Abstract
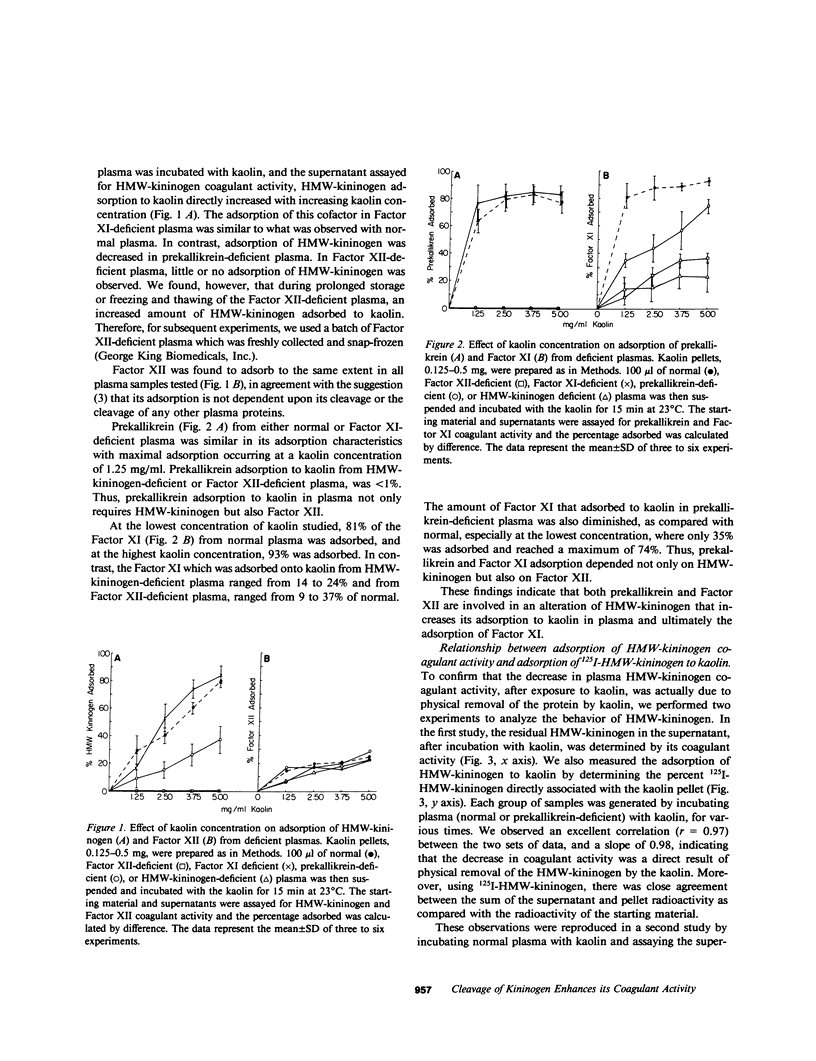
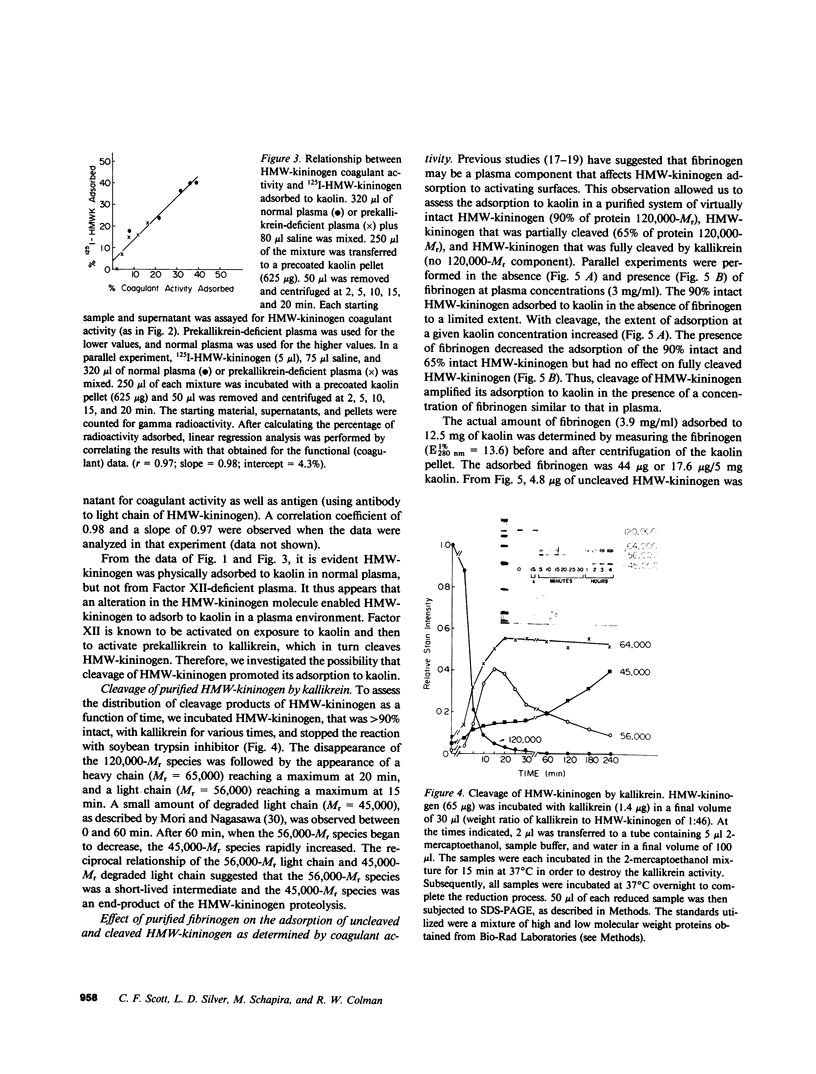
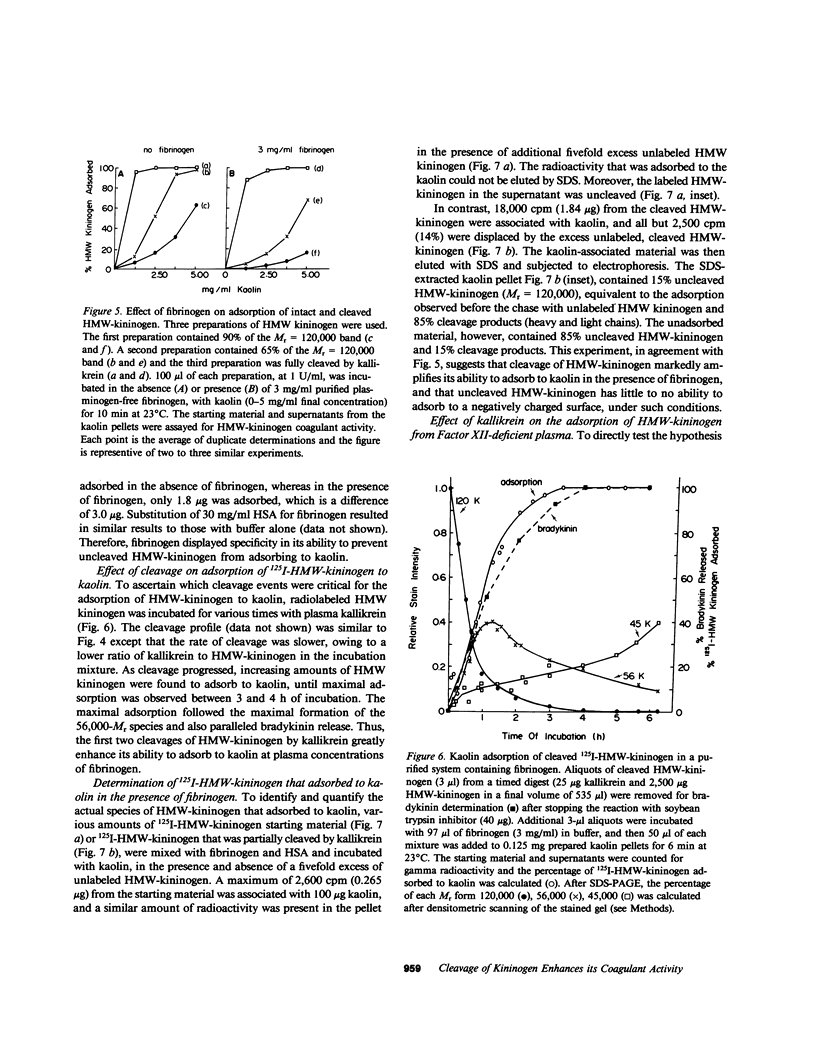
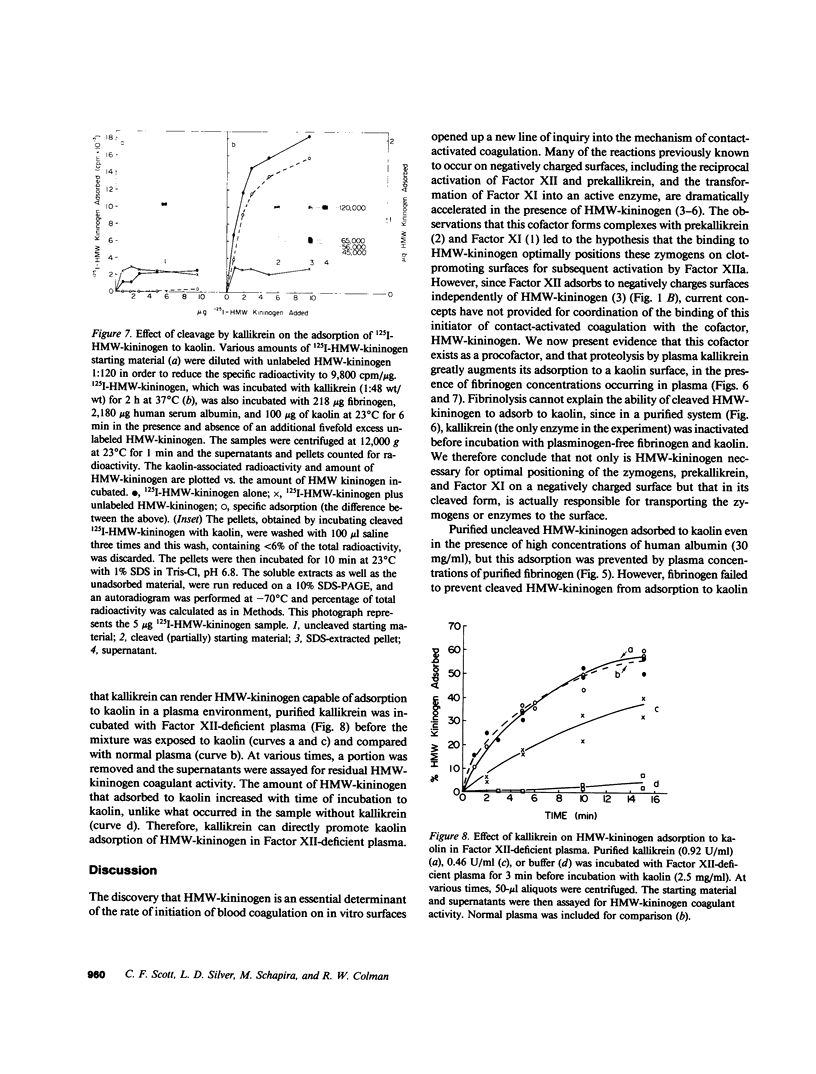
High molecular weight kininogen (HMW)-kininogen, the cofactor of contact-activated blood coagulation, accelerates the activation of Factor XII, prekallikrein, and Factor XI on a negatively charged surface. Although prekallikrein and Factor XI circulate as a complex with HMW-kininogen, no physical association has been demonstrated between Factor XII and HMW-kininogen, nor has the order of adsorption to surfaces of these proteins been fully clarified. In this report we explore the requirements for adsorption of HMW-kininogen to a clot-promoting surface (kaolin), in purified systems, as well as in normal plasma and plasma genetically deficient in each of the proteins of the contact system. The fraction of each coagulant protein associated with the kaolin pellet was determined by measuring the difference in coagulant activity between the initial sample and supernatants after incubation with kaolin, or by directly quantifying the amount of 125I-HMW-kininogen that was associated with the kaolin pellet. In normal plasma, the adsorption of HMW-kininogen to kaolin increased as the quantity of kaolin was increased in the incubation mixture. However, the HMW-kininogen in Factor XII-deficient plasma did not absorb appreciably to kaolin. Furthermore, the quantity of HMW-kininogen from prekallikrein-deficient plasma that adsorbed to kaolin was decreased as compared with normal plasma. These observations suggested that HMW-kininogen in plasma must be altered by a reaction involving both Factor XII and prekallikrein in order for HMW-kininogen to adsorb to kaolin, and to express its coagulant activity. Subsequently, the consequence of the inability of HMW-kininogen to associate with a negatively charged surface results in decreased surface activation. This assessment was derived from the further observation of the lack of prekallikrein adsorption and the diminished Factor XI adsorption in both Factor XII-deficient and HMW-kininogen-deficient plasmas, since these two zymogens (prekallikrein and Factor XI) are transported to a negatively charged surface in complex with HMW-kininogen. The percentage of HMW-kininogen coagulant