Abstract
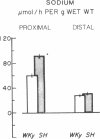
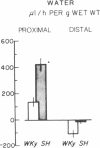
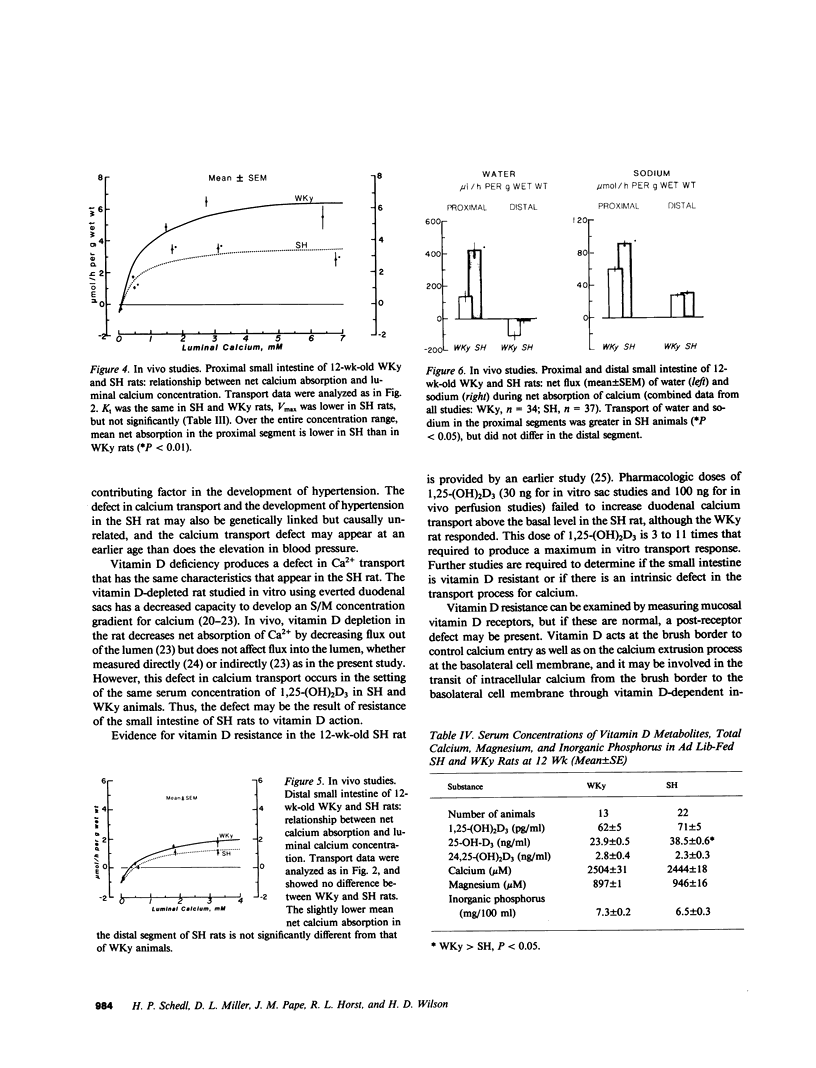
Serum ionized calcium levels are lower and immunoreactive parathyroid hormone levels are higher in the spontaneously hypertensive (SH) rat than in the normotensive Wistar-Kyoto (WKy) control. We postulated that there is either a defect in the regulation of vitamin D metabolism by parathyroid hormone or that the gut target organ for vitamin D in the SH rat is unresponsive. To test these hypotheses we measured serum concentrations of vitamin D metabolites and intestinal transport of calcium and sodium. Compared with that of WKy controls, in vitro calcium transport by duodenal sacs of the SH rat was decreased (P less than 0.001) at 5 wk, before the development of hypertension, and at 12 wk, after hypertension was well established. When measured in vivo in the most proximal 20 cm of small intestine, maximum velocity (Vmax) for calcium transport was decreased (P less than 0.05) and net absorption of sodium and water was increased (P less than 0.05) in SH rats as compared with WKy rats. Vmax for calcium transport was also decreased (P less than 0.05) in the most distal 20 cm of small intestine of SH rats, but net sodium and water transport were the same in SH and WKy rats. At 12 wk, serum concentration of 1,25-dihydroxycholecalciferol [1,25-(OH)2D3] was the same in both SH and WKy groups, but its precursor, 25-hydroxycholecalciferol, was increased (P less than 0.05) in the SH rat. We conclude that in the SH rat: (a) the concentration of 1,25-(OH)2D3 is inappropriately low in relation to the elevated immunoreactive parathyroid hormone and the depressed calcium absorption, suggesting a defect in the regulation of vitamin D metabolism; and (b) the depressed calcium absorption, in the setting of normal concentrations of [1,25-(OH)2D3], demonstrates unresponsiveness of the gut to vitamin D and may explain in part the low serum ionized calcium found in earlier studies. The presence of these abnormalities before we found a significant difference in blood pressure suggests that they may be causal, not secondary, to the hypertension.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayachi S. Increased dietary calcium lowers blood pressure in the spontaneously hypertensive rat. Metabolism. 1979 Dec;28(12):1234–1238. doi: 10.1016/0026-0495(79)90136-7. [DOI] [PubMed] [Google Scholar]
- Belizán J. M., Villar J. The relationship between calcium intake and edema-, proteinuria-, and hypertension-getosis: an hypothesis. Am J Clin Nutr. 1980 Oct;33(10):2202–2210. doi: 10.1093/ajcn/33.10.2202. [DOI] [PubMed] [Google Scholar]
- Eastin W. C., Wilson H. D., Schedl H. P. Intestinal resection and calcium absorption in the rat. Proc Soc Exp Biol Med. 1980 Apr;163(4):553–557. doi: 10.3181/00379727-163-40813. [DOI] [PubMed] [Google Scholar]
- Friedman S. M. Evidence for enhanced sodium transport in the tail artery of the spontaneously hypertensive rat. Hypertension. 1979 Nov-Dec;1(6):572–582. doi: 10.1161/01.hyp.1.6.572. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. Non-inverted versus inverted plots in enzyme kinetics. Nature. 1959 Oct 24;184:1296–1298. doi: 10.1038/1841296b0. [DOI] [PubMed] [Google Scholar]
