Abstract
Background:
Oxidative stress has been implicated in various disorders including epilepsy. We studied the antioxidant status in patients with epilepsy and aimed at determining whether there was any difference in the antioxidant levels between patients and controls, patients who are not on antiepileptic drugs (AEDs), and on treatment, between individual AEDs and patients on monotherapy and polytherapy.
Materials and Methods:
Antioxidant levels like catalase, glutathione peroxidase (GPx), vitamin E, glutathione (GSH), thiol group (SH), uric acid, and total antioxidant capacity (TAC) were compared between 100 patients with epilepsy and equal number of controls. Twenty-five patients who were not on AEDs were compared with patients on AEDs and the control group. Patients were divided into monotherapy and polytherapy group and antioxidant status was compared between the two groups and between individual drugs.
Results:
Catalase, SH, vitamin E, and TAC were significantly low in patients with epilepsy than those in the control group (P < 0.001). GSH and uric acid did not show any difference; GPx in patients was significantly higher than those in the control group There were no differences in the antioxidant levels between the treated and the untreated groups; however, it was lower in untreated patients than controls (P < 0.001), suggesting that AEDs do not modify the oxidative stress. Patients on Valproate (VPA) showed higher catalase and GPx levels. Catalase was higher in the monotherapy than polytherapy group (P < 0.04).
Conclusion:
Our study found significantly low levels of antioxidant in patients as compared to controls. AED did not influence the antioxidant status suggesting that seizures induce oxidative stress.
Keywords: Antiepileptic drugs, catalase, epilepsy, glutathione, glutathione peroxidase, seizures, thiol goup, total antioxidant capacity, uric acid, vitamin E
Introduction
Epilepsy is one of the oldest neurological disorders known to mankind with social and economic burden to the patient. Epileptogenesis refers to a dynamic process that occurs in the brain during the period between the original insult and the first seizure.[1] Various causes have been identified for epileptogenesis in the symptomatic[2] and also in few genetic types of epilepsy.[3] Recurrent seizures, producing brain injury, and neuronal death are dynamic processes suggesting that epileptogenesis might continue even after the diagnosis of epilepsy.[4,5] This suggests that epileptogenesis is involved in the progression and modification of the disease. Several factors like genetic, excitotoxicity-induced mitochondrial dysfunction, altered cytokine levels, and oxidative stress have been implicated in epileptogenesis.[6]
Oxidative stress occurs when the body is unable to eliminate the free radicals which are implicated in the pathogenesis of various neurological disorders. Maintenance of oxidative balance in the brain is tightly regulated by antioxidants. In recent years, role of oxidative stress in epilepsy has been attracting considerable attention. With the background knowledge that brain is vulnerable to oxidative stress and that oxidative stress being one of the factors in epileptogenesis, we aimed to determine the antioxidant status in patients. As neuronal death is the major neurobiological abnormality in epileptogenesis, determination of antioxidant status will give further insights into the treatment of epilepsy. We determined the antioxidant status in patients with epilepsy and compared with healthy controls. We also studied the antioxidant status in patients who are not on antiepileptic drugs and evaluated the effects of monodrug or multidrug antiepileptic drugs (AEDs).
Materials and Methods
The study prospectively enrolled 100 patients with idiopathic generalized epilepsy. One hundred age and sex matched healthy volunteers served as the control group. Patients were recruited over a period of 6 months. We followed the convenient sampling method. Patients who were seizure-free for more than three days were included in the study. All patients underwent a detailed examination which included demographics, seizure frequency, duration of illness, and treatments. Patients with chronic medical illnesses such as hypertension, diabetes, rheumatoid arthritis, collagen vascular disease, chronic neurological illness, or localisation related epilepsy; those who were smokers, alcoholics, tobacco chewers; and those taking any other type of medications were excluded from the study. All patients underwent electroencephalography (EEG) and several patients underwent neuroimaging for a definitive diagnosis.
The antioxidant levels were compared between patients and the control group. Antioxidants were divided into enzymatic [Catalase and glutathione peroxidase (GPx)] and nonenzymatic[vitamin E, Glutathione (GSH), thiol (SH) and uric acid] groups. Total antioxidant capacity (TAC) was also analyzed. Twenty-five patients who were not on AEDs were compared with patients on AEDs and the control group to eliminate the effects of the antiepileptic drugs. Depending upon treatment, the patients were divided into monotherapy and polytherapy group.
Those patients on monotherapy were treated with one of the following antiepileptic drugs: Carbamazepine (CBZ), Phenytoin (PHT), Valproate (VPA), Clobazam (CLB), Phenobarbital (PB), Lamotrigine (LTG), Topiramate (TPM), or Levetiracetam (LEV). Polytherapy group was composed of patients who are on more than one type of drug. Patients on monotherapy were analyzed among each other for the antioxidant status. All patients were provided written-informed consent and the study was approved by the institutional ethical committee.