activity that adsorbed to kaolin closely correlated (r = 0.98, slope = 0.97) with the amount of 125I-HMW-kininogen adsorbed, suggesting that adsorption of HMW-kininogen results in the expression of its coagulant activity. Since kallikrein, which is known to cleave HMW-kininogen, is generated when kaolin is added to plasma, we tested the hypothesis that proteolysis by kallikrein was responsible for the enhanced adsorption of HMW-kininogen to kaolin. When purified HMW-kininogen was incubated with purified kallikrein, its ability to absorb to kaolin increased with time of digestion until a maximum was reached. Moreover, (125)I-HMW-kininogen, after cleavage by kallikrein, had markedly increased affinity for kaolin than the uncleaved starting material. Furthermore, fibrinogen, at plasma concentration (3 mg/ml), markedly curtailed the adsorption of a mixture of cleaved and uncleaved HMW-kininogen to kaolin, but was unable to prevent fully cleaved HMW-kininogen from adsorbing to the kaolin. Addition of purified kallikrein to Factor XII-deficient plasma, which bypasses Factor XII-dependent contact-activation amplified the ability of its HMW-kininogen to adsorb to kaolin. These observations indicate that HMW-kininogen is a procofactor that is activated by kallikrein, a product of a reaction which it accelerates. This cleavage, which enhances its association with a clot-promoting surface in a plasma environment, is an event that is necessary for expression of its cofactor activity. These interactions would allow coordination of HMW-kininogen adsorption with the adsorption of Factor XII, which adsorbs independently of cleavage, to the same negatively charged surface.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cochrane C. G., Revak S. D. Dissemination of contact activation in plasma by plasma kallikrein. J Exp Med. 1980 Sep 1;152(3):608–619. doi: 10.1084/jem.152.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman R. W., Bagdasarian A., Talamo R. C., Scott C. F., Seavey M., Guimaraes J. A., Pierce J. V., Kaplan A. P. Williams trait. Human kininogen deficiency with diminished levels of plasminogen proactivator and prekallikrein associated with abnormalities of the Hageman factor-dependent pathways. J Clin Invest. 1975 Dec;56(6):1650–1662. doi: 10.1172/JCI108247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman R. W. The effect of proteolytic enzymes on bovine factor V. I. Kinetics of activation and inactivation by bovine thrombin. Biochemistry. 1969 Apr;8(4):1438–1445. doi: 10.1021/bi00832a019. [DOI] [PubMed] [Google Scholar]
