Abstract
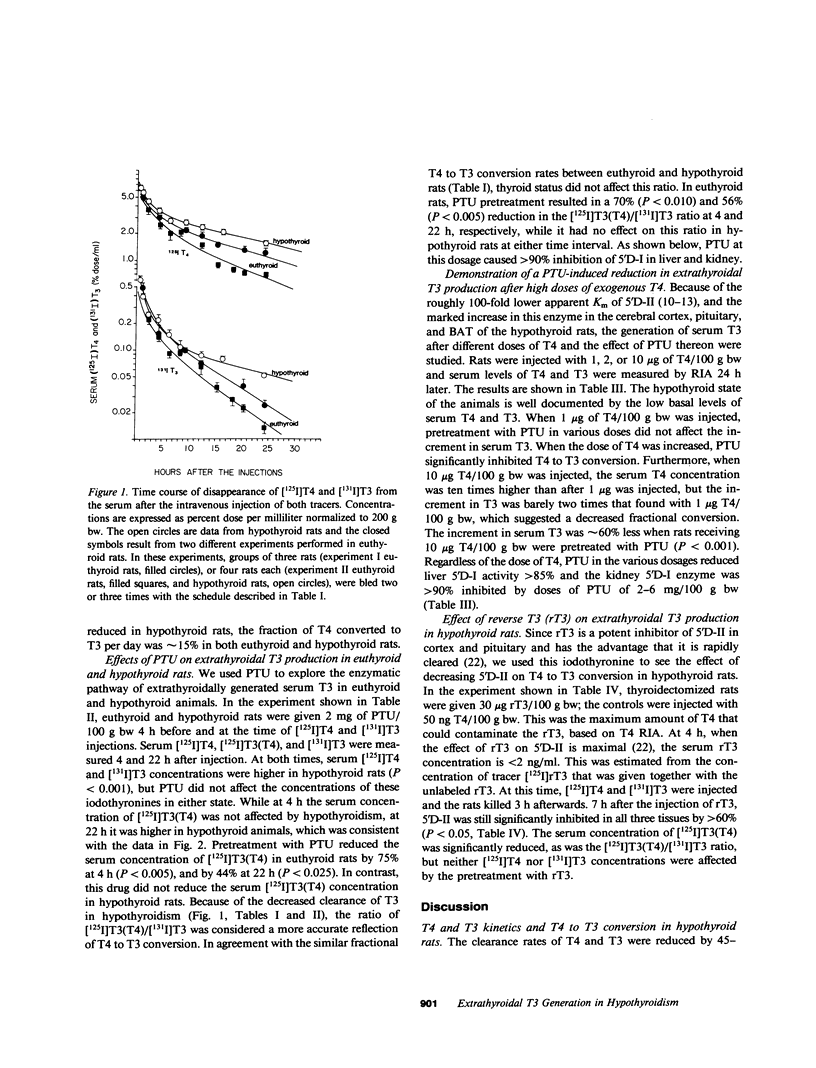
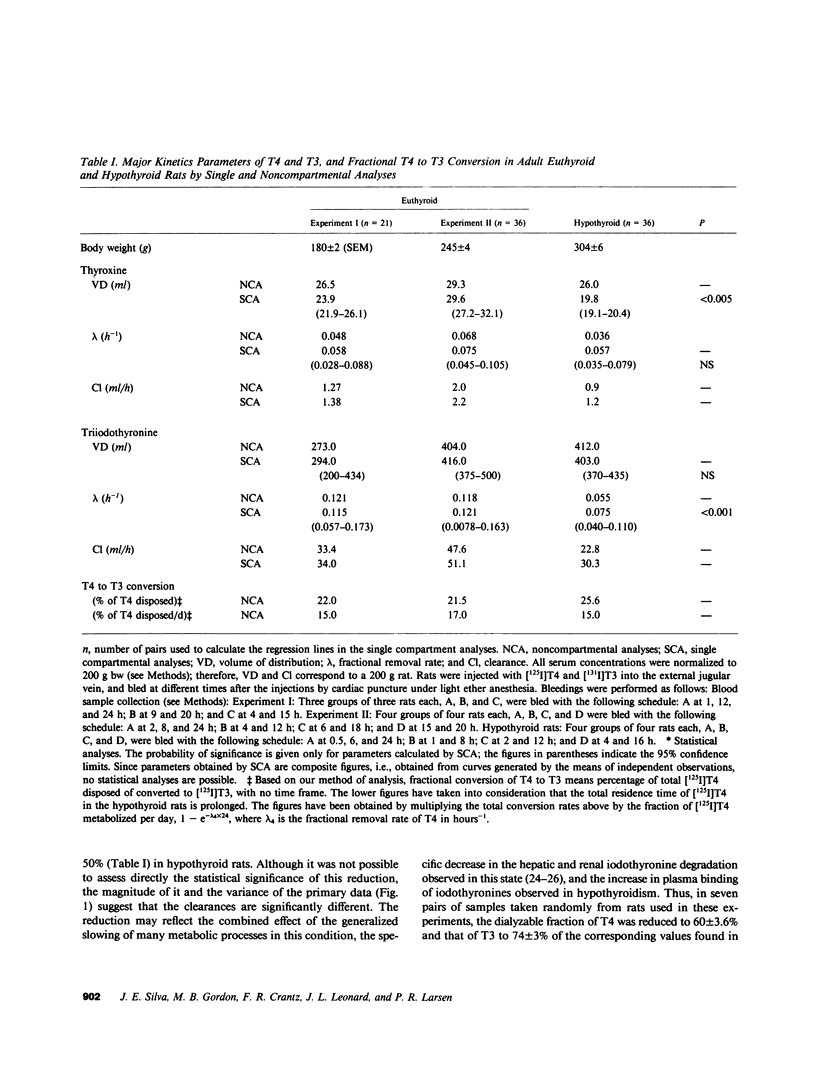
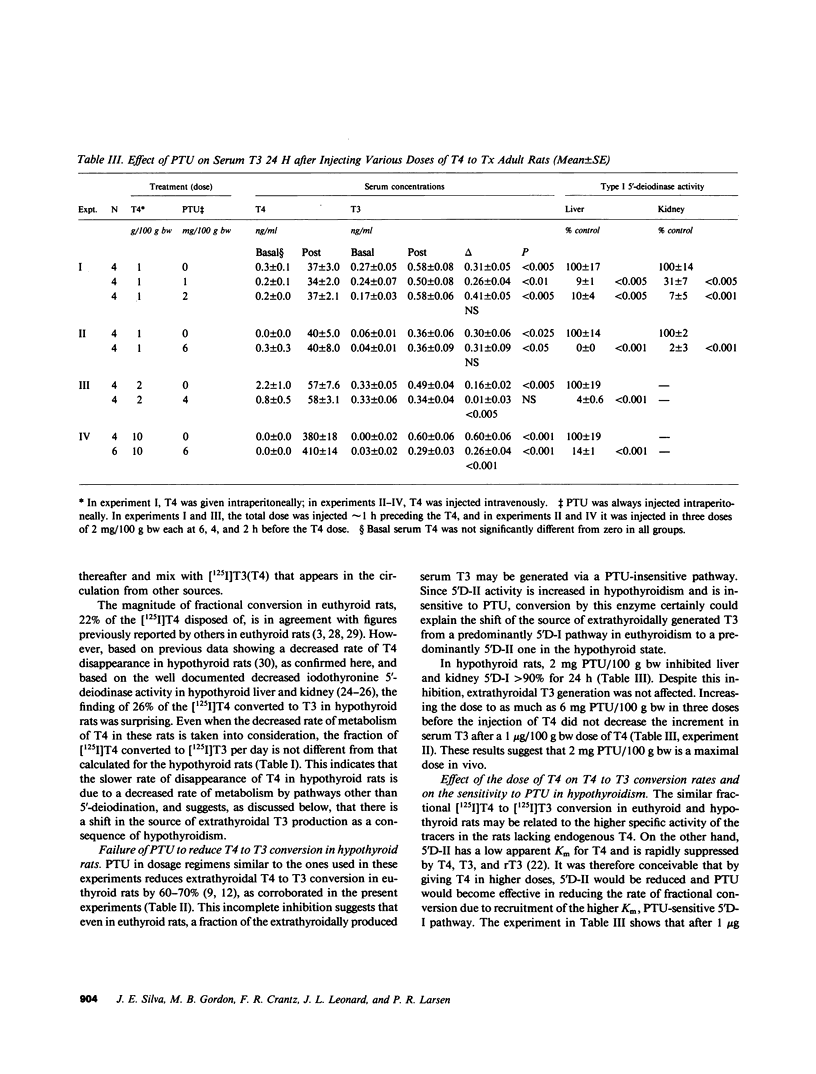
Propylthiouracil (PTU) in maximally inhibitory doses for liver and kidney iodothyronine 5'-deiodinase activity (5'D-I), reduces extrathyroidal T4 to T3 conversion by only 60-70% in euthyroid rats. A second pathway of T4 to T3 conversion (5'D-II) has been found in pituitary, central nervous system, and brown adipose tissue. 5'D-II is insensitive to PTU and increases in hypothyroidism, whereas 5'D-I decreases in hypothyroid rats. Thyroxine (T4) and triiodothyronine (T3) kinetics were assessed in euthyroid and thyroidectomized rats by noncompartmental analysis after injecting [125I]T4 and [131I]T3. Neither the volume of distribution nor the rate of fractional removal of plasma T4 was affected by the thyroid status, but the fractional removal rate of T3 was approximately 50% reduced in hypothyroid rats (P less than 0.001). Fractional T4 to T3 conversion was 22% in euthyroid and 26% in hypothyroid rats. In euthyroid rats, sufficient PTU to inhibit liver and kidney 5'D-I greater than 90% reduced serum [125I]T3 after [125I]T4 (results given as percent dose per milliliter X 10(-3) +/- SEM): 4 h, control 16 +/- 2 vs. PTU 4 +/- 1, P less than 0.005, and 22 h, control 6.4 +/- 0.4 vs. PTU 3.6 +/- 0.7, P less than 0.025. In thyroidectomized rats, the same dose of PTU also inhibited 5'D-I in liver and kidney, but had no effect on the generation of serum [125I]T3 from [125I]T4. Similarly, after 1 microgram T4/100 g bw was given to thyroidectomized rats, serum T3 (radioimmunoassay) increased by 0.30 +/- 0.6 ng/ml in controls and 0.31 +/- 0.09 ng/ml in PTU-treated rats. However, when the dose of T4 was increased to 2-10 micrograms/100 g bw, PTU pretreatment significantly reduced the increment in serum T3. T3 clearance was not affected by PTU in hypothyroid rats. The 5'D-II in brain, pituitary, and brown adipose tissue was reduced to less than or equal to 60% of control by 30 micrograms/100 g bw reverse T3 (rT3), an effect that lasted for at least 3 h after rT3 had been cleared. In rT3-pretreated thyroidectomized rats, the