Abstract
Background:
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement is a set of recommendations about what should be included in a more accurate and complete description of observational studies.
Aim:
The aim was to assess the quality of reporting of cross-sectional studies by evaluating the extent to which they adhere to the STROBE statement.
Materials and Methods:
This study has a cross-sectional design. All the articles published as original articles in Indian Journal of Community Medicine from January 2010 to September 2011 were downloaded from the journal website. A total of 96 articles were downloaded out of which 80 were found to have a cross-sectional design. Variables were: (1) Percentage of STROBE items included in a report and (2) percentage of articles reporting each item in the STROBE checklist. Data analysis was done by descriptive statistics using frequencies and percentages.
Results:
A total of 80 articles were evaluated. About 46% (37/80) articles reported 12–15 items of the STROBE checklist. Bias, nonparticipants and reasons for nonparticipation, other analyses done, generalizability, and source of funding were reported by < 25% of studies. The most frequently reported items of the checklist were summary of what was done and what was found in the abstract, background/rationale, objectives, setting, outcome data, key results in discussion, interpretation of results. None of the articles reported all items of the STROBE checklist.
Conclusion:
This study reveals that the quality of reporting cross-sectional studies in Indian Journal of Community Medicine is not satisfactory and there is room for improvement.
Keywords: Cross sectional studies, Reporting, Strengthening the Reporting of Observational Studies in Epidemiology statement
Introduction
In biomedical research randomized controlled trials are considered the gold standard because this type of design controls bias to a significant degree. Much of the research conducted, however, is in the form of observational studies.[1] The results of these studies should be reported as transparently as possible “so that readers can follow what was planned, what was done, what was found, and what conclusions were drawn”.[2] In practice reporting of observational studies is not detailed enough and readers are often unable to judge the strengths and weaknesses in the studies.[3] To address this problem and to improve reporting of observational studies a group of experts developed a checklist of items known as Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.[2] Items relate to title, abstract, introduction, methods, results and discussion sections of articles. This checklist can prove helpful to authors for complete reporting of observational research as well as to reviewers and editors and in the long run it can lead to improvement in the quality of articles being published by journals.[4,5] Keeping in view the importance of complete reporting this study was planned to assess the extent in which items in the STROBE checklist are reported in articles being published in Indian Journal of Community Medicine which is a renowned journal in the field of public health in India and also to highlight specific areas of improvement.
Materials and Methods
Study design
The study was conducted in November 2011 and we used a cross-sectional study design.
All original articles published in Indian Journal of Community Medicine from January 2010 to September 2011 were downloaded from the journal website. The articles were then screened for cross-sectional studies. Out of the 96 original articles published in the journal in the said time, 80 articles were of cross-sectional design and all of the articles were included in the study. Cross-sectional studies were chosen for the study on account of the fact that these are easier and cheaper as they are less time consuming and do not need follow-up of study participants. All of the articles were then reviewed by two reviewers independently. The reviewer's independently made decision on whether the articles had reported items in the STROBE checklist. No blinding about the article name or author name was done. Any disagreement between the two reviewers was resolved by a third reviewer.
Variables: (1) Percentage of STROBE items included in a report. (2) Percentage of articles reporting each item in the STROBE checklist.
Statistics
Descriptive statistics using frequencies and percentages were computed. Percentages were rounded off to the nearest one decimal place.
Results
A total of 80 articles were evaluated using STROBE statement as a guideline. Proportion of articles reporting each item in the STROBE checklist is shown in the Table 1. The data suggests that there is a tendency to not report some important information about the research process which is signified by underreporting of items like source of funding or the way the sample size was achieved. Proportion of reported items per article is depicted in Table 2. Over 58% of articles (47/80) reported < 15 items in the STROBE checklist. Mostly articles reported about 12–17 items in the STROBE checklist. Table 3 summarizes the items that were reported most often and the items that were underreported most often. Items 9, 13, 17, 21, 22 were reported by least number of articles.
Table 1.