Blood sample collection
Subjects under this study were advised to fast overnight (12 hours) and sample was collected in fasting condition. Ten millilitres of venous blood was collected and part of it was transferred to EDTA coated vacutainers for estimation of red blood cell (RBC) GSH levels (RBC-GSH), GPx, and catalase activity as well as hematological analysis. Remaining blood was allowed to clot to obtain serum and stored at −20°C to measure rest of the analytes.
Antioxidants assay
Estimation of erythrocyte reduced glutathione
The erythrocyte reduced GSH content was determined by the method of Beutler and Kelley (Beutler and Kelley, 1963). In this method, reduced GSH present in the RBC reacts with di-thio-nitrobenzoic acid (DTNB) and the intensity of the color is measured at 412 nm. The reduced GSH concentrations was expressed as mg/g Hb.[7]
Estimation of erythrocyte catalase activity
The catalase activity in erythrocytes was estimated by the method of Aebi, et al. In this method, catalase present in RBC utilizes hydrogen peroxide (H2O2) present in the reaction mixture. The decomposition of H2O2 was measured by monitoring the decrease in absorbance at 240 nm. The catalase activity was expressed as rate constant (k/ml).[8]
Estimation of erythrocyte glutathione peroxidase activity
The GPx activity in erythrocytes was estimated by the method described by Rotruck et al. The reaction was initiated by the addition of H2O2 and the GPx utilizes the reduced GSH present in the reaction mixture. The reduced GSH content present in the reaction mixture was measured using DTNB. The activity of GPx is expressed as U/g Hb of erythrocyte lysate.[9]
Total thiol group assay
Total SH groups were measured using DTNB. This reagent reacts with the SH groups to produce a yellow colored complex which has peak absorbance at 412 nm.[10]
Measurement of total antioxidant capacity
TAC of plasma was determined by ferric reducing antioxidant power assay — whereby at low pH, reduction of a ferric tripyridyl triazine (Fe3+-TPTZ) complex (Sigma Aldrich, St. Louis, MO, USA) to a ferrous form which has an intense blue color that can be monitored by measuring the absorbance at 593 nm using a spectrophotometer. It is directly related to the combined or total reducing power of the electron donating antioxidants present in the reaction mixture. The results were expressed as μM/L.[11]
Serum uric acid
Estimation of uric acid was carried out by enzymatic, colorimetric 4-aminophenazone (PAP) method using a commercially available kit, Humastar 300 chemistry analyzer (Human, GmbH, Germany).[12]
Vitamin-E (α-Tocopherol)
Estimation of serum α-tocopherol is carried out as described by Andre r et al., using High Performance Liquid Cromatography (HPLC) system at 292 nm with isocratic gradient pump.[13]
Statistical analysis
The data were analyzed with the statistical package SPSS for Windows, version 20. Descriptive analyses are expressed as mean ± S. D. Paired t-tests were performed between patients and controls, patients before treatment and controls, and patients before and after treatment. One way analysis of variance (ANOVA) was used to estimate the differences between the study groups.
Results
The study included 100 patients with epilepsy (56 males and 44 females) and 100 control subjects (52 males and 48 females). The mean ages of the patients and control group were 26 ± 10 and 26 ± 8 years respectively. The mean duration of illness in the patients was 7 ± 6 yrs. All patients had idiopathic generalized epilepsy. Twenty-five percent of the patients were not on treatment, 66% were on monotherapy (CBZ 16%, PHT 20%, VPA 17%, others 13%), and 34% were on polytherapy. The demographics and epilepsy profiles are shown in Table 1. The biochemical and antioxidant parameters are shown in Table 2. The total cholesterol, low density lipoprotein (LDL), and alkaline phosphatase levels was significantly higher in patients than in the control group Table 2. Rest of the biochemical parameters were normal.
Table 1.
