Abstract
Cisplatin-based, cyclic balloon-occluded arterial infusion, neoadjuvant chemotherapy (NAC) has previously been reported to enable hysterectomy in patients with locally advanced cervical cancer. Sirtuin1 (SIRT1) is a nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase that deacetylates a number of proteins and is overexpressed in several human malignancies. Upregulation of SIRT1 has been reported to induce tumorigenesis and chemoresistance. To assess the role of SIRT1 in uterine cervical cancer, the outcomes in 62 patients aged <70 years with locally advanced International Federation of Gynecology and Obstetrics (FIGO) stage IIIA-IIIB uterine cervical cancer were reviewed between 1995 and 2010. Tumor samples were obtained by biopsy prior to NAC. The patients were separated into two groups. One group comprised of the patients in which NAC was effective, surgery and radiotherapy were performed (NAC+OP+R group; n=35), and the second group contained patients in which NAC was ineffective and radiation therapy was performed (NAC+R group; n=27). SIRT1 and p53 expression was assessed immunohistochemically in paraffin-embedded sections. SIRT1 expression was significantly higher in the NAC+R compared to the NAC+OP+R group (P<0.001), as was p53 expression (P=0.001). The overall survival time was significantly longer in the NAC+OP+R compared to the NAC+R group (P=0.001). Following the division of patients into two groups based on SIRT1 level, low (weighted score ≤4, n=30), and high level (weighted score ≥6, n=32) groups, the former group was significantly more sensitive to NAC (P<0.001). Collectively, these results indicate that SIRT1 expression may predict the efficacy of NAC as a treatment for locally advanced uterine cervical cancer.
Keywords: sirtuin1, neoadjuvant chemotherapy, uterine cervical cancer
Introduction
Sirtuin1 (SIRT1), one of the seven members (SIRT1-7) of the silent information regulator 2 (Sir2) family in mammals, has activity as a nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase (HDAC) (1–3). SIRT1 deacetylates several key proteins that regulate the cell cycle and apoptosis, including Foxo family proteins, Ku70 and p53, and plays important roles in cell survival (4–7).
SIRT1 was previously reported to be upregulated in several tumor cell lines and human tumors (8–11). Upregulation of SIRT1 may induce tumorigenesis and resistance to certain chemotherapeutic agents (12). When normal cells undergo stress, such as DNA damage, p53 is activated, which results in the transcription of the hypermethylated in cancer 1 (HIC1) gene (13). HIC1 represses transcription of the gene encoding SIRT1, inducing pathways leading to cell senescence or apoptosis. However, during the early stage of tumor progression, epigenetic silencing of HIC1 leads to upregulation of SIRT1. Upregulated SIRT1 inactivates p53 by deacetylation, impairing the functions of p53 and leading to a defective apoptotic response to DNA damage. This allows cells to reproduce in the presence of damaged DNA, resulting in the accumulation of mutations, including p53. Upregulated mutant p53 interferes with the functions of wild-type p53, disrupting cell-cycle control and promoting tumor progression (14, 15).
Locally advanced uterine cervical cancer is extremely difficult to treat. The standard treatment for patients with International Federation of Gynecology and Obstetrics (FIGO) stage IIIA, IIIB, and IVA uterine cervical cancer consists of concurrent chemoradiotherapy (CCRT) (16, 17), but patient prognosis is poor (18, 19). Successful neoadjuvant chemotherapy (NAC), followed by hysterectomy, has been reported to be effective in patients with locally advanced, uterine cervical cancer (20), with a prognosis equal to that of CCRT. However, the prognosis is worse if NAC is unsuccessful, as hysterectomy cannot be performed, and consequently, the treatment strategy must be changed from surgery to radiation therapy, resulting in a crucial delay (21, 22). Thus, it is important to identify prognostic factors in patients with locally advanced cervical cancer that predict whether NAC is likely to be successful (23–27).
Thus far, the expression of SIRT1 has not been assessed in patients with locally advanced uterine cervical cancer. Therefore, the present study was designed to examine the correlation between SIRT1 expression and the efficacy of NAC for locally advanced, uterine cervical cancer.
