Abstract
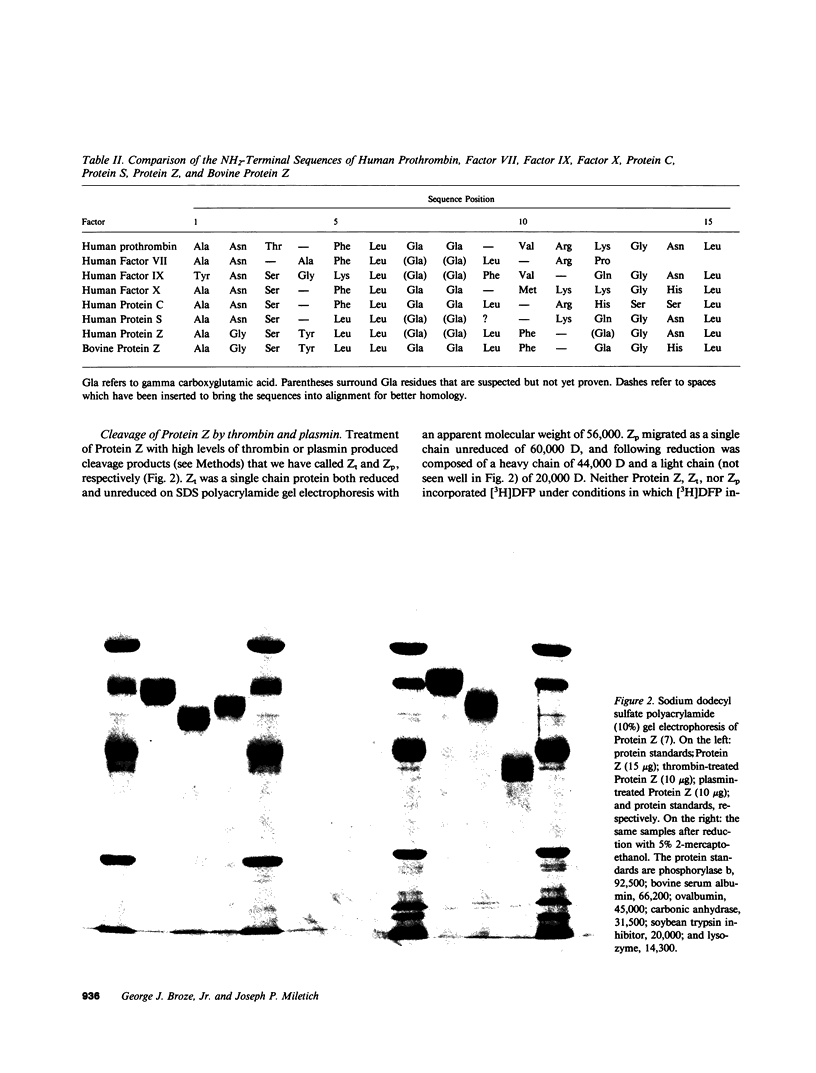
Protein Z was purified from human plasma by a four-step procedure which included barium citrate adsorption, ammonium sulfate fractionation, DEAE-Sepharose chromatography, and blue agarose chromatography with a yield of 20%. It is a 62,000 mol wt protein with an extinction coefficient of 12.0. The concentration of Protein Z in pooled, citrated plasma is 2.2 micrograms/ml and its half-life in patients starting warfarin anticoagulation therapy is estimated to be less than 2.5 d. The NH2-terminal sequence is Ala-Gly-Ser-Tyr-Leu-Leu-(Gla)-(Gla)-Leu-Phe-(Gla)-Gly-Asn-Leu. Neither Protein Z nor its cleavage products, which were obtained by treatment of Protein Z with thrombin or plasmin, incorporated [3H]diisopropyl fluorophosphate. The physiological function of Protein Z remains unknown.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babul J., Stellwagen E. Measurement of protein concentration with interferences optics. Anal Biochem. 1969 Apr 4;28(1):216–221. doi: 10.1016/0003-2697(69)90172-9. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Broze G. J., Jr, Majerus P. W. Purification and properties of human coagulation factor VII. J Biol Chem. 1980 Feb 25;255(4):1242–1247. [PubMed] [Google Scholar]
- Castellino F. J., Powell J. R. Human plasminogen. Methods Enzymol. 1981;80(Pt 100):365–378. doi: 10.1016/s0076-6879(81)80031-6. [DOI] [PubMed] [Google Scholar]
- Castellino F. J., Sodetz J. M. Rabbit plasminogen and plasmin isozymes. Methods Enzymol. 1976;45:273–286. doi: 10.1016/s0076-6879(76)45026-7. [DOI] [PubMed] [Google Scholar]
- Dahlbäck B. Purification of human vitamin K-dependent protein S and its limited proteolysis by thrombin. Biochem J. 1983 Mar 1;209(3):837–846. doi: 10.1042/bj2090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Scipio R. G., Hermodson M. A., Yates S. G., Davie E. W. A comparison of human prothrombin, factor IX (Christmas factor), factor X (Stuart factor), and protein S. Biochemistry. 1977 Feb 22;16(4):698–706. doi: 10.1021/bi00623a022. [DOI] [PubMed] [Google Scholar]
- DiScipio R. G., Davie E. W. Characterization of protein S, a gamma-carboxyglutamic acid containing protein from bovine and human plasma. Biochemistry. 1979 Mar 6;18(5):899–904. doi: 10.1021/bi00572a026. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Suttie J. W., Jackson C. M. The functional significance of vitamin K action. Difference in phospholipid binding between normal and abnormal prothrombin. J Biol Chem. 1975 Jun 10;250(11):4095–4099. [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Analysis of phenylthiohydantoins by ultrasensitive gradient high-performance liquid chromatography. Methods Enzymol. 1983;91:486–493. doi: 10.1016/s0076-6879(83)91045-5. [DOI] [PubMed] [Google Scholar]
- Højrup P., Roepstorff P., Petersen T. E. Amino-acid sequence of the vitamin-K-dependent part of protein Z. Eur J Biochem. 1982 Aug;126(2):343–348. doi: 10.1111/j.1432-1033.1982.tb06784.x. [DOI] [PubMed] [Google Scholar]