Demographic profile of patients and controls
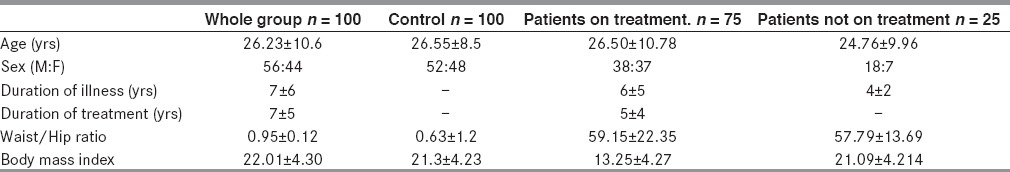
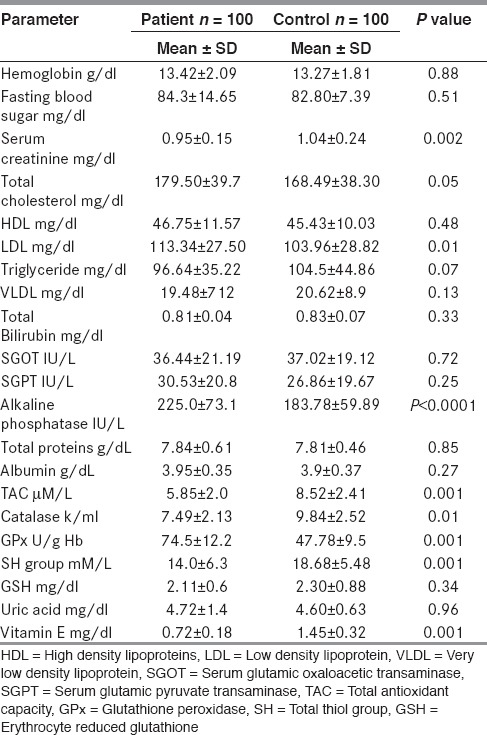
Table 2.
Biochemical and antioxidant parameters in patients and controls
Comparison between patients and controls
TAC in patients (5.85 ± 2 μM/L) was significantly lower than those in the control group (8.52 ± 2.41 μMl/L) (P < 0.001). Catalase levels in patients (7.49 ± 2.13 k/ml) were significantly lower than those in the control group (9.84 ± 2.52 k/ml) (P< 0.001). GPx in patients (74.5 ± 12.2 U/g Hb) was significantly higher than those in the control group (47.78 ± 9.50 U/g Hb) (P < 0.001). SH group in patients (14.0 ± 6.35 mM/L) was significantly lower than those in the control group (18.68 ± 5.48 mM/L) (P < 0.001). GSH did not show any difference between patients (2.11 ± 0.60 mg/g Hb) and controls (2.30 ± 0.88 mg/g Hb) (P = 0.11). Uric acid did not show any difference between patient (4.72 ± 1.40 mg/dl) and controls (4.60 ± 0.63 mg/dl) (P = 0.40). Vitamin E was significantly lower in patients (0.72 ± 0.18 mg/dl) than in controls (1.45 ± 0.32 mg/dl) (P < 0.001).
We evaluated the antioxidant levels in 25 patients who were not on AEDs and compared with controls as well as the treated group. This way we could eliminate the antioxidant effect of the AEDs.
Comparison between untreated patients and controls
The mean ages of untreated patients and control group were 24.76 ± 9.96 and 25.72 ± 7.77, respectively.
TAC in untreated patients (5.73 ± 1.34 μM/L) was significantly lower than those in the control group (8.52 ± 2.41 μM/L) (P < 0.001). Catalase level in untreated patients (7.94 ± 2.57 k/ml) was significantly lower than those in the control group (9.84 ± 2.52 k/ml) (P < 0.01). GPx in untreated patients (75.6 ± 8.65 U/g Hb) was higher than those in the control group (47.78 ± 9.5 U/g Hb) (P < 0.001). SH group in patients (12.9 ± 4.67 mM/L) was lower than those in the control group (18.68 ± 5.48 mM/L) (P < 0.001). GSH did not show any difference between patients (2.06 ± 0.61 mg/g Hb) and controls (2.30 ± 0.88 mg/g Hb) (P = 0.34). Uric acid did not show any difference between patients (4.61 ± 1.36 mg/dl) and controls (4.60 ± 0.63 mg/dl) (P = 0.96). Vitamin E was significantly lower in patients (0.72 ± 0.15 mg/dl) than in controls (1.45 ± 0.32 mg/dl) (P < 0.001).
Comparison between untreated and treated patients; in monotherapy and polytherapy groups
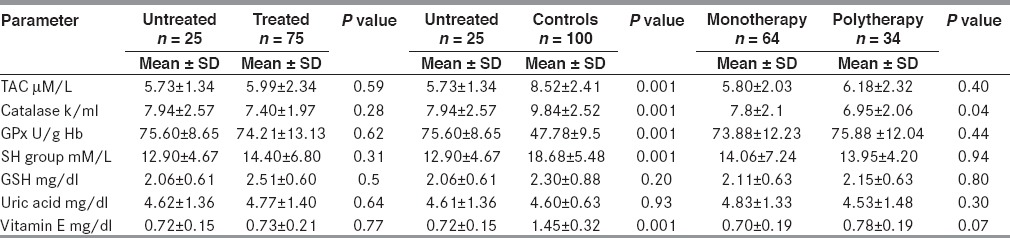
There were no differences in the antioxidant levels between the treated and the untreated groups. We compared the antioxidant levels between patients on monotherapy and those on polytherapy. Catalase was higher in the montherapy patients (7.8 ± 2.1 k/ml) than in the polytherapy (6.95 ± 2.06 k/ml) (P < 0.04) [Table 3].