- Davie E. W., Fujikawa K., Kurachi K., Kisiel W. The role of serine proteases in the blood coagulation cascade. Adv Enzymol Relat Areas Mol Biol. 1979;48:277–318. doi: 10.1002/9780470122938.ch6. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Griffin J. H., Cochrane C. G. Mechanisms for the involvement of high molecular weight kininogen in surface-dependent reactions of Hageman factor. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2554–2558. doi: 10.1073/pnas.73.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultin M. B., Nemerson Y. Activation of factor X by factors IXa and VIII; a specific assay for factor IXa in the presence of thrombin-activated factor VIII. Blood. 1978 Nov;52(5):928–940. [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Kaplan A. P. Initiation of the intrinsic coagulation and fibrinolytic pathways of man: the role of surfaces, hageman factor, prekallikrein, high molecular weight kininogen, and factor XI. Prog Hemost Thromb. 1978;4:127–175. [PubMed] [Google Scholar]
- Kato H., Sugo T., Ikari N., Hashimoto N., Maruyama I., Han Y. N., Iwanaga S., Fujii S. Role of bovine high-molecular-weight (HMW) kininogen in contact-mediated activation of bovine Factor XII. Adv Exp Med Biol. 1979;120B:19–37. [PubMed] [Google Scholar]
- Kerbiriou D. M., Griffin J. H. Human high molecular weight kininogen. Studies of structure-function relationships and of proteolysis of the molecule occurring during contact activation of plasma. J Biol Chem. 1979 Dec 10;254(23):12020–12027. [PubMed] [Google Scholar]
- Kirby E. P., McDevitt P. J. The binding of bovine factor XII to kaolin. Blood. 1983 Apr;61(4):652–659. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mandle R. J., Colman R. W., Kaplan A. P. Identification of prekallikrein and high-molecular-weight kininogen as a complex in human plasma. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4179–4183. doi: 10.1073/pnas.73.11.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier H. L., Pierce J. V., Colman R. W., Kaplan A. P. Activation and function of human Hageman factor. The role of high molecular weight kininogen and prekallikrein. J Clin Invest. 1977 Jul;60(1):18–31. doi: 10.1172/JCI108754. [DOI] [PMC free article] [PubMed] [Google Scholar]
