Abstract
Context:
Parkinson's disease (PD) is associated with sleep disturbances, attributed to the neurodegenerative process and therapeutic drugs. Studies have found levodopa to increase wakefulness in some patients while increasing sleepiness in others.
Aims:
To confirm sleep disturbances in drug naïve PD patients and understand the impact of levodopa on their sleep.
Materials and Methods:
Twenty-three drug naïve PD patients and 31 age-gender matched controls were compared using the Parkinson's Disease Sleep Scale (PDSS) and Epworth Sleepiness Scale (ESS). A polysomnogram objectively compared sleep quality. Of the 23 patients, the 12 initiated on levodopa were reassessed subjectively and through polysomnography after 2 months of therapy.
Statistical Analysis:
Data was expressed as mean ± standard deviation, median, and range. Continuous variables were analyzed by Student's T test for normally distributed data and Mann–Whitney U test for skewed data. Discrete variables were compared by Chi Square tests (Pearson Chi square Test or Fisher's Exact Test). Wilcoxon signed ranks test was applied in the analysis of paired data pre- and post-levodopa. A P value < 0.05 was considered as statistically significant. Statistical analysis of the data was done using the Statistical Package for the Social Sciences (SPSS) version 12.
Results:
Drug naïve PD patients had lower PDSS scores than controls. The sleep architecture changes observed on polysomnogram were reduced NREM Stage III and REM sleep and increased sleep latency and wake after sleep onset time. Following levodopa, improved sleep efficiency with reduced sleep latency and wake after sleep onset time was noted, coupled with improved PDSS scores. However, NREM Stage III and REM sleep duration did not increase.
Discussion:
PD patients take longer to fall asleep and have difficulty in sleep maintenance. Sleep maintenance is affected by nocturia, REM behavioral disorder, nocturnal cramps, akinesia, and tremors, as observed in PDSS scores. Levodopa improves sleep efficiency by improving motor scores without altering sleep architecture.
Conclusions:
Poor sleep quality and sleep architecture changes occur secondary to the neurodegenerative process in PD patients. Though levodopa improves sleep quality by reducing rigidity and tremor, it does not reverse sleep architecture changes.
Keywords: Levodopa on Parkinson's disease sleep, levodopa on sleep, sleep in Parkinson's disease
Introduction
Sleep disturbances have been reported in approximately 98% patients[1] of Idiopathic Parkinson's disease (PD). Nocturnal cramps, nocturia, early morning dystonia, nocturnal/early morning tremor, restless legs syndrome, and REM behavioral disorder (RBD) are some of the factors disrupting sleep in PD patients.
The objectives of our study were to confirm sleep disturbances in drug naïve PD patients and to check for reduced or additional sleep disturbance with the administration of levodopa. Both subjective methods (questionnaires) and objective (polysomnography) were used.
Materials and Methods
This prospective study was conducted in the Department of Neurology, Postgraduate Institute of Medical Education and Research, Chandigarh. The protocol of the study was approved by the Ethical Committee of the Institute. Twenty-three drug naïve PD patients satisfying the UK Parkinson's disease Brain Bank clinical diagnostic criteria[9] were enrolled into the study after obtaining informed consent. It was mandatory that no patient should have received levodopa or a dopamine agonist before inclusion into the study. The thirty-one age and gender matched healthy controls included hospital staff and relatives of the patient, other than the immediate caregiver, who did not suffer from any chronic ailment or did not have a history of chronic sedative, antidepressant, or alcohol use. Patients with coexisting chronic diseases that could affect sleep such as chronic obstructive pulmonary disease, ischemic heart disease, stroke, arthritis, diabetes mellitus, chronic liver disease, and chronic renal disease were excluded from the study.
With help of a face-to-face interview with patients and controls, information on demographic variables, symptoms, medication intake, and sleep difficulties were recorded. Semi-quantitative scales viz. Parkinson's Disease Sleep Scale (PDSS)[10] and Epworth sleepiness Scale (ESS)[11] were used to assess nighttime sleep problems and excessive daytime somnolence, respectively, in both patients and controls. PDSS is a visual analogue scale that addresses 15 problems commonly affecting sleep in Parkinson's disease patients. The patient has to answer each question by giving a score from zero to ten wherein zero indicates worst score and ten best score. Both scales were self-administered by educated patients and controls while for the uneducated patients, it was administered by the investigator during the interview. Patients and controls were also questioned during the interview for symptoms suggestive of REM behavioral disorder (RBD) and restless legs syndrome. Screening for coexisting depression was done using the Beck Depression Inventory (BDI) and dementia using the Mini Mental State Exam (MMSE) and patients or controls with severe depression (BDI >20) were excluded from the study. Patients were further assessed for severity of motor dysfunction by Unified Parkinson's Disease Rating Scale (UPDRS-III) motor score that assesses the extent of disability due to tremor, rigidity, bradykinesia, posture, gait, and postural stability.