Patients and methods
Patients and samples
The retrospective study included 62 patients aged <70 years with locally advanced uterine cervical cancer (FIGO stages IIIA and IIIB), initially treated at the Osaka City University Medical School Hospital (Osaka, Japan) between 1995 and 2010. Tumor samples were obtained by biopsy prior to NAC. The patients were divided into two groups: One in which NAC was effective, surgery was possible and radiation therapy was performed (NAC+OP+R group; n=35), and the second in which NAC was ineffective and, therefore, radiation therapy alone was performed (NAC+R group; n=27). Additionally, patients were further divided into groups that attained complete/partial remission (CR+PR) and stable/progressive disease (SD+PD) in response to NAC. Written informed consent was obtained from all the patients prior to immunohistochemical examination. The study was approved by the Ethics Committee of Osaka City University (IRB no. 2581).
Balloon-occluded arterial infusion chemotherapy (BOAI) for NAC
Pelvic angiography was performed under local anesthesia using Seldinger's technique (28) to localize the tumor and feeder vessels. A balloon-wedge single-pressure catheter (5F, 80 cm in length; Dispomedica GmbH, Hamburg, Germany) was inserted into each femoral artery and subsequently into the internal iliac artery. The balloon catheters were advanced until they reached the vicinity of the feeder vessel (usually the uterine artery), where the balloon was inflated to interrupt local blood flow. cis-Diamminedichloroplatinum (CDDP) was slowly infused intra-arterially through the two catheters over a period of 30 min (28). The two ovarian arteries were blocked following the first round of BOAI to increase the intratumor concentration of CDDP. BOAI was performed three times in each patient to shrink the tumor. Adequate hydration was ensured prior to and following CDDP administration, and anti-emetics and diuretics were administered as appropriate. CDDP was administered at doses of 50, 75, or 100 mg/m2, depending on patient age and renal function. The efficacy of CDDP arterial infusion therapy was evaluated by cytology, histology, serum tumor marker level and magnetic resonance imaging (MRI) prior to the initiation of CDDP treatment. The results were compared with those obtained following the completion of each arterial infusion. MRI was used to estimate tumor regression by measuring its size in two dimensions (29, 30). Tumor tissue was obtained from all the patients who had undergone punch biopsy or surgery.
Immunohistochemical analysis
The expression of SIRT1 and p53 was examined in paraffin-embedded sections using antibodies to SIRT1 and p53, respectively, and the avidin-biotin peroxidase complex method. Briefly, 4-µm paraffin sections were deparaffinized and immersed in 3% hydrogen peroxidase in methanol to block endogenous peroxidase activity. The antigen was retrieved by immersing the slides in 10 mM citrate buffer (pH 6.0) and heating in an autoclave at 110°C for 20 min, followed by washing in phosphate-buffered solutions (PBS). The manufacturers' instructions were followed for the Dako LSAB 2 peroxidase kit (Dako, Kyoto, Japan). The sections were incubated with a 1:100 dilution of polyclonal rabbit anti-human SIRT1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or a 1:100 dilution of monoclonal mouse anti-human p53 (Santa Cruz Biotechnology, Inc.) overnight at 4°C. The sections were washed with PBS for 15 min and incubated for 10 min with biotinylated goat anti-mouse or anti-rabbit immunoglobulin G (Dako). The sections were incubated with the streptavidin-peroxidase complex, with 3,3′;-diaminobenzidine used as the chromogen. Finally, the sections were counterstained with Mayer's hematoxylin. The specificity of the immunohistochemical reactions was checked by omitting the primary antibody. SIRT1 and p53 expression was quantitatively analyzed as described (31). The mean percentage of positive tumor cells was determined in five separate areas (magnification, x400), with positivity rates of <5, 5–25, 25–50, 50–75 and >75% scored as 0–4, respectively. The staining intensity was scored as weak (1+), moderate (2+), or intense (3+). For each specimen, the percentage of positive tumor cells was multiplied by the staining intensity to yield a weighted score.