- Kisiel W. Human plasma protein C: isolation, characterization, and mechanism of activation by alpha-thrombin. J Clin Invest. 1979 Sep;64(3):761–769. doi: 10.1172/JCI109521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisiel W., McMullen B. A. Isolation and characterization of human factor VIIa. Thromb Res. 1981 May 1;22(3):375–380. doi: 10.1016/0049-3848(81)90130-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Mattock P., Esnouf M. P. A form of bovine factor X with a single polypeptide chain. Nat New Biol. 1973 Mar 21;242(116):90–92. doi: 10.1038/newbio242090a0. [DOI] [PubMed] [Google Scholar]
- McMullen B. A., Fujikawa K., Kisiel W., Sasagawa T., Howald W. N., Kwa E. Y., Weinstein B. Complete amino acid sequence of the light chain of human blood coagulation factor X: evidence for identification of residue 63 as beta-hydroxyaspartic acid. Biochemistry. 1983 Jun 7;22(12):2875–2884. doi: 10.1021/bi00281a016. [DOI] [PubMed] [Google Scholar]
- Mecham R. P., Lange G. Antigenicity of elastin: characterization of major antigenic determinants on purified insoluble elastin. Biochemistry. 1982 Feb 16;21(4):669–673. doi: 10.1021/bi00533a013. [DOI] [PubMed] [Google Scholar]
- Miletich J. P., Broze G. J., Jr, Majerus P. W. The synthesis of sulfated dextran beads for isolation of human plasma coagulation factors II, IX, and X. Anal Biochem. 1980 Jul 1;105(2):304–310. doi: 10.1016/0003-2697(80)90462-5. [DOI] [PubMed] [Google Scholar]
- Miletich J. P., Jackson C. M., Majerus P. W. Properties of the factor Xa binding site on human platelets. J Biol Chem. 1978 Oct 10;253(19):6908–6916. [PubMed] [Google Scholar]
- Nelsestuen G. L., Zytkovicz T. H., Howard J. B. The mode of action of vitamin K. Identification of gamma-carboxyglutamic acid as a component of prothrombin. J Biol Chem. 1974 Oct 10;249(19):6347–6350. [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Petersen T. E., Thłgersen H. C., Sottrup-Jensen L., Magnusson S., Jörnvall H. Isolation and N-terminal amino acid sequence of protein Z, a gamma-carboxyglutamic acid containing protein from bovine plasma. FEBS Lett. 1980 Jun 2;114(2):278–282. doi: 10.1016/0014-5793(80)81133-1. [DOI] [PubMed] [Google Scholar]
- Prowse C. V., Esnouf M. P. The isolation of a new warfarin-sensitive protein from bovine plasma. Biochem Soc Trans. 1977;5(1):255–256. doi: 10.1042/bst0050255. [DOI] [PubMed] [Google Scholar]
- Seegers W. H., Ghosh A. Activation of prothrombin and factor X: function of previously unrecognized plasma protein. Thromb Res. 1980 Jan 1;17(1-2):71–81. doi: 10.1016/0049-3848(80)90295-9. [DOI] [PubMed] [Google Scholar]
- Seegers W. H., Ghosh A. The activation of factor X and factor X beta with factor VII or protein M. Thromb Res. 1980 Feb 1;17(3-4):501–506. doi: 10.1016/0049-3848(80)90085-7. [DOI] [PubMed] [Google Scholar]
- Stenflo J., Fernlund P., Egan W., Roepstorff P. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2730–2733. doi: 10.1073/pnas.71.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]