as a possible complication of hepatitis B vaccination in a child. J Health Popul Nutr. 2009;27:707–8. doi: 10.3329/jhpn.v27i5.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paul R, Stassen LF. Transient facial nerve paralysis (Bell's palsy) following administration of hepatitis B recombinant vaccine: A case report. Br Dent J. 2014;216:69–71. doi: 10.1038/sj.bdj.2014.1. [DOI] [PubMed] [Google Scholar]