generation of [125I]T3 from tracer [125I]T4 was reduced in the serum: 6 +/- 1 vs. 12 +/- 1 X 10(-3)% dose/ml, P less than 0.01, during this 3-h period. We conclude that virtually all the T3 produced from low doses of exogenous T4 given to hypothyroid rats is generated via a PTU-insensitive pathway, presumably catalyzed by the 5'D-II. This is a consequence of the enhanced activity of this low Km enzyme together with the concomitant decrease in the hepatic and renal 5'D-I characteristic of the hypothyroid state. The results indicate that in some circumstances, 5D-II activity may contribute to the extracellular, as well as intracellular, T3 pool.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balsam A., Sexton F., Ingbar S. H. The effect of thyroidectomy, hypophysectomy, and hormone replacement on the formation of triiodothyronine from thyroxine in rat liver and kidney. Endocrinology. 1978 Nov;103(5):1759–1767. doi: 10.1210/endo-103-5-1759. [DOI] [PubMed] [Google Scholar]
- Chiraseveenuprapund P., Buergi U., Goswami A., Rosenberg I. N. Conversion of L-thyroxine to triiodothyronine in rat kidney homogenate. Endocrinology. 1978 Feb;102(2):612–622. doi: 10.1210/endo-102-2-612. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. A study of extrathyroidal conversion of thyroxine (T4) to 3,3',5-triiodothyronine (T3) in vitro. Endocrinology. 1977 Aug;101(2):453–463. doi: 10.1210/endo-101-2-453. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Solomon D. H., Chopra U., Wu S. Y., Fisher D. A., Nakamura Y. Pathways of metabolism of thyroid hormones. Recent Prog Horm Res. 1978;34:521–567. doi: 10.1016/b978-0-12-571134-0.50018-1. [DOI] [PubMed] [Google Scholar]
- Conversion of thyroxine into tri-iodothyronine by rat liver homogenate. Biochem J. 1975 Sep;150(3):489–493. doi: 10.1042/bj1500489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crantz F. R., Silva J. E., Larsen P. R. An analysis of the sources and quantity of 3,5,3'-triiodothyronine specifically bound to nuclear receptors in rat cerebral cortex and cerebellum. Endocrinology. 1982 Feb;110(2):367–375. doi: 10.1210/endo-110-2-367. [DOI] [PubMed] [Google Scholar]