Statistical analysis
Data are presented as mean ± standard deviation. The Kaplan-Meier and log-rank tests were performed for prognostic analysis. Weighted scores were compared using the Mann-Whitney U test. Student's t-test and the χ2 test were performed as appropriate for between-group comparisons. SPSS software, version 21.0 (IBM, Armonk, NY, USA), was used for all the statistical analyses. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
In total, 35 of the 62 patients with locally advanced, uterine cervical cancer were assigned to the NAC+OP+R group and 27 to the NAC+R group. The mean ages were 49.3 (range, 24–69 years) and 52.3 years (range, 36–68 years), respectively. Of the 35 patients in the NAC+OP+R group, one was classified as stage IIIA and 34 as stage IIIB, whereas all the 27 patients in the NAC+R group were classified as stage IIIB. Histologically, 30 patients in the NAC+OP+R group were classified as having squamous cell carcinoma and five as having adenocarcinoma. A total of 22 patients in the NAC+R group were classified as having squamous cell carcinoma, three as having adenocarcinoma, and one each as having adenosquamous carcinoma and glassy cell carcinoma. There were no significant differences between the two groups (Table I).
Table I.
Characteristics of patients in the NAC+OP+R and NAC+R groups.
| NAC+OP+R | NAC+R | P-value | |
|---|---|---|---|
| Patients, n | 35 | 27 | |
| Age, years | |||
| Mean ± SD | 49.3±12.7 | 52.3±11.1 | 0.322a |
| Range | 24–69 | 36–68 | |
| FIGO stage, n | |||
| IIIA | 1 | 0 | 0.376b |
| IIIB | 34 | 27 | |
| Histology, n | |||
| SCC | 30 | 22 | 0.433b |
| A | 5 | 3 | |
| AS | 0 | 1 | |
| Others | 0 | 1 | |
Student's t-test
χ2 test; NAC+OP+R, neoadjuvant chemotherapy + surgery + radiotherapy; NAC+R, neoadjuvant chemotherapy + radiotherapy; FIGO, International Federation of Gynecology and Obstetrics; SCC, squamous cell carcinoma; A, adenocarcinoma; AS, adenosquamous carcinoma; SD, standard deviation.