- Horst R. L., Littledike E. T., Riley J. L., Napoli J. L. Quantitation of vitamin D and its metabolites and their plasma concentrations in five species of animals. Anal Biochem. 1981 Sep 1;116(1):189–203. doi: 10.1016/0003-2697(81)90344-4. [DOI] [PubMed] [Google Scholar]
- Krawitt E. L., Schedl H. P. In vivo calcium transport by rat small intestine. Am J Physiol. 1968 Feb;214(2):232–236. doi: 10.1152/ajplegacy.1968.214.2.232. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Ito Y., Suzuki H., Kitamura K., Itoh T. Factors modifying contraction-relaxation cycle in vascular smooth muscles. Am J Physiol. 1982 Nov;243(5):H641–H662. doi: 10.1152/ajpheart.1982.243.5.H641. [DOI] [PubMed] [Google Scholar]
- Masironi R., Koirtyohann S. R., Pierce J. O., Schamschula R. G. Calcium content of river water, trace element concentrations in toenails, and blood pressure in village populations in New Guinea. Sci Total Environ. 1976 Jul;6(1):41–53. doi: 10.1016/0048-9697(76)90005-x. [DOI] [PubMed] [Google Scholar]
- McCarron D. A. Blood pressure and calcium balance in the Wistar-Kyoto rat. Life Sci. 1982 Feb 15;30(7-8):683–689. doi: 10.1016/0024-3205(82)90284-3. [DOI] [PubMed] [Google Scholar]
- McCarron D. A. Low serum concentrations of ionized calcium in patients with hypertension. N Engl J Med. 1982 Jul 22;307(4):226–228. doi: 10.1056/NEJM198207223070405. [DOI] [PubMed] [Google Scholar]
- McCarron D. A., Morris C. D., Cole C. Dietary calcium in human hypertension. Science. 1982 Jul 16;217(4556):267–269. doi: 10.1126/science.7089566. [DOI] [PubMed] [Google Scholar]
- McCarron D. A., Pingree P. A., Rubin R. J., Gaucher S. M., Molitch M., Krutzik S. Enhanced parathyroid function in essential hypertension: a homeostatic response to a urinary calcium leak. Hypertension. 1980 Mar-Apr;2(2):162–168. doi: 10.1161/01.hyp.2.2.162. [DOI] [PubMed] [Google Scholar]
- Orlov S. N., Postnov Y. V. Ca2+ binding and membrane fluidity in essential and renal hypertension. Clin Sci (Lond) 1982 Sep;63(3):281–284. doi: 10.1042/cs0630281. [DOI] [PubMed] [Google Scholar]
- Petith M. M., Schedl H. P. Duodenal and ileal adaptation to dietary calcium restriction: in vivo studies in the rat. Am J Physiol. 1976 Sep;231(3):865–871. doi: 10.1152/ajplegacy.1976.231.3.865. [DOI] [PubMed] [Google Scholar]
- Petith M. M., Schedl H. P. Effects of diabetes on cecal and colonic calcium transport in the rat. Am J Physiol. 1978 Dec;235(6):E699–E702. doi: 10.1152/ajpendo.1978.235.6.E699. [DOI] [PubMed] [Google Scholar]
- Petith M. M., Wenger J. R., Schedl H. P. Vitamin D dependence and aboral gradient of in vivo intestinal calcium transport in the rat. Am J Dig Dis. 1978 Oct;23(10):943–947. doi: 10.1007/BF01072472. [DOI] [PubMed] [Google Scholar]
- Postnov YuV, Orlov S. N., Pokudin N. I. Alteration of the intracellular calcium pool of adipose tissue in spontaneously hypertensive rats. No effect of peripheral immunosympathectomy. Pflugers Arch. 1981 Jun;390(3):256–259. doi: 10.1007/BF00658271. [DOI] [PubMed] [Google Scholar]
- Postnov Y. U., Orlov S., Gulak P., Shevchenko A. Altered permeability of the erythrocyte membrane for sodium and potassium ions in spontaneously hypertensive rats. Pflugers Arch. 1976 Sep 30;365(2-3):257–263. doi: 10.1007/BF01067026. [DOI] [PubMed] [Google Scholar]