Table 3.
Comparison between antioxidant parameters in untreated and treated patients; monotherapy and polytherapy
Comparison between individual AEDs
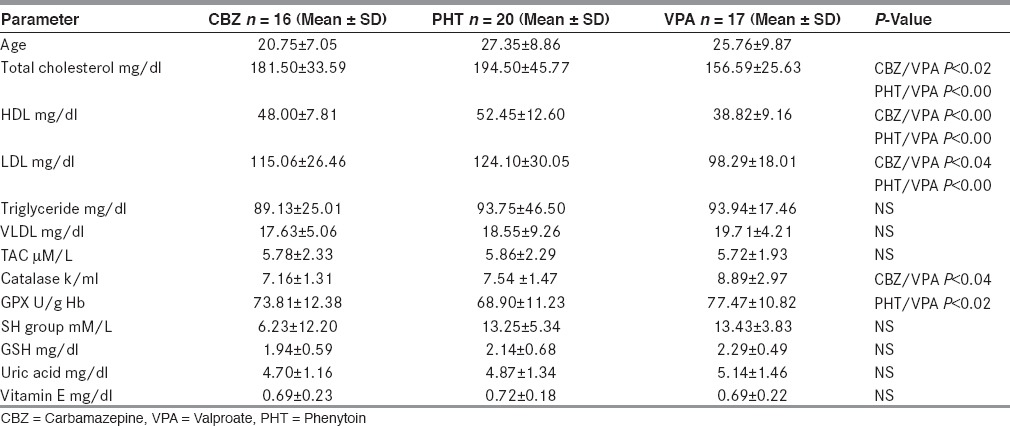
The antioxidant levels of patients on CBZ, PHT, and VPA are shown in [Table 4]. ANOVA analysis showed only GPx levels to be higher in VPA group than PHT groups (P < 0.02), catalase was found to be higher in VPA group than CBZ group. Rest of the analysis did not show any difference. However, there was a difference in lipid parameters; the total cholesterol, high density lipoproteins (HDL), and LDL levels were highest in patients taking PHT and lowest in patients taking CBZ.
Table 4.
Demographic, lipid profile and oxidative parameters in patients on various AEDs
Discussion
Our results showed that antioxidant levels were significantly low in patients with epilepsy. Patients had low levels of catalase, SH group, vitamin E, and TAC as compared to controls (P < 0.001). Earlier studies have found oxidative stress in patients with epilepsy.[14,15,16,17,18] However, our study did not show any difference between GSH (P = 0.11) and uric acid levels (P = 0.40) between patients and controls. GPx showed higher levels in controls than in patients (P < 0.001). Twenty-five patients who were not on AED also had low levels of catalase, SH group, vitamin E, and TAC (P < 0.001) as compared to controls. When we compared patients on AEDs to patients not on AEDs, we found no difference in the antioxidant status, thereby suggesting that AEDs do not modify the oxidative stress.
Brain is susceptible to oxidative stress because of the high lipid content and oxidative metabolism.[15,19,20] This was proven in experimental animals which showed oxidative damage after electro-shock[21,22] and Pentylenetetrazole-induced seizure (PTZ) models.[23] Yet another study proved that a deficiency in glutamine synthetase may be more important in the pathogenesis of mesial-tempotal lobe epilepsy than the patterned loss of hippocampal neurons which are characteristic of the disease.[24] Recurrent seizures increase the reactive oxygen species (ROS) in the brain. Oxidative stress is considered one of the mechanisms that could independently contribute to the disease progression in addition to serving as processes that underlie neuronal injury.[25] Antioxidant system prevents free radical damage and also plays an important part in disease progression in the body.[26] We studied catalase, GPx, vitamin E, GSH, SH group, uric acid, and TAC in patients and controls. Catalase is naturally produced in the body catalyzing the reduction of H2O2 which prevents oxidative damage in human cells. GSH exists in two forms. GPx catalyzes H2O2 reduction using GSH, thus protecting mammalian cells against oxidative damage. In our study, catalase was lower in patients whereas GSH did not show any change and GPx was higher in patients. The higher GPx levels in patients is not surprising; as experimental induced seizure models has also shown conflicting results with few studies showing increased GPx[27,28] and few showing lower levels.[29,30]
Uric acid is, by far, the antioxidant present in highest concentration in human blood.[31] Uric acid functions as a paradox as it acts as an antioxidant in plasma or pro-oxidant within the cell.[32,33] The role of uric acid as a marker of oxidative stress and its role as antioxidant is still under debate.[34] Indeed, our study also showed no difference in uric acid levels between patients and controls.