Expression of SIRT1
SIRT1 was expressed in the nuclei of the tumor cells (Fig. 1). The weighted scores in the two groups are shown in Table II. The mean weighted score for SIRT1 expression was significantly lower in the NAC+OP+R compared to the NAC+R group (3.97 vs. 8.67, P<0.001; Fig. 2). In total, 30 of the 62 patients had weighted scores of 0–4 (low expression) and 32 had weighted scores of 6–12 (high expression). There were no significant differences between these two groups (Table III).
Figure 1.
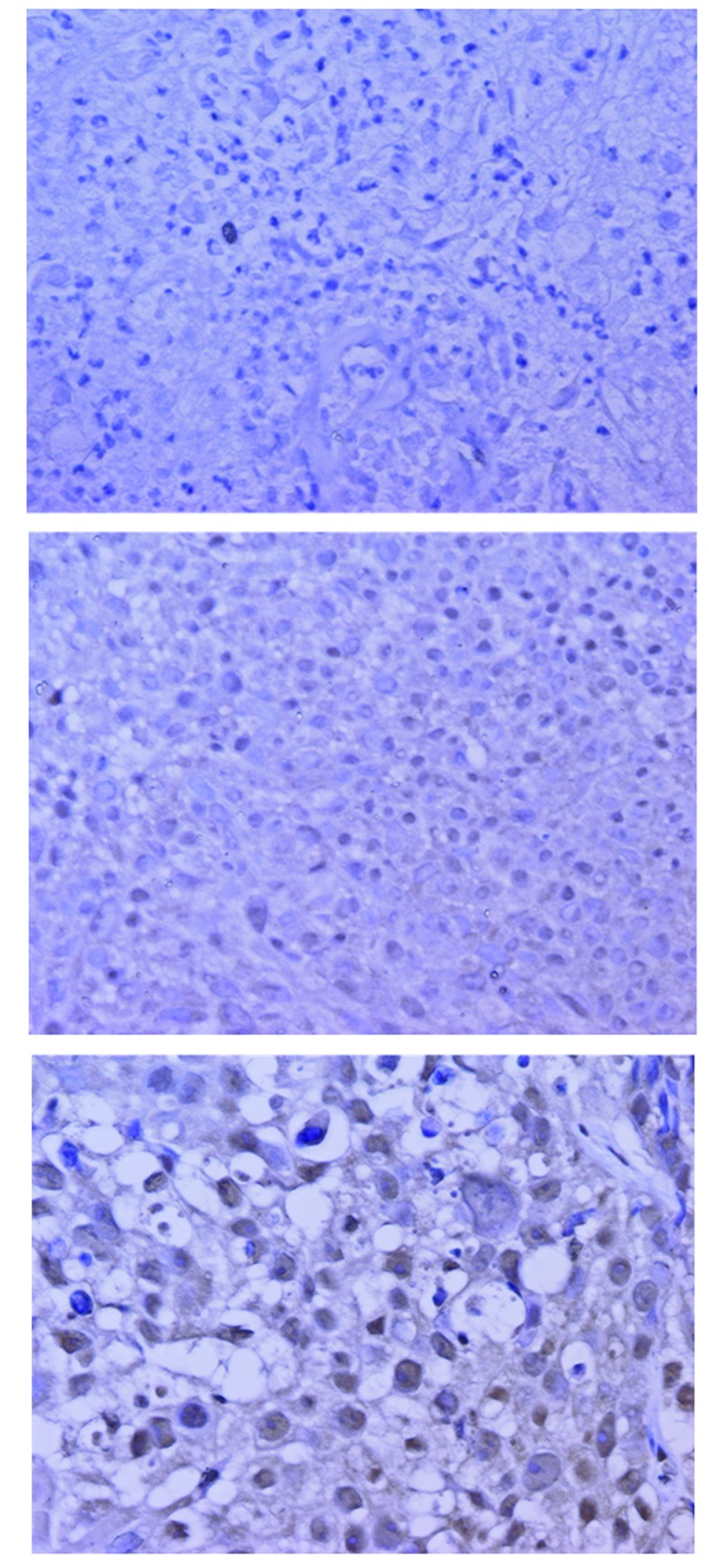
Immunohistochemical staining of SIRT1 in locally advanced cervical cancer. (A) Negative control. (B) Score 1, NAC+OP+R group. (C) Score 2, NAC+OP+R group (A-C, H&E; magnification, x400). SIRT1 was expressed in the nuclei of the tumor cells. SIRT1, sirtuin 1; NAC+OP+R, neoadjuvant chemotherapy + surgery + radiotherapy; H&E, hematoxylin and eosin.
Table II.
Weighted scores of SIRT1 expression in the NAC+OP+R and NAC+R groups.
| Patients, n | ||
|---|---|---|
| Weighted score | NAC+OP+R | NAC+R |
| 0 | 1 | 1 |
| 1 | 2 | 1 |
| 2 | 10 | 1 |
| 3 | 6 | 0 |
| 4 | 7 | 1 |
| 6 | 5 | 3 |
| 8 | 1 | 4 |
| 9 | 1 | 5 |
| 12 | 2 | 11 |
| Total | 35 | 27 |
| Weighted score, mean | 3.97 | 8.67 |
SIRT1, sirtuin1; NAC+OP+R, neoadjuvant chemotherapy + surgery + radiotherapy; NAC+R, neoadjuvant chemotherapy + radiotherapy.
Figure 2.
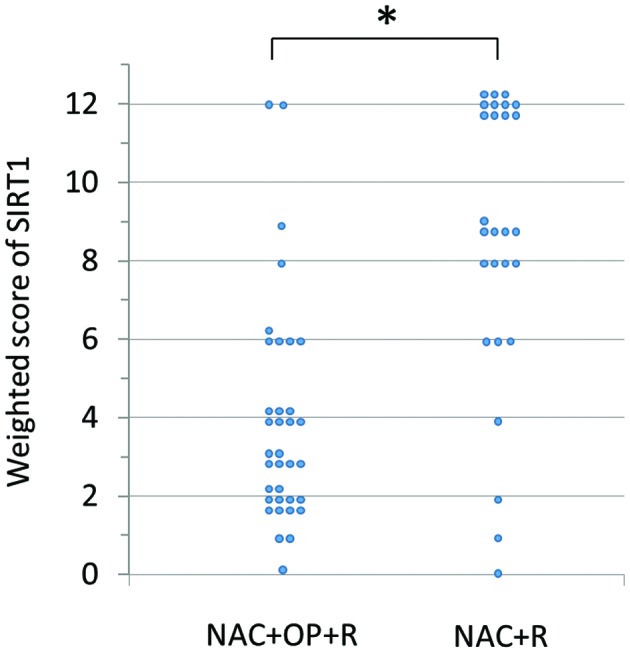
Weighted score for SIRT1 expression in tumor samples from patients with locally advanced cervical cancer. SIRT1 expression was significantly higher in the NAC+R compared to the NAC+OP+R group. *P<0.001 (Mann-Whitney U test). SIRT1, sirtuin 1; NAC+R, neoadjuvant chemotherapy + radiotherapy; NAC+OP+R, neoadjuvant chemotherapy + surgery + radiotherapy.
