Abstract
Fungi are a relatively uncommon cause of brain abscess in neonates and early infancy. They are usually associated with predisposing factors like prematurity, low birth weight, use of broad-spectrum antibiotics, and prolonged stay in the intensive care unit. Candida tropicalis (C. tropicalis) is rapidly emerging as a nosocomial threat in the neonatal intensive care settings. This case report describes a neonate with C. tropicalis brain abscess who was diagnosed early and managed aggressively with a favorable outcome. Inadvertent use of intravenous antibiotics can have serious complications such as invasive fungal infection. Correct microbiological diagnosis is the key to successful treatment of deep-seated pyogenic infection. Fungal etiology should always be studied in relevant clinical settings.
Keywords: Brain abscess, Candida tropicalis, fungal, neonate
Introduction
Brain abscess refers to a focal pyogenic infection of the brain parenchyma, fungi being a relatively uncommon cause. Predisposing risk factors include low birth weight, prematurity, immunodeficiency, prolonged intensive care stay and ventilation, extensive use of broad-spectrum antibiotics, and sepsis.[1]
Global data in pediatric brain abscess reports fungal etiology in 20% cases with a very high mortality (almost 80%).[2] Indian data shows a prevalence of even less than 1%.[3] Most commonly, brain abscess in a neonate is caused by Gram-negative bacilli.[4,5] This case report describes an extremely rare cause for cerebral abscess in a neonate and how timely and appropriate intervention resulted in a favorable outcome.
Case Report
A full-term male neonate was born in a nursing home (not the current centre) to a second gravida mother by lower segment caesarean section (LSCS) (indication: Previous LSCS) with a birth weight of 3.2 kg and delayed cry at birth (Apgar score: 7, 8, 9) without significant antenatal complaints. The baby had poor suck and feeding since birth and was admitted to the neonatal intensive care unit (NICU) of that nursing home. There was no respiratory distress/jaundice/bleeding/skin rashes/ear discharge/delayed separation of cord/umbilical discharge/convulsions. Sepsis screen (Hemoglobin: 12.5 g%, Total leukocyte count: 14,500/mm3, Platelet count: 2.4 lacs/mm3, peripheral smear for band count: <10%, microESR: 9 mm/hour, C reactive protein: 6 g/L) was negative and blood culture was sterile. However, the baby received intravenous antibiotics in the form of ceftriaxone (50 mg/kg/day in two divided doses) and amikacin (15 mg/kg/day in three divided doses) through a peripheral line. By the fifth day, he was alert, active, and discharged from the nursing home with an advice to continue intravenous antibiotics for the following nine days. The reason for administration of antibiotics was not clearly mentioned in the discharge card; however, it seemed that they were administered as the baby was admitted in NICU.
He remained asymptomatic for one week after the completion of antibiotics and thereafter developed vomiting with decreased feed intake. Intravenous antibiotics (ceftriaxone and amikacin in the same dosage) were restarted from the primary nursing home and continued for next two weeks. He continued to have frequent vomiting episodes with poor weight gain on exclusive breast feeds. There was no history of fever/abnormal movements or posturing/worsening of sensorium. He presented for the first time to the current centre on day 40 of life.
On examination, the baby had stable vitals with level and pulsatile anterior fontanelle. Otoscopic and fundus examination were normal. On central nervous system examination, he was an alert baby with preserved state to state variability, full extraocular movements, normal tone, and no paucity of movements. In view of persistent vomiting, prior to lumbar puncture, contrast enhanced-CT brain was done which revealed a 5 × 5 cm enhancing, well-defined hypodense lesion in the left frontal region with hydrocephalus [Figure 1a]. The child was started on intravenous meropenem and vancomycin. Complete hemogram (Hb - 12.9 gm%, TLC: 10900, DLC: P62L35M3, Platelet count: 2.2 lacs/mm3), blood and urine cultures (bacterial and fungal, all four cultures sterile), liver function tests (T. Bilirubin: 0.8 mg%, SGOT: 28, SGPT: 33, ALP: 160, total protein: 6.4 gm%, albumin: 4.0 gm%) and renal parameters (Blood urea: 34 mg%, serum creatinine: 0.7 gm%) did not reveal any abnormality. In view of the large size and hydrocephalus, the abscess was drained through a burr hole and Omaya reservoir was placed. The pus culture grew Candida tropicalis. There was no evidence of oropharyngeal or perineal thrush. Ultrasound pelvis did not reveal any urinary tract fungal balls. Echocardiography and ophthalmological evaluation was normal. Mother's HIV status and the infant's immunodeficiency work up (complete immunoglobulin profile: IgA – 125 mg/L, IgD – 31 mg/L, IgE – 122 kIU/L, IgG - 7.2 g/L, IgM – 192 mg/L; CD4 and CD8 lymphocyte count - 1700 cells/mm3, and 900 cells/mm3, nitroblue tetrazolium test: >90% activity as compared to control) were normal. Intravenous antibacterials were stopped within four days, and intravenous antifungals (amphotericin B 1 mg/kg/day and flucystosine 120 mg/kg/day in four divided doses) were administered for six weeks. Hemogram, serum electrolytes, and liver and kidney function tests were monitored biweekly, and no abnormality was detected. The Omaya reservoir was used only for cerebrospinal fluid analysis; no antibiotic was instilled through it. There was no family history of repeated infection suggestive of immunodeficiency. Repeat neuroimaging showed significant reduction in the size of abscess [Figure 1b]. Cerebrospinal fluid analysis done after six weeks of intravenous antibiotics was acellular and sterile. Omaya reservoir was removed and child underwent ventriculoperitoneal shunt placement. At discharge, the child was asymptomatic. Presently, he is eight months old, except for gross motor delay he is gaining milestones normally, does not have seizures, and has normal head circumference, vision, and hearing.