- Cullen M. J., Doherty G. F., Ingbar S. H. The effect of hypothyroidism and thyrotoxicosis on thyroxine metabolism in the rat. Endocrinology. 1973 Apr;92(4):1028–1033. doi: 10.1210/endo-92-4-1028. [DOI] [PubMed] [Google Scholar]
- DiStefano J. J., 3rd, Jang M., Malone T. K., Broutman M. Comprehensive kinetics of triiodothyronine production, distribution, and metabolism in blood and tissue pools of the rat using optimized blood-sampling protocols. Endocrinology. 1982 Jan;110(1):198–213. doi: 10.1210/endo-110-1-198. [DOI] [PubMed] [Google Scholar]
- DiStefano J. J., 3rd, Malone T. K., Jang M. Comprehensive kinetics of thyroxine distribution and metabolism in blood and tissue pools of the rat from only six blood samples: dominance of large, slowly exchanging tissue pools. Endocrinology. 1982 Jul;111(1):108–117. doi: 10.1210/endo-111-1-108. [DOI] [PubMed] [Google Scholar]
- Foster D. O., Frydman M. L. Nonshivering thermogenesis in the rat. II. Measurements of blood flow with microspheres point to brown adipose tissue as the dominant site of the calorigenesis induced by noradrenaline. Can J Physiol Pharmacol. 1978 Feb;56(1):110–122. doi: 10.1139/y78-015. [DOI] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Vagenakis A. G., Braverman L. E. Effect of starvation, nutriment replacement, and hypothyroidism on in vitro hepatic T4 to T3 conversion in the rat. Metabolism. 1978 Nov;27(11):1680–1690. doi: 10.1016/0026-0495(78)90290-1. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Tatro J. B., Breitbart R., Larsen P. R. Comparison of thyroxine and 3,3',5'-triiodothyronine metabolism in rat kidney and liver homogenates. Metabolism. 1979 Nov;28(11):1139–1146. doi: 10.1016/0026-0495(79)90153-7. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in liver and kidney homogenates from hyperthyroid and hypothyroid rats. Endocrinology. 1978 Jul;103(1):156–161. doi: 10.1210/endo-103-1-156. [DOI] [PubMed] [Google Scholar]
- Kochupillai N., Yalow R. S. Preparation, purification, and stability of high specific activity 125I-labeled thyronines. Endocrinology. 1978 Jan;102(1):128–135. doi: 10.1210/endo-102-1-128. [DOI] [PubMed] [Google Scholar]