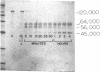
- Mori K., Nagasawa S. Studies on human high molecular weight (HMW) kininogen. II. Structural change of HMW kininogen by the action of human plasma kallikrein. J Biochem. 1981 May;89(5):1465–1473. doi: 10.1093/oxfordjournals.jbchem.a133339. [DOI] [PubMed] [Google Scholar]
- Proud D., Togias A., Naclerio R. M., Crush S. A., Norman P. S., Lichtenstein L. M. Kinins are generated in vivo following nasal airway challenge of allergic individuals with allergen. J Clin Invest. 1983 Nov;72(5):1678–1685. doi: 10.1172/JCI111127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnoff O. D., Saito H. Evidence that Fitzgerald factor counteracts inhibition by kaolin or ellagic acid of the amidolytic properties of a plasma kallikrein. Blood. 1976 Feb;47(2):243–251. [PubMed] [Google Scholar]
- Saito H. Purification of high molecular weight kininogen and the role of this agent in blood coagulation. J Clin Invest. 1977 Sep;60(3):584–594. doi: 10.1172/JCI108810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira M., Scott C. F., Colman R. W. Protection of human plasma kallikrein from inactivation by C1 inhibitor and other protease inhibitors. The role of high molecular weight kininogen. Biochemistry. 1981 May 12;20(10):2738–2743. doi: 10.1021/bi00513a006. [DOI] [PubMed] [Google Scholar]
- Schapira M., Scott C. F., James A., Silver L. D., Kueppers F., James H. L., Colman R. W. High molecular weight kininogen or its light chain protects human plasma kallikrein from inactivation by plasma protease inhibitors. Biochemistry. 1982 Feb 2;21(3):567–572. doi: 10.1021/bi00532a024. [DOI] [PubMed] [Google Scholar]
- Scott C. F., Kirby E. P., Schick P. K., Colman R. W. Effect of surfaces on fluid-phase prekallikrein activation. Blood. 1981 Mar;57(3):553–560. [PubMed] [Google Scholar]
- Scott C. F., Liu C. Y., Colman R. W. Human plasma prekallikrein: a rapid high-yield method for purification. Eur J Biochem. 1979 Oct;100(1):77–83. doi: 10.1111/j.1432-1033.1979.tb02035.x. [DOI] [PubMed] [Google Scholar]