Following the interview and clinical examination, both patients and controls underwent a standard in-laboratory overnight polysomnography. Any patient who was on a sedative/antidepressant was weaned off the drug and was assessed fifteen days after the drug was stopped. The polysomnogram included a 6 channel electroencephalogram (EEG), electrooculogram (EOG), chin and bilateral tibilais anterior surface electromyogram, electrocardiogram, airflow measurement by thermistor, thoracic and abdominal movements during respiration, snore microphone, and body position sensor. Parameters were assessed as per the American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events (2007).[12]
Out of the 23 patients included, 12 were started on levodopa therapy. Doses were slowly titrated as per patient requirement in order to reduce tremor and rigidity. These 12 patients were again assessed for sleep disturbances after two months using the same protocol as detailed above. Six of the remaining 11 patients were less than 50 years old and were started on anticholinergics with dopamine agonist therapy since it was decided to delay the initiation of levodopa therapy. The remaining 5 patients were not compliant on treatment. These 11 patients were not reassessed after 2 months.
Data was expressed as mean ± standard deviation, median, and range. Continuous variables were analyzed by Student's T test for normally distributed data and Mann–Whitney U test for skewed data. Discrete variables were compared by Chi Square tests (Pearson Chi square Test or Fisher's Exact Test). Wilcoxon signed ranks test was applied in the analysis of paired data pre- and post-levodopa. A P value < 0.05 was considered as statistically significant. Statistical analysis of the data was done using the Statistical Package for the Social Sciences (SPSS) version 12.
Results
Drug naïve PD patients had significantly poorer sleep efficiencies (mean 74%) as compared to controls (mean 87%). Patients had longer sleep latencies and reduced duration of slow wave sleep (NREM 3) and REM sleep [Table 1]. They spent longer periods of time awake after onset of the first sleep cycle (WASO) although they did not have greater arousal indices as compared to controls. The REM latencies were prolonged in patients but the difference was not statistically significant.
Table 1.
Sleep efficiency comparison between drug naïve and control
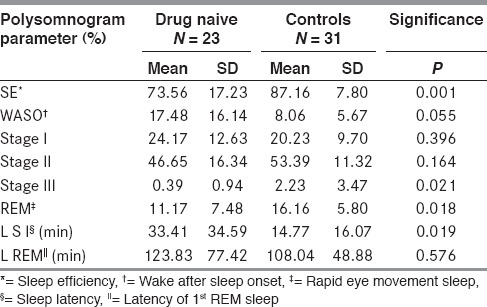
Periodic limb movement indices (PLMS Index) and Apnea Hypopnea indices (AHI) were higher than controls, although not statistically significant.
Drug naïve patients had lower scores as compared to controls on total PDSS score as well as on most of the individual items of the PDSS. The scores were significantly lower on PDSS 3 (difficulty in sleep maintenance), PDSS 6 (distressing nighttime dreams), PDSS 8 (nocturia), PDSS 11 (nocturnal muscle cramps), PDSS 12 (painful dystonia), and PDSS 13 (tremor on awakening) [Table 2]. Patients also frequently complained of un-refreshing sleep (PDSS 14).
Table 2.
PDSS* comparison drug naïve vs. control
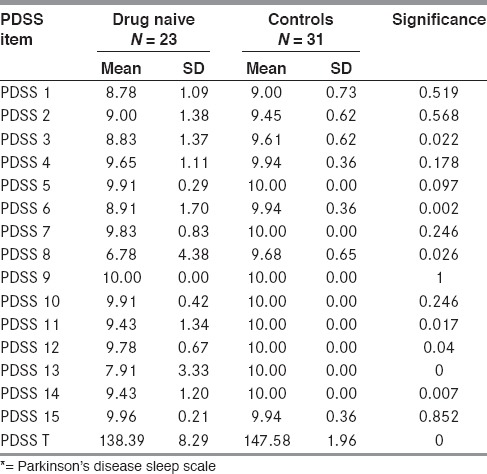
REM behavioral disorder (history of dream enactment behavior and loss of chin atonia during REM sleep documented on polysomnography) was found in 22% PD patients as compared to being absent in controls.
PD patients did not complain of excessive daytime sleepiness. Although ESS scores were higher in PD patients, they were not found to be statistically significant compared to controls.