- Larsen P. R., Frumess R. D. Comparison of the biological effects of thyroxine and triiodothyronine in the rat. Endocrinology. 1977 Apr;100(4):980–988. doi: 10.1210/endo-100-4-980. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Mellen S. A., Larsen P. R. Thyroxine 5'-deiodinase activity in brown adipose tissue. Endocrinology. 1983 Mar;112(3):1153–1155. doi: 10.1210/endo-112-3-1153. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Rosenberg I. N. Iodothyronine 5'-deiodinase from rat kidney: substrate specificity and the 5'-deiodination of reverse triiodothyronine. Endocrinology. 1980 Nov;107(5):1376–1383. doi: 10.1210/endo-107-5-1376. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Rosenberg I. N. Thyroxine 5'-deiodinase activity of rat kidney: observations on activation by thiols and inhibition by propylthiouracil. Endocrinology. 1978 Dec;103(6):2137–2144. doi: 10.1210/endo-103-6-2137. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Shapiro H. C., Bernstein G., Surks M. I. Differences in primary cellular factors influencing the metabolism and distribution of 3,5,3'-L-triiodothyronine and L-thyroxine. J Clin Invest. 1970 May;49(5):1016–1024. doi: 10.1172/JCI106301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Determination of common parameters fo iodothyronine metabolism and distribution in man by noncompartmental analysis. J Clin Endocrinol Metab. 1975 Aug;41(2):319–324. doi: 10.1210/jcem-41-2-319. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Propylthiouracil inhibits the conversion of L-thyroxine to L-triiodothyronine. An explanation of the antithyroxine effect of propylthiouracil and evidence supporting the concept that triiodothyronine is the active thyroid hormone. J Clin Invest. 1972 Sep;51(9):2493–2497. doi: 10.1172/JCI107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman C. S., Chambers J. B., Jr, Read V. H. The extrathyroidal conversion rate of thyroxine to triiodothyronine in normal man. J Clin Invest. 1971 Jun;50(6):1187–1196. doi: 10.1172/JCI106596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz H. L., Surks M. I., Oppenheimer J. H. Quantitation of extrathyroidal conversion of L-thyroxine to 3,5,3'-triiodo-L-thyronine in the rat. J Clin Invest. 