- Scott C. F., Schapira M., James H. L., Cohen A. B., Colman R. W. Inactivation of factor XIa by plasma protease inhibitors: predominant role of alpha 1-protease inhibitor and protective effect of high molecular weight kininogen. J Clin Invest. 1982 Apr;69(4):844–852. doi: 10.1172/JCI110524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg M., Nicoll J. E., Kaplan A. P. The mechanism by which the light chain of cleaved HMW-kininogen augments the activation of prekallikrein, factor XI and Hageman factor. Thromb Res. 1980 Oct 15;20(2):173–189. doi: 10.1016/0049-3848(80)90383-7. [DOI] [PubMed] [Google Scholar]
- Sugo T., Kato H., Iwanaga S., Fujii S. The accelerating effect of bovine plasma HMW kininogen on the surface-mediated activation of factor XII: generation of a derivative form (active kininogen) with maximal cofactor activity by limited proteolysis. Thromb Res. 1981 Nov 15;24(4):329–337. doi: 10.1016/0049-3848(81)90006-2. [DOI] [PubMed] [Google Scholar]
- Thompson R. E., Mandle R., Jr, Kaplan A. P. Association of factor XI and high molecular weight kininogen in human plasma. J Clin Invest. 1977 Dec;60(6):1376–1380. doi: 10.1172/JCI108898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. E., Mandle R., Jr, Kaplan A. P. Studies of binding of prekallikrein and Factor XI to high molecular weight kininogen and its light chain. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4862–4866. doi: 10.1073/pnas.76.10.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy P. B., Peterson J. M., Nesheim M. E., McDuffie F. C., Mann K. G. Interaction of coagulation factor V and factor Va with platelets. J Biol Chem. 1979 Oct 25;254(20):10354–10361. [PubMed] [Google Scholar]
- Vroman L., Adams A. L., Fischer G. C., Munoz P. C. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood. 1980 Jan;55(1):156–159. [PubMed] [Google Scholar]
- Wiggins R. C., Bouma B. N., Cochrane C. G., Griffin J. H. Role of high-molecular-weight kininogen in surface-binding and activation of coagulation Factor XI and prekallikrein. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4636–4640. doi: 10.1073/pnas.74.10.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins R. C. Kinin release from high molecular weight kininogen by the action of Hageman factor in the absence of kallikrein. J Biol Chem. 1983 Jul 25;258(14):8963–8970. [PubMed] [Google Scholar]