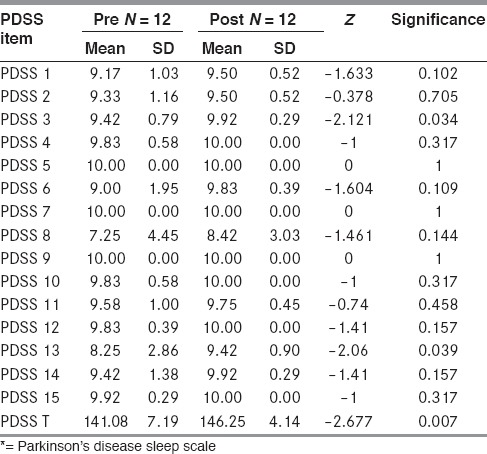
In the 12 patients who received levodopa therapy, a significant improvement in sleep efficiency (75.3% vs. 86.4%) was observed after two months of levodopa. An improvement was observed on most items of the PDSS but the improvements in scores were statistically significant for total PDSS, PDSS 3, and PDSS 13 [Table 3].
Table 3.
PDSS* comparison Pre vs. Post levodopa
The sleep architecture, however, did not change significantly except for a reduction in WASO, shorter sleep latency, and a marginal increase in Stage I NREM sleep. The amount of NREM Stage II and III as well as REM sleep remained unchanged [Table 4]. REM latency was prolonged (168.21 ±82.95 min) after levodopa as compared to before initiation of the drug (114.27 ± 68.22 min). A mild increase in periodic limb movements in sleep as well as the periodic limb movement arousal index was observed after levodopa. This increase in mean PLMS index was mainly attributable to a single patient who developed periodic limb movements in sleep (PLMS) after levodopa. This patient, however, did not complain of restless legs and was not aware of PLMS.
Table 4.
Sleep efficiency comparison between pre vs. post
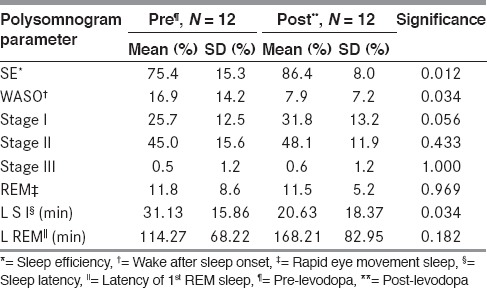
Arousal indices and AHI did not differ before and after levodopa therapy. Patients and their relatives also did not complain of an increase in distressing nighttime dreams or dream enactment behavior or nighttime hallucinations after levodopa.
No change in daytime alertness was noted as seen by same scores on ESS.
All the 12 patients responded to levodopa therapy, with improvement in the mean UPDRS-III motor score at two months being 14.5 points (P = 0.002). Becks depression scores were significantly higher in the patient group at baseline as compared to controls. There was a significant improvement in the Becks depression score in the 12 patients after levodopa therapy (P = 0.01).
Discussion
Drug naïve PD patients report sleep disturbances more frequently than age-matched healthy controls. They have difficulties in sleep maintenance, and while their arousal indices are lower than those of controls, they remain awake for longer periods of time after every nocturnal awakening. This difficulty in sleep maintenance could be due to nocturia, REM behavioral disorder, nocturnal cramps, akinesia, and tremors disturbing sleep. A prior study performed by the same investigator group on IPD patients who were already receiving dopaminomimetic drugs found similar factors affecting sleep. They also found that the factors remained the same irrespective of disease duration except for increasing bladder dysfunction with longer disease duration. Nocturia was worse and patients complained of bed-wetting due to motor off symptoms as the disease progressed. Dhawan et al.[5] reported similar results in a study comparing drug naive PD patients and advanced PD with controls.
Following levodopa, an improvement was noted in most items of the PDSS score but this was statistically significant for PDSS 3 (better sleep maintenance), PDSS 13 (tremor on awakening), and PDSS Total. The simultaneous improvement in the UPDRS-III motor score could have contributed in reducing the akinesia and tremor that disturb sleep. There was no change in distressing dreams or nighttime hallucinations and no increase in the incidence of RBD after levodopa. Nocturia also improved after levodopa therapy indicating a partial response to dopaminergic therapy as reported in earlier animal studies.[13]
The sleep architectural changes in the form of reduced Stage III NREM, REM sleep and prolonged sleep latency, and increased wake after sleep onset time has also been reported by Kales et al.[2] in a study of 6 PD patients before initiation of levodopa.