1971 May;50(5):1124–1130. doi: 10.1172/JCI106584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Comparison of iodothyronine 5'-deiodinase and other thyroid-hormone-dependent enzyme activities in the cerebral cortex of hypothyroid neonatal rat. Evidence for adaptation to hypothyroidism. J Clin Invest. 1982 Nov;70(5):1110–1123. doi: 10.1172/JCI110699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Leonard J. L., Crantz F. R., Larsen P. R. Evidence for two tissue-specific pathways for in vivo thyroxine 5'-deiodination in the rat. J Clin Invest. 1982 May;69(5):1176–1184. doi: 10.1172/JCI110554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surks M. I., Schadlow A. R., Stock J. M., Oppenheimer J. H. Determination of iodothyronine absorption and conversion of L-thyroxine (T 4 ) to L-triiodothyronine (T 3 ) using turnover rate techniques. J Clin Invest. 1973 Apr;52(4):805–811. doi: 10.1172/JCI107244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser T. J., Kaplan M. M., Leonard J. L., Larsen P. R. Evidence for two pathways of iodothyronine 5'-deiodination in rat pituitary that differ in kinetics, propylthiouracil sensitivity, and response to hypothyroidism. J Clin Invest. 1983 Apr;71(4):992–1002. doi: 10.1172/JCI110854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser T. J., Leonard J. L., Kaplan M. M., Larsen P. R. Kinetic evidence suggesting two mechanisms for iodothyronine 5'-deiodination in rat cerebral cortex. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5080–5084. doi: 10.1073/pnas.79.16.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke J., Orskov H. Synthesis of 125I monolabelled 3, 5, 3'-triiodothyronine and thyroxine of maximum specific activity for radioimmunoassay. Scand J Clin Lab Invest. 1973 Dec;32(4):357–360. doi: 10.3109/00365517309084359. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. J., Izumi M., Larsen P. R. Isolation of labeled triiodothyronine from serum using affinity chromatography: application to the extimation of the peripheral T4 to T3 conversion in rats. Metabolism. 1978 Mar;27(3):303–313. doi: 10.1016/0026-0495(78)90110-5. [DOI] [PubMed] [Google Scholar]