SH groups are highly-reactive constituents of protein molecules and important scavengers of oxygen-derived free radicals.[35,36] Vitamin E is one of the most important lipid-soluble antioxidant which is also known as the chain breaking antioxidant. It protects membranes from oxidation by reacting with lipid radicals produced in the lipid peroxidation chain reaction.[37,38,39] Vitamin E is reported to delay the onset of seizures induced by intracerebral ferrous chloride injection; moreover, it was found that addition of Vitamin E to the drug treatment improves the electroencephalographic findings.[40,41] Our study showed lower levels of vitamin E in patients which was in accordance with earlier studies.[42] Recent studies have shown that PTZ/pilocarpine induced epileptic rats have low levels of vitamin E which was increased after antioxidant administration.[43,44] This study also implies the protective effect of vitamin E. The measurement of individual antioxidant assay helps us in summarizing the total antioxidant status. TAC is a more accurate measure of the antioxidant capacity. Though our study did not find low levels of GPx, GSH and uric acid; TAC was found to be significantly low in patients as compared to controls. Earlier studies have also shown low TAC levels in patients with epilepsy,[45] and TAC can be considered as a cumulative index of the complete antioxidant status.
Few studies have explored the role of AEDs in modulating oxidative stress.[17,46,48] In our study, we compared antioxidant levels between patients and controls and found low antioxidant status in patients. Thereafter, we compared patients who were not on treatment to patients on AEDs and control group. There were no differences in the antioxidant levels between the treated and the untreated groups, however, the antioxidant levels (catalase, SH, Vitamin E) were lower in untreated patients when compared to controls. Hence, this study implies that AED did not have any effect on the antioxidant levels and suggests that seizures induced oxidative stress. We also attempted to know whether individual AEDs can alter the antioxidant status and found that intake of VPA showed higher catalase and GPx levels than PHT and CBZ intake. However, earlier studies have shown varied results about the role of AED on oxidative stress.[16,49,50,51] We also analyzed antioxidant levels between patients on monotherapy and polytherapy and found that catalase levels were higher in patients on monotherapy. Interestingly, a recent study concluded that anti-epileptic drugs had a positive effect on oxidative stress.[52] Our study had only few patients in each AED group and hence it is difficult to conclude about the superiority of an individual AED on antioxidant status. Moreover, failure to demonstrate a difference in antioxidant status between treated and untreated patients could be due to an inadequate sample size. Further studies are required to prove this. However, it does seem that some of the AEDs may have a positive antioxidant property. Indeed, earlier finding as well as ours shows that AEDs with positive antioxidant effect will have a positive bearing on the treatment.[53]
Our result of low antioxidant status in patients with epilepsy has treatment implications. This is in the light of studies which showed that adding antioxidant to epilepsy treatment reduces the severity and frequency of seizures.[53,54,55] Experimentally-induced seizure models also showed decreased levels of superoxide dismutase (SOD) and catalase which was normalized after antioxidant injection.[56,57] The results of these studies suggest that antioxidants may have anticonvulsive effects too. However, these findings need further confirmation.
We also detected higher cholesterol and LDL levels in patients when compared to controls. Oxidative stress has been seen to modify the lipoproteins by oxidation. Lipoproteins are shown to be taken up by macrophages and transformed into foam cells through degradation of the oxidized-LDL (Ox-LDL) via the scavenger receptors in the arterial wall[58] resulting in atherosclerotic streak formation leading to development and progression of atherogenesis.[59] Studies have shown that patients with epilepsy had increased carotid artery intima-media thickness and increased vascular risk factors.[60,61] Hence, patients with epilepsy can have long term atherosclerotic complications and oxidative stress could be one of the implicating causes which need to be addressed in further studies. Ketogenic diet is yet another area being explored.
In conclusion, our study found significantly low antioxidant levels in patients with epilepsy as compared to controls. Our study also showed that AED did not have any influence on the antioxidant levels as there were no differences between the treated and the untreated groups; with antioxidant levels being lower in untreated patients when compared to controls. Our previous study demonstrated significantly increased level of oxidative markers in person with epilepsy independent of AED suggesting that seizures induce oxidative stress.[18] The fact that oxidative stress is present in patients with epilepsy seems convincing from the present and earlier studies. Limitation of our study was that there were few patients in each individual AED groups. Hence, conclusive evidence about the antioxidant potential of individual AED could not be drawn This study adds further knowledge to the prevailing information, however, more research needs to be undertaken to prove the association of oxidative stress in seizure induction, propagation, and its atherogenic potential in epilepsy patients. With more knowledge, future AEDs could definitely be designed with antioxidant potentials as well.
Acknowledgements
The study was supported by a grant from the International Epilepsy Trust.
Footnotes
Source of Support: The study was supported by a grant from the International Epilepsy Trust
Conflict of Interest: None declared.