In the present study, after levodopa treatment, although there was an 11% improvement in sleep efficiency, there were no significant changes in slow wave sleep and REM sleep. Sleep latency and WASO reduced after levodopa, and there was a small increase in amount of Stage I NREM sleep. REM latency increased although the increase was not statistically significant. A similar improvement in sleep efficiency, reduction in WASO with a significant increase in Stage II sleep, and no alteration in REM sleep was seen in a study by Askenasy et al.[8] Kales et al.,[2] after administering levodopa to six PD patients and controls for one month, observed no significant changes in parameters evaluating sleep initiation and sleep maintenance or REM sleep in both groups.
An increase in periodic limb movements was observed after levodopa in a single patient. No significant change in arousal index and apnea hypopnea index was noted after levodopa. No change was observed in daytime alertness in PD patients after levodopa therapy.
Thus, it appears that sleep disturbances and sleep architectural changes in Idiopathic Parkinson's disease are universal to all stages of the illness and occur primarily due to the neurodegenerative changes of the disease rather than levodopa. Levodopa can have a minor role in improving the subjective quality of sleep by reducing the disability caused by nocturnal akinesia and tremors that often disturb sleep. However, levodopa does not appear to change the underlying de-structuring of the sleep architecture in PD patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Factor SA, McAlarney T, Sanchez-Ramos JR, Weiner WJ. Sleep disorders and sleep effect in Parkinson's disease. Mov Disord. 1990;5:280–5. doi: 10.1002/mds.870050404. [DOI] [PubMed] [Google Scholar]
- 2.Kales A, Ansel RD, Markham CH, Scharf MB, Tan TL. Sleep in patients with Parkinson's disease and normal subjects prior to and following levodopa administration. Clin Pharmacol Ther. 1971;12:397–406. doi: 10.1002/cpt1971122part2397. [DOI] [PubMed] [Google Scholar]
- 3.Shpirer I, Miniovitz A, Klein C, Goldstein R, Prokhorov T, Theitler J, et al. Excessive daytime sleepiness in patients with Parkinson's disease: A polysomnography study. Mov Disord. 2006;21:1432–8. doi: 10.1002/mds.21002. [DOI] [PubMed] [Google Scholar]
- 4.Diederich NJ, Vaillant M, Mancuso G, Lyen P, Tiete J. Progressive sleep ‘destructuring’ in Parkinson´s disease. A polysomnographic study in 46 patients. Sleep Med. 2005;6:313–8. doi: 10.1016/j.sleep.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Dhawan V, Dhoat S, Williams AJ, Dimarco A, Pal S, Forbes A, et al. The range and nature of sleep dysfunction in untreated Parkinson's diasease (PD). A comparative controlled clinical study using the Parkinson's disease sleep scale and selective polysomnography. J Neurol Sci. 2006;248:158–62. doi: 10.1016/j.jns.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson AN, Pascoe PA. Dopaminergic transmission and the sleep-wakefulness continiuum in man. Neuropharmacology. 1990;29:411–7. doi: 10.1016/0028-3908(90)90101-v. [DOI] [PubMed] [Google Scholar]
- 7.van Hilten B, Hoff JI, Middelkoop HA, van der Velde EA, Kerkhof GA, Wauguier A, et al. Sleep disruption in Parkinson's disease. Assessment by continuous activity monitoring. Arch Neurol. 1994;51:922–8. doi: 10.1001/archneur.1994.00540210094018. [DOI] [PubMed] [Google Scholar]
- 8.Askenasy JJ, Yahr MD. Reversal of sleep disturbance in Parkinson's disease by antiparkinsonian therapy: A preliminary study. Neurology. 1985;35:527–32. doi: 10.1212/wnl.35.4.527. [DOI] [PubMed] [Google Scholar]
- 9.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhuri KR, Pal S, Dimarco A, Whately-Smith C, Bridgman K, Mathew R, et al. The Parkinson's disease sleep scale: A new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002;73:629–35. doi: 10.1136/jnnp.73.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johns M, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep. 1997;20:844–9. doi: 10.1093/sleep/20.10.844. [DOI] [PubMed] [Google Scholar]
- 12.Iber C, Ancoli-Israel S, Chessonn A, Quan SF. 1st ed. Westchester: American Academy of Sleep Medicine; 2007. In The AASM Manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. [Google Scholar]
- 13.Yoshimura N, Mizuta E, Yoshida O, Kuno S. Therapeutic effects of dopamine D1/D2 receptor agonists on detrusor hyperreflexia in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned parkinsoniancynomolgus monkeys. J Pharmacol Exp Ther. 1998;286:228–33. [PubMed] [Google Scholar]