- Postnov Y. V., Orlov S. N. Evidence of altered calcium accumulation and calcium binding by the membranes of adipocytes in spontaneously hypertensive rats. Pflugers Arch. 1980 May;385(1):85–89. doi: 10.1007/BF00583919. [DOI] [PubMed] [Google Scholar]
- Postnov Y. V., Orlov S. N., Pokudin N. I. Decrease of calcium binding by the red blood cell membrane in spontaneously hypertensive rats and in essential hypertension. Pflugers Arch. 1979 Mar 16;379(2):191–195. doi: 10.1007/BF00586947. [DOI] [PubMed] [Google Scholar]
- SCHACHTER D., DOWDLE E. B., SCHENKER H. Active transport of calcium by the small intestine of the rat. Am J Physiol. 1960 Feb;198:263–268. doi: 10.1152/ajplegacy.1960.198.2.263. [DOI] [PubMed] [Google Scholar]
- SCHACHTER D., KIMBERG D. V., SCHENKER H. Active transport of calcium by intestine: action and bio-assay of vitamin D. Am J Physiol. 1961 Jun;200:1263–1271. doi: 10.1152/ajplegacy.1961.200.6.1263. [DOI] [PubMed] [Google Scholar]
- SCHEDL H. P., CLIFTON J. A. Small intestinal absorption of steroids. Gastroenterology. 1961 Nov;41:491–499. [PubMed] [Google Scholar]
- Schneider L. E., Wasserman R. H., Schedl H. P. Depressed duodenal calcium absorption in the diabetic rat: restoration by Solanum malacoxylon. Endocrinology. 1975 Sep;97(3):649–653. doi: 10.1210/endo-97-3-649. [DOI] [PubMed] [Google Scholar]
- Toraason M. A., Wright G. L. Transport of calcium by duodenum of spontaneously hypertensive rat. Am J Physiol. 1981 Oct;241(4):G344–G347. doi: 10.1152/ajpgi.1981.241.4.G344. [DOI] [PubMed] [Google Scholar]
- Urban E., Schedl H. P. Comparison of in vivo and in vitro effects of vitamin D on calcium transport in the rat. Am J Physiol. 1969 Jul;217(1):126–130. doi: 10.1152/ajplegacy.1969.217.1.126. [DOI] [PubMed] [Google Scholar]
- Urban E., Schedl H. P. Vitamin D, tissue calcium, and calcium transport in the in vivo rat small intestine. Am J Physiol. 1970 Oct;219(4):944–951. doi: 10.1152/ajplegacy.1970.219.4.944. [DOI] [PubMed] [Google Scholar]
- Webb R. C., Bohr D. F. Potassium relaxation of vascular smooth muscle from spontaneously hypertensive rats. Blood Vessels. 1979;16(2):71–79. [PubMed] [Google Scholar]
- Wei J. W., Janis R. A., Daniel E. E. Calcium accumulation and enzymatic activities of subcellular fractions from aortas and ventricles of genetically hypertensive rats. Circ Res. 1976 Jul;39(1):133–140. doi: 10.1161/01.res.39.1.133. [DOI] [PubMed] [Google Scholar]
- Wright G. L., Rankin G. O. Concentrations of ionic and total calcium in plasma of four models of hypertension. Am J Physiol. 1982 Sep;243(3):H365–H370. doi: 10.1152/ajpheart.1982.243.3.H365. [DOI] [PubMed] [Google Scholar]
- Wright G. L., Toraason M. A., Barbe J. S., Crouse W. The concentrations of ionic and total calcium in plasma of the spontaneously hypertensive rat. Can J Physiol Pharmacol. 1980 Dec;58(12):1494–1499. doi: 10.1139/y80-225. [DOI] [PubMed] [Google Scholar]
- Younoszai M. K., Urban E., Schedl H. P. Vitamin D and intestinal calcium fluxes in vivo in the rat. Am J Physiol. 1973 Aug;225(2):287–292. doi: 10.1152/ajplegacy.1973.225.2.287. [DOI] [PubMed] [Google Scholar]
- Zsotér T. T., Wolchinsky C., Henein N. F., Ho L. C. Calcium kinetics in the aorta of spontaneously hypertensive rats. Cardiovasc Res. 1977 Jul;11(4):353–357. doi: 10.1093/cvr/11.4.353. [DOI] [PubMed] [Google Scholar]