References
- 1.Engel J, Jr, Pedley TA. Introduction: What is epilepsy? In: Engel J Jr, Pedley TA, editors. Epilepsy: A comprehensive textbook. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 1–7. [Google Scholar]
- 2.International League Against Epilepsy (ILAE) A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: Report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 3.Zara F, Bianchi A. The impact of genetics on the classification of epilepsy syndromes. Epilepsia. 2009;50:11–4. doi: 10.1111/j.1528-1167.2009.02113.x. [DOI] [PubMed] [Google Scholar]
- 4.Pitkanen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1:173–81. doi: 10.1016/s1474-4422(02)00073-x. [DOI] [PubMed] [Google Scholar]
- 5.Pitkanen A, Lukasiuk K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 2009;14:16–25. doi: 10.1016/j.yebeh.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Ferriero DM. Protecting neurons. Epilepsia. 2005;46:45–51. doi: 10.1111/j.1528-1167.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 7.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 8.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 9.Roturck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 10.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 11.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 12.Barham D, Trinder P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst. 1972;97:142–5. doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- 13.De Leenheer AP, De Bevere VO, Cruyl AA, Claeys AE. Determination of serum alpha-tocopherol (vitamin E) by high-performance liquid chromatography. Clin Chem. 1978;24:585–90. [PubMed] [Google Scholar]
- 14.Yürekli VA, Nazıroğlu M. Selenium and topiramate attenuates blood oxidative toxicity in patients with epilepsy: A clinical pilot study. Biol Trace Elem Res. 2013;152:180–6. doi: 10.1007/s12011-013-9616-9. [DOI] [PubMed] [Google Scholar]
- 15.Sudha K, Rao AV, Rao A. Oxidative stress and antioxidants in epilepsy. Clin Chim Acta. 2001;303:19–24. doi: 10.1016/s0009-8981(00)00337-5. [DOI] [PubMed] [Google Scholar]
- 16.Niketić V, Ristić S, Saicić ZS, Spasić M, Buzadzić B, Stojković M. Activities of antioxidant enzymes and formation of the glutathione adduct of hemoglobin (Hb ASSG) in epileptic patients with long-term antiepileptic therapy. Farmaco. 1995;50:811–3. [PubMed] [Google Scholar]
- 17.Yüksel A, Cengiz M, Seven M, Ulutin T. Erythrocyte glutathione, glutathione peroxidase, superoxide dismutase and serum lipid peroxidation in epileptic children with valproate and carbamzepine monotherapy. J Basic Clin Physiol Pharmacol. 2000;11:73–81. doi: 10.1515/jbcpp.2000.11.1.73. [DOI] [PubMed] [Google Scholar]
- 18.Menon B, Ramalingam K, Kumar RV. Oxidative stress in patients with epilepsy is independent of antiepileptic drugs. Seizure. 2012;21:780–4. doi: 10.1016/j.seizure.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Choi BH. Oxygen, antioxidants and brain disfunction. Yonsei Med J. 1993;34:1–10. doi: 10.3349/ymj.1993.34.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Oliver CN, Starke-Reed PE, Stadtman ER, Liu GJ, Carney JM, Floyd RA. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci U S A. 1990;87:5144–7. doi: 10.1073/pnas.87.13.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rola R, Swiader M, Czuczwar SJ. Electroconvulsions elevate the levels of lipid peroxidation process in mice. Pol J Pharmacol. 2002;54:521–4. [PubMed] [Google Scholar]
- 22.Barichello T, Bonatto F, Agostinho FR, Reinke A, Moreira JC, Dal-Pizzol F, et al. Structure-related oxidative damage in rat brain after acute and chronic electroshock. Neurochem Res. 2004;29:1749–53. doi: 10.1023/b:nere.0000035811.06277.b3. [DOI] [PubMed] [Google Scholar]
- 23.Rauca C, Zerbe R, Jantze H. Formation of free hydroxyl radicals after pentylenetetrazol-induced seizure and kindling. Brain Res. 1999;847:347–51. doi: 10.1016/s0006-8993(99)02084-3. [DOI] [PubMed] [Google Scholar]
- 24.Eid T, Ghosh A, Wang Y, Beckström H, Zaveri HP, Lee TS, et al. Recurrent seizures and brain pathology after inhibition of glutamine synthetase in the hippocampus in rats. Brain. 2008;131:2061–70. doi: 10.1093/brain/awn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azam F, Prasad MV, Thangavel N. Targeting oxidative stress component in the therapeutics of epilepsy. Curr Top Med Chem. 2012;12:994–1007. doi: 10.2174/156802612800229224. [DOI] [PubMed] [Google Scholar]