Table III.
Characteristics of the patients in the low and high SIRT1 expression groups.
| Characteristics | ≤4a | ≥6a | P-value |
|---|---|---|---|
| Patients, n | 30 | 32 | |
| Age, years | |||
| Mean ± SD | 49.8±12.4 | 51.3±11.8 | 0.633b |
| Range | 24–69 | 24–68 | |
| FIGO stage, n | |||
| IIIA | 0 | 1 | 0.329c |
| IIIB | 30 | 31 | |
| Histology, n | |||
| SCC | 26 | 26 | 0.585c |
| A | 4 | 4 | |
| AS | 0 | 1 | |
| Others | 0 | 1 | |
Weighted score
Student's t-test
χ2 test; NAC+OP+R, neoadjuvant chemotherapy + surgery + radiotherapy; NAC+R, neoadjuvant chemotherapy + radiotherapy; FIGO, International Federation of Gynecology and Obstetrics; SCC, squamous cell carcinoma; A, adenocarcinoma; AS, adenosquamous carcinoma; SD, standard deviation.
Expression of p53
p53 was expressed in the nuclei of the tumor cells. The weighted scores are shown in Table IV. The mean weighted score for p53 expression was also significantly lower in the NAC+OP+R group compared to the NAC+R group (4.23 vs. 6.70; P<0.001).
Table IV.
Weighted scores of p53 expression in the NAC+OP+R and NAC+R groups.
| Patients, n | ||
|---|---|---|
| Weighted score | NAC+OP+R | NAC+R |
| 0 | 0 | 1 |
| 1 | 2 | 0 |
| 2 | 10 | 0 |
| 3 | 1 | 3 |
| 4 | 12 | 4 |
| 6 | 5 | 7 |
| 8 | 3 | 6 |
| 9 | 1 | 2 |
| 12 | 1 | 4 |
| Total | 35 | 27 |
| Weighted score, mean | 4.23 | 6.70 |
NAC+OP+R, neoadjuvant chemotherapy + surgery + radiotherapy; NAC+R, neoadjuvant chemotherapy + radiotherapy.
Correlation between expression of SIRT1 and p53
In total, 19 of the 62 patients showed low expression of SIRT1 and p53, and 18 showed high expression. There was a weak correlation between expression of SIRT1 and p53 (|r|=0.247).
Correlation between expression of SIRT1 and effects of NAC
Of the 35 patients in the NAC+OP+R group, 26 (74%) showed low SIRT1 expression, whereas nine (26%) showed high SIRT1 expression. The group with low SIRT1 expression was significantly more sensitive to NAC (P=0.001; Table V).
Table V.
Numbers of patients with low and high SIRT1 expression in the NAC+OP+R and NAC+R groups.
| Expression | NAC+OP+R, n (%) | NAC+R, n (%) | P-value |
|---|---|---|---|
| Low, ≤4 | 26 (87) | 4 (13) | <0.001a |
| High, ≥6 | 9 (28) | 23 (72) |
χ2 test. SIRT1, sirtuin1; NAC+OP+R, neoadjuvant chemotherapy + surgery + radiotherapy; NAC+R, neoadjuvant chemotherapy + radiotherapy.
Survival
The overall survival time was significantly longer in the NAC+OP+R compared to the NAC+R group (Fig. 3). However, overall survival was similar in patients with low and high SIRT1 expression (Fig. 4).
Figure 3.