Figure 1.
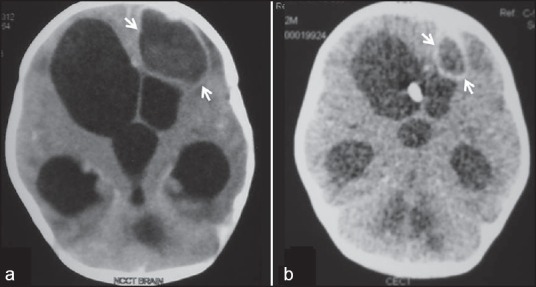
Contrast enhanced CT scan of the head: Contrast enhanced axial CT scan image of the brain (a) shows a welldefined peripheral enhancing cystic lesion (arrows) in left frontal lobe with surrounding edema consistent with abscess with marked hydrocephalus. Follow-up CT scan after antifungal treatment (b) shows near complete resolution of the abscess
Conclusion
Candida species are currently one of the commonest cause of nosocomial infection in NICUs with C. tropicalis emerging as a frequent offending agent.[6] Systemic fungal disease in a neonate is usually secondary to Candida species, particularly Candida albicans (C. albicans).[7] C. albicans causing neonatal brain abscess and meningitis has been previously reported.[8] In the current case, C. tropicalis was identified in pus cultured from brain abscess of an infant which to our knowledge has not been reported as a causative strain for fungal brain abscess. However, in the past, an outbreak of C. tropicalis fungemia in a NICU from India has been described. Sixteen neonates grew the organism in their blood and 14 of them also demonstrated it in their urine cultures. Environmental sampling done from mattresses and blankets used for neonates also yielded the same organism.[6] Primary candidiasis of the brain is rare, usually it is secondary to hematogenous dissemination.[9] Even then, only 4% of neonates with candidial sepsis develop brain abscess.[10] Interestingly in this case there was no evidence of candidemia.
The various risk factors described with neonatal-onset invasive candidiasis are prematurity, broad-spectrum antibiotics and steroid therapy, prolonged endotracheal intubation, central venous catheters, and parenteral alimentation.[6] In the current case, the prolonged nonjudicoius administration of antibiotics through the peripheral line could have introduced the infection. Intracerebral abscess should be considered in an infant presenting with altered sensorium, raised intracranial tension, and new onset focal neurological deficits. In early infancy, focal neurological deficits may be difficult to elicit; therefore, non-specific features such as vomiting and refusal to feed may be the only indicators of an intracranial pathology, as in this case.
The brain lesions described with candidiasis are focal infarctions, cerebritis, abscesses, and granulomas. Classically, cerebral microabscesses are described.[1] Cerebral microabscesses secondary to C. albicans have been described previously in preterm neonates.[10] The peculiarity in the current case was a solitary abscess in the left frontal lobe.
There is insufficient evidence to make specific recommendations for antifungal treatment of cerebral abscess. The duration of antibiotic therapy (usually 6-8 weeks) depends on the organism and response to treatment.[11] Systemic Amphotericin B is the first line of treatment for invasive fungal infections. Echinocandins and pyrimidine analogues (5-Flucytosine) are used as second line or add on therapy.[1] Lately, Fluconazole is being used sparingly, particularly in invasive candidiasis, in view of emerging resistance.[6] In the current case, intravenous Amphotericin B and 5-Flucytosine were given for six weeks with documented significant response and no adverse effects.
Ideally, craniotomy is recommended for fungal brain abscess. However in neonatal brain abscess, burrhole puncture, aspiration, and irrigation is the preferred method, craniotomy is recommended only for multiloculated, large abscesses usually of posttraumatic etiology with foreign material.[4,12] Till date controversies exist between complete medical management and add on surgical management of brain abscess, evidence is gathering that aspiration of abscess aids in microbiological diagnosis and appropriate antibiotic administration.[13] In the current case, although burrhole drainage was done because of the size (>2.5 cm),[11] eventually it played the most important role in deciding management.