Proportion of articles reporting each item in the STROBE checklist
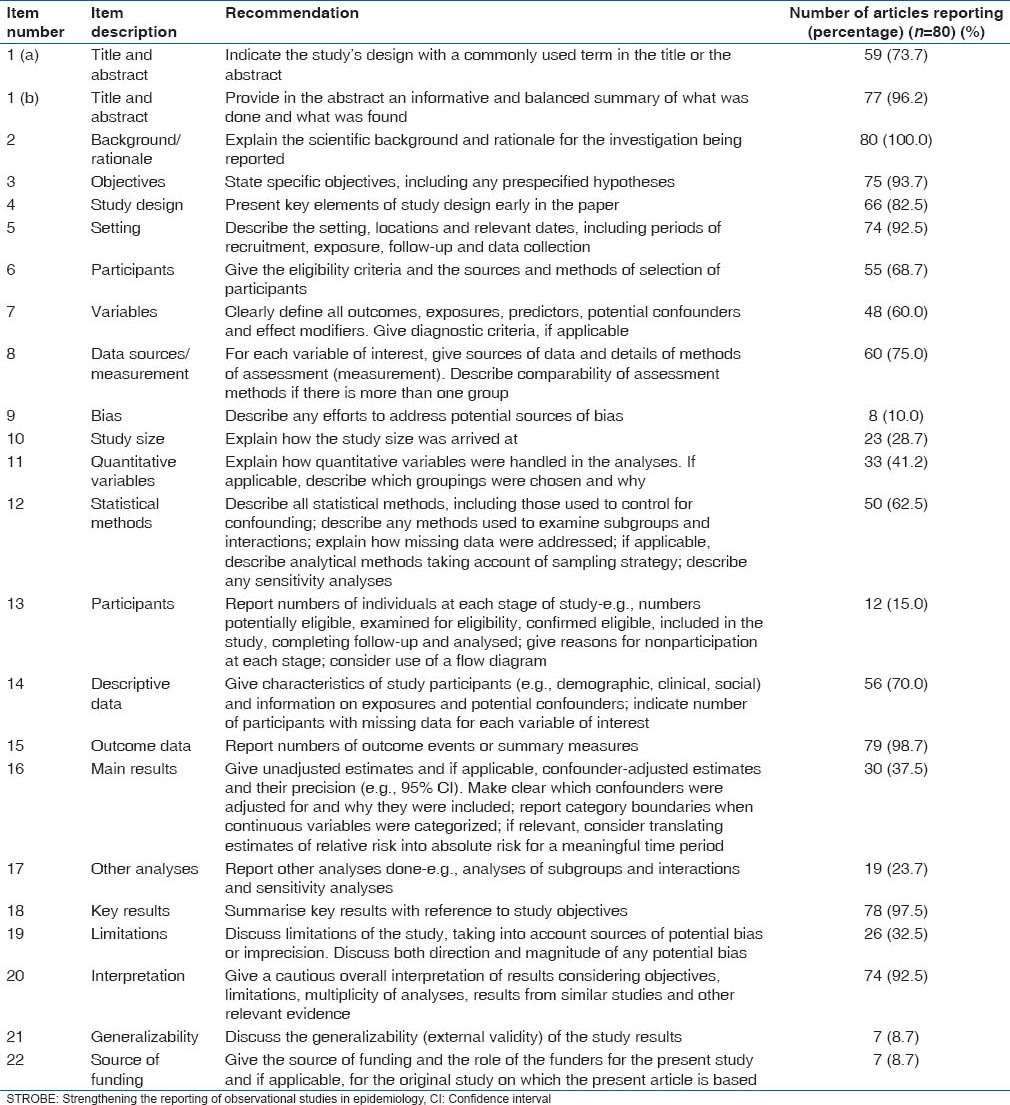
Table 2.
Proportion of reported items per article
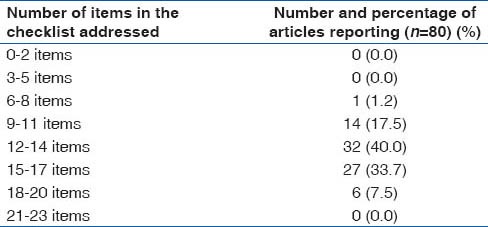
Table 3.
Items in the checklist usually reported and usually not reported

Discussion
Our study evaluated the reporting quality of cross-sectional observational studies published in Indian Journal of Community Medicine from January 2010 to September 2011 using STROBE checklist as a reference. Reporting of different items was very varied with some items being reported almost consistently while some items like information regarding bias and nonparticipants being consistently underreported. The study has highlighted areas where reporting is good in cross-sectional studies along with areas which require improvement. Over 58% of articles reported < 15 items in the STROBE checklist which needs to be improved to give readers a clear idea as to what was planned and what was done. There is a need for studies to improve their reporting of sample size calculations, statistical methods, and details of numbers and characteristics of participants. Nonparticipation and reasons of nonparticipation need to be clearly stated as they affect the external validity of the study.
Strengths and limitations of this study: This is a novel study and in our view the first study which analyzed cross-sectional observational studies in the field of public health in India. The results have direct relevance for authors, readers and editors of biomedical research. It brings to light those areas of our research which are not being consistently reported by authors. A key limitation is the fact that inter-rater agreement was not ascertained.
Interpretation and generalizability: The results of the present study represent the reporting of cross-sectional studies published in a prestigious public health journal that generally accepts well-done and well-written studies. However, there are numerous observational studies, the results of which are published in other less fastidious peer-reviewed medical journals. Thus, it is expected that the quality of reporting of such studies is much poorer than what is reported in the present study, although the result of present study is not desirable enough. Furthermore, cohort studies are much more expensive and take longer follow-up time than cross-sectional studies. Hence, the reporting of cohort studies is generally expected to be of substantially superior quality to other observational studies.[6] Accordingly, if this survey had been planned to assess reporting of case-control or cohort studies, the estimated result may have been much desirable.
Conclusion
We conclude that reporting of cross-sectional studies published in Indian Journal of Community Medicine from January 2010 to September 2011 is not clear and desirable enough yet. This issue should be the focus of both authors’ and editors’ special attention when reporting and/or reviewing the reports of observational studies.
Acknowledgment
Indian Journal of Community Medicine for providing free access to articles published in their journal.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Glasziou P, Vandenbroucke JP, Chalmers I. Assessing the quality of research. BMJ. 2004;328:39–41. doi: 10.1136/bmj.328.7430.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. The strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tooth L, Ware R, Bain C, Purdie DM, Dobson A. Quality of reporting of observational longitudinal research. Am J Epidemiol. 2005;161:280–8. doi: 10.1093/aje/kwi042. [DOI] [PubMed] [Google Scholar]
- 4.Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Ann Intern Med. 2001;134:663–94. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 5.Poorolajal J, Cheraghi Z, Irani AD, Rezaeian S. Quality of cohort studies reporting post the strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. Epidemiol Health. 2011;33:e2011005. doi: 10.4178/epih/e2011005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordis L. 4th ed. Philadelphia: Saunders; 2008. Epidemiology. [Google Scholar]