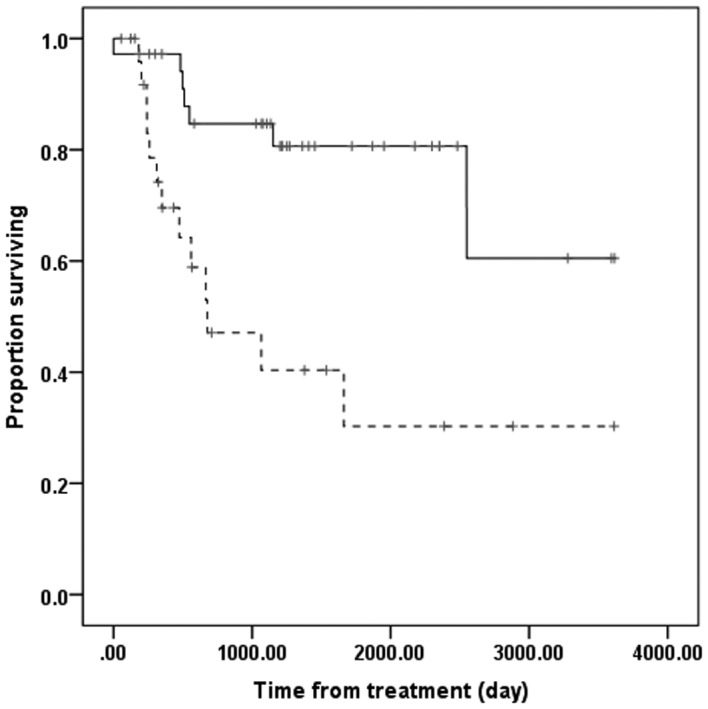
Overall survival rate in the NAC+OP+R (n=35) and NAC+R (n=27) groups. Solid line, NAC+OP+R; dashed line, NAC+R. NAC+OP+R group showed significantly improved overall survival time compared to NAC+R group (P=0.001, Kaplan-Meier and log-rank tests). NAC+OP+R, neoadjuvant chemotherapy + surgery + radiotherapy; NAC+R, neoadjuvant chemotherapy + radiotherapy.
Figure 4.
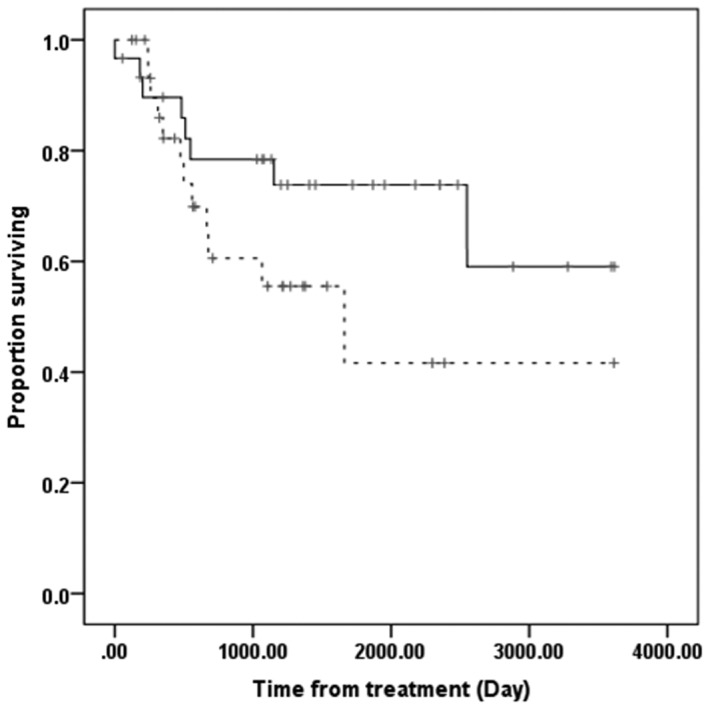
Overall survival rate in the low (n=30) and high SIRT1 expression (n=32) groups. Solid line, low SIRT1 expression; dashed line, high SIRT1 expression. There was no significant difference in overall survival between the two groups (P=0.143, Kaplan-Meier and log-rank tests). SIRT1, sirtuin1.
Discussion
The results of the present study show the association between the expression of SIRT1 and the efficacy of NAC. NAC was ineffective in the majority of patients with high SIRT1 expression, who were unable to undergo surgery. Overall survival time was significantly longer in the NAC+OP+R compared to the NAC+R group. These results are in agreement with findings showing that prognosis is worse when NAC is unsuccessful (21, 22). By contrast, overall survival time did not differ significantly in the groups of patients with high and low SIRT1 expression.