Overall mortality rate in systemic candidiasis ranges from 35%-80% because of delayed onset of therapy, severe underlying disease, and drug resistance. However, timely management causes drastic reduction in mortality.[6,14,15] Sterile CSF, normal size of ventricles on CT scan, absence of seizure, and early aspiration are associated with favorable outcome in neonatal brain abscess.[5] Except for presence of hydrocephalus all other criteria were fulfilled in the current case.
This case highlights the emergence of neonatal candidemia as a nosocomial menace particularly in NICU set up with a shift towards non-albicans (tropicalis being the leading pathogen) species in recent years.[6] Hospitals should follow stringent policies regarding asepsis maintenance, antibiotic administration, use of invasive procedures and length of stay in the intensive care units. However in many cases these scenarios are unavoidable and hence a reasonable index of suspicion is warranted for fungal infections. In this case, definite microbiological diagnosis aided by surgical drainage and aspiration of abscess coupled with prompt and appropriate antibiotic administration resulted in favorable outcome in a case of extremely rare and fatal brain abscess.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Raman Sharma R. Fungal infections of the nervous system: Current perspective and controversies in management. Int J Surg. 2010;8:591–601. doi: 10.1016/j.ijsu.2010.07.293. [DOI] [PubMed] [Google Scholar]
- 2.Goodkin HP, Harper MB, Pomeroy SL. Intracerebral abscess in children: Historical trends at children's hospital Boston. Pediatrics. 2004;113:1765–70. doi: 10.1542/peds.113.6.1765. [DOI] [PubMed] [Google Scholar]
- 3.Prasad KN, Mishra AM, Gupta D, Husain N, Husain M, Gupta RK. Analysis of microbial etiology and mortality in patients with brain abscess. J Infect. 2006;53:221–7. doi: 10.1016/j.jinf.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Krajewski R, Stelmasiak Z. Brain abscess in infants. Childs Nerv Syst. 1992;8:279–80. doi: 10.1007/BF00300796. [DOI] [PubMed] [Google Scholar]
- 5.Renier D, Flandin C, Hirsch E, Hirsch JF. Brain abscesses in neonates. A study of 30 cases. J Neurosurg. 1988;69:877–82. doi: 10.3171/jns.1988.69.6.0877. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhary A, Becker K, Fegeler W, Gugnani HC, Kapoor L, Randhawa VS, et al. An outbreak of candidemia due to Candida tropicalis in a neonatal intensive care unit. Mycoses. 2003;46:287–92. doi: 10.1046/j.1439-0507.2003.00883.x. [DOI] [PubMed] [Google Scholar]
- 7.Rao S, Ali U. Systemic fungal infections in neonates. J Postgrad Med. 2005;51:27–9. [PubMed] [Google Scholar]
- 8.Ancalle IM, Rivera JA, Garcia I, Garcia L, Valcarcel M. Candida albicans meningitis and brain abscesses in a neonate: A case report. Bol Asoc Med PR. 2010;102:45–8. [PubMed] [Google Scholar]
- 9.Khandelwal N, Gupta V, Singh P. Central nervous system fungal infections in tropics. Neuroimag Clin N Am. 2011;21:859–66. doi: 10.1016/j.nic.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Pahud BA, Greenhow TL, Piecuch B, Weintrub PS. Preterm neonates with candidal brain microabscesses: A case series. J Perinatol. 2009;29:323–6. doi: 10.1038/jp.2008.201. [DOI] [PubMed] [Google Scholar]
- 11.Honda H, Warren DK. Central nervous system infections: Meningitis and brain abscess. Infect Dis Clin North Am. 2009;23:609–23. doi: 10.1016/j.idc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 12.de Oliviera RS, Pinho VF, Madureira JF, Machado HR. Brain abscess in a neonate: An unusual presentation. Childs Nerv Syst. 2007;23:139–42. doi: 10.1007/s00381-006-0239-8. [DOI] [PubMed] [Google Scholar]
- 13.Smith SJ, Ughratdar I, MacArthur DC. Never go to sleep on undrained pus: A retrospective review of surgery for intraparenchymal cerebral abscess. Br J Neurosurg. 2009;23:412–7. doi: 10.1080/02688690902887549. [DOI] [PubMed] [Google Scholar]
- 14.Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Hospital acquired candidaemia: Attributable mortality and excess lenth of stay. Arch Intern Med. 1988;148:2642–5. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]
- 15.Stratov I, Gottlieb T, Bradbury R, O’Kane GM. Candidaemia in an Australian teaching hospital: Relationship to central line and TPN use. J Infect. 1998;36:203–7. doi: 10.1016/s0163-4453(98)80014-5. [DOI] [PubMed] [Google Scholar]