- 26.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 27.Tejada S, Sureda A, Roca C, Gamundí A, Esteban S. Antioxidant response and oxidative damage in brain cortex after high dose of pilocarpine. Brain Res Bull. 2007;71:372–5. doi: 10.1016/j.brainresbull.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Bellissimo MI, Amado D, Abdalla DS, Ferreira EC, Cavalheiro EA, Naffah-Mazzacoratti MG. Superoxide dismutase, glutathione peroxidase activities and the hydroperoxides concentration are modified in the hippocampus of epileptic rats. Epilepsy Res. 2001;46:121–8. doi: 10.1016/s0920-1211(01)00269-8. [DOI] [PubMed] [Google Scholar]
- 29.Erakovic V, Zupan G, Varljen J, Simonic A. Pentylenetetrazole-induced seizures and kindling: Changes in free fatty acids, superoxide dismutase, and glutathione peroxidase activity. Neurochem Int. 2003;42:173–8. doi: 10.1016/s0197-0186(02)00070-0. [DOI] [PubMed] [Google Scholar]
- 30.Patsoukis N, Zervoudakis G, Georgiou CD, Angelatou F, Matsokis NA, Panagopoulos NT. Effect of pentylenetetrazole-induced epileptic seizure on thiol redox state in the mouse cerebral cortex. Epilepsy Res. 2004;62:65–74. doi: 10.1016/j.eplepsyres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Enomoto A, Endou H. Roles of organic anion transporters (OATs) and a urate transporter (URAT1) in the pathophysiology of human disease. Clin Exp Nephrol. 2005;9:195–205. doi: 10.1007/s10157-005-0368-5. [DOI] [PubMed] [Google Scholar]
- 32.Sautin YY, Johnson RJ. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27:608–19. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becker BF. Towards the physiological function of uric acid. Free Radic Biol Med. 1993;14:615–31. doi: 10.1016/0891-5849(93)90143-i. [DOI] [PubMed] [Google Scholar]
- 34.Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11:4145–51. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 35.Jensen EV. Sulfhydryl-disulfide interchanges. Science. 1959;130:1319–23. doi: 10.1126/science.130.3385.1319. [DOI] [PubMed] [Google Scholar]
- 36.Del Maestro RF. An approach to free radicals in medicine and biology. Acta Physiol Scand Suppl. 1980;492:153–68. [PubMed] [Google Scholar]
- 37.Herrera E, Barbas C. Vitamin E: Action, metabolism and perspectives. J Physiol Biochem. 2001;57:43–56. [PubMed] [Google Scholar]
- 38.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberta JW, Timothy JP. Free radicals. In: Marshall WJ, Bangert SK, editors. Clinical bio-chemistry: Metabolic and clinical aspects. New York: Churchill Livingstone Publishers; 1995. pp. 765–777. [Google Scholar]
- 40.Mori A, Yokoi I, Noda Y, Willmore LJ. Natural antioxidants may prevent post traumatic epilepsy: A pro-posal based on experimental animal studies. Acta Med Okayama. 2004;58:111–8. doi: 10.18926/AMO/32111. [DOI] [PubMed] [Google Scholar]
- 41.Higashi A, Tamari H, Ikeda T, Ohtani Y, Matsukura M, Miyoshino S, et al. Serum vitamin E concentration in patients with severe multiple handicaps treated with anticonvulsants. Pediatr Pharmacol (New York) 1980;1:129–34. [PubMed] [Google Scholar]
- 42.Ogunmekan AO. Relationship between age and vitamin E level in epileptic and normal children. Am J Clin Nutr. 1979;32:2269–71. doi: 10.1093/ajcn/32.11.2269. [DOI] [PubMed] [Google Scholar]
- 43.Nazıroğlu M, Akay MB, Çelik Ö, Yıldırım MÝ, Balcı E, Yürekli VA. Capparis ovata modulates brain oxidative toxicity and epileptic seizures in pentylentetrazol-induced epileptic rats. Neurochem Res. 2013;38:780–8. doi: 10.1007/s11064-013-0978-3. [DOI] [PubMed] [Google Scholar]
- 44.dos Santos PS, Costa JP, Tomé Ada R, Saldanha GB, de Souza GF, Feng D, et al. Oxidative stress in rat striatum after pilocarpine-induced seizures is diminished by alpha-tocopherol. Eur J Pharmacol. 2011;668:65–71. doi: 10.1016/j.ejphar.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 45.Mahle C, Dasgupta A. Decreased total ntioxidant capacity and elevated lipid hydroperoxide concentrations in sera of epileptic patients receiving phenytoin. Life Sci. 1997;61:437–43. doi: 10.1016/s0024-3205(97)00401-3. [DOI] [PubMed] [Google Scholar]
- 46.Aycicek A, Iscan A. The effects of carbamazepine, valproic acid and phenobarbital on the oxidative and antioxidative balance in epileptic children. Eur Neurol. 2007;57:65–9. doi: 10.1159/000098053. [DOI] [PubMed] [Google Scholar]
- 47.Verrotti A, Basciani F, Trotta D, Pomilio MP, Morgese G, Chiarelli F. Serum copper, zinc, selenium, glutathione peroxidase and superoxide dismutase levels in epileptic children before and after 1 year of sodium valproate and carbamazepine therapy. Epilepsy Res. 2002;48:71–5. doi: 10.1016/s0920-1211(01)00322-9. [DOI] [PubMed] [Google Scholar]
- 48.Maertens P, Dyken P, Graf W, Pippenger C, Chronister R, Shah A. Free radicals, anticonvulsants and the neuronal ceroid-lipofuscinoses. Am J Med Genet. 1995;57:225–8. doi: 10.1002/ajmg.1320570222. [DOI] [PubMed] [Google Scholar]
- 49.Schulpis KH, Lazaropoulou C, Regoutas S, Karikas GA, Margeli A, Tsakiris S, et al. Valproic acid monotherapy induces DNA oxidative damage. Toxicology. 2006;217:228–32. doi: 10.1016/j.tox.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Verrotti A, Scardapane A, Franzoni E, Manco R, Chiarelli F. Increased oxidative stress in epileptic children treated with valproic acid. Epilepsy Res. 2008;78:171–7. doi: 10.1016/j.eplepsyres.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Hamed SA, Abdellah MM, El-Melegy N. Blood levels of trace elements, electrolytes and oxidative stress/antioxidant systems in epileptic patients. J Pharmacol Sci. 2004;96:465–73. doi: 10.1254/jphs.fpj04032x. [DOI] [PubMed] [Google Scholar]
- 52.Ercegovac M, Jovic N, Simic T, Bumbasirevic LB, Sokic D, Radojevic AR. Antiepileptic drugs affect protein, lipid and DNA oxidative damage and antioxidant defense in patients with epilepsy. J Med Biochem. 2013;32:121–30. [Google Scholar]
- 53.Xavier SM, Barbosa CO, Barros DO, Silva RF, Oliveira AA, Freitas RM. Vitamin C antioxidant effects in hippocampus of adult Wistar rats after seizures and status epilepticus induced by pilocarpine. Neurosci Lett. 2007;420:76–9. doi: 10.1016/j.neulet.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 54.Ayyildiz M, Coskun S, Yildirim M, Agar E. The effects of ascorbic acid on penicillin-induced epileptiform activity in rats. Epilepsia. 2007;48:1388–95. doi: 10.1111/j.1528-1167.2007.01080.x. [DOI] [PubMed] [Google Scholar]
- 55.Sumanont Y, Murakami Y, Tohda M, Vajragupta O, Watanabe H, Matsumoto K. Prevention of kainic acid-induced changes in nitric oxide level and neuronal cell damage in the rat hippocampus by manganese complexes of curcumin and diacetylcurcumin. Life Sci. 2006;78:1884–91. doi: 10.1016/j.lfs.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 56.Liu SH, Chang CD, Chen PH, Su JR, Chen CC, Chaung HC. Docosahexaenoic acid and phosphatidylserine supplementations improve antioxidant activities and cognitive functions of the developing brain on pentylenetetrazol-induced seizure model. Brain Res. 2012;1451:19–26. doi: 10.1016/j.brainres.2012.02.060. [DOI] [PubMed] [Google Scholar]
- 57.Júnior JS, de Almeida AA, Tomé Ada R, Citó AM, Saffi J, de Freitas RM. Evaluation of possible antioxidant and anticonvulsant effects of the ethyl acetate fraction from Platonia insignis Mart.(Bacuri) on epilepsy models. Epilepsy Behav. 2011;22:678–84. doi: 10.1016/j.yebeh.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 58.Hulthe J, Fagerberg B. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR study) Arteriocler Thromb Vasc Biol. 2002;22:1162–7. doi: 10.1161/01.atv.0000021150.63480.cd. [DOI] [PubMed] [Google Scholar]
- 59.Holvoet P, Van Cleemput J, Collen D, Vanhaecke J. Oxidized low density lipoprotein is a prognostic marker of transplant-associated coronary artery disease. Arterioscler Thromb Vasc Biol. 2000;20:698–702. doi: 10.1161/01.atv.20.3.698. [DOI] [PubMed] [Google Scholar]
- 60.Hamed SA, Hamed EA, Hamdy R, Nabeshima T. Vascular risk factors and oxidative stress as independent predictors of asymptomatic atherosclerosis in adult patients with epilepsy. Epilepsy Res. 2007;74:183–92. doi: 10.1016/j.eplepsyres.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 61.Tan TY, Lu CH, Chuang HY, Lin TK, Liou CW, Chang WN, et al. Long-term antiepileptic drug therapy contributes to the acceleration of atherosclerosis. Epilepsia. 2009;50:1579–86. doi: 10.1111/j.1528-1167.2009.02024.x. [DOI] [PubMed] [Google Scholar]