In general, CCRT is considered the standard treatment for patients with locally advanced, uterine cervical cancer. However, limited clinical studies have assessed CCRT in Japanese patients with locally advanced, uterine cervical cancer. Although surgery following NAC has been reported effective (20), NAC is not currently recommended, as if NAC is not effective, surgery is difficult to perform and radiation therapy is required. Radiation therapy following chemotherapy has shown poorer prognosis compared to radiation alone (21, 22). Thus, identifying factors prognostic of the efficacy of NAC is important in patients with locally advanced uterine cervical cancer.
SIRT1 is a member of the silent information regulator 2 (Sir2) family in mammals, with activity as an NAD+-dependent HDAC (1–3). SIRT1 deacetylates several key cell-cycle and apoptosis regulating proteins (4–7). SIRT1 expression has been reported to increase in various human malignant tumors. SIRT1 is considered a tumor promoter, as it inhibits tumor suppressor genes such as p53 (14, 15). SIRT1 overexpression has been associated with primary tumorigenesis, metastasis, chemoresistance and patient prognosis. However, other studies have reported that SIRT1 may act as a tumor suppressor (32, 33).
The present study is the first to report a correlation between SIRT1 expression and locally advanced, uterine cervical cancer. These findings indicate that NAC may be more effective in patients with low compared to high SIRT1 expression, suggesting that SIRT1 expression may predict the efficacy of NAC in patients with locally advanced, uterine cervical cancer. As overexpression of SIRT1 has been associated with chemoresistance, lower SIRT1 expression may result in tumor susceptibility to treatment. When the correlation between p53 expression and NAC was assessed in patients with locally advanced, uterine cervical cancer, the observed results were similar to those for SIRT1 and NAC (P=0.001; data not shown). A weak correlation was also observed between SIRT1 and p53 expression. Human papillomavirus (HPV) infection causes the majority of uterine cervical cancers, with the viral E6 and E7 proteins playing important roles in tumor progression (34). The E6 protein targets p53, inducing a loss of p53 tumor suppressor activity, such as apoptosis (35, 36). By contrast, HPV E7 protein has been reported to activate SIRT1 expression, leading to a defective apoptotic response (37). Thus, HPV infection enhances SIRT1 and p53 expression, providing further evidence for the significant role of SIRT1 in cervical cancer.
If NAC is not successful in patients with locally advanced, uterine cervical cancer, their prognosis becomes worse. Therefore, it is important to identify factors prognostic of the success of NAC in these patients. SIRT1 expression may predict the efficacy of NAC as a treatment for locally advanced, uterine cervical cancer. Our previous study reported that the expression of bax, bcl-xL, and MAD2 (mitotic arrest deficiency 2) proteins may predict the efficacy of NAC in patients with locally advanced, uterine cervical cancer (25, 38). Taken together, a combination of these factors may more effectively predict the efficacy of NAC in these patients.
References
- 1.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 2.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 3.Voelter-Mahlknecht S, Mahlknecht U. Cloning, chromosomal characterization and mapping of the NAD-dependent histone deacetylases gene sirtuin 1. Int J Mol Med. 2006;17:59–67. [PubMed] [Google Scholar]
- 4.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 5.Cohen HY, Lavu S, Bitterman KJ, Hekking B, Imahiyerobo TA, Miller C, Frye R, Ploegh H, Kessler BM, Sinclair DA. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell. 2004;13:627–638. doi: 10.1016/s1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- 6.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 7.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 8.Hida Y, Kubo Y, Murao K, Arase S. Strong expression of a longevity-related protein, SIRT1, in Bowen's disease. Arch Dermatol Res. 2007;299:103–106. doi: 10.1007/s00403-006-0725-6. [DOI] [PubMed] [Google Scholar]
- 9.Huffman DM, Grizzle WE, Bamman MM, Kim JS, Eltoum IA, Elgavish A, Nagy TR. SIRT1 is significantly elevated in mouse and human prostate cancer. Cancer Res. 2007;67:6612–6618. doi: 10.1158/0008-5472.CAN-07-0085. [DOI] [PubMed] [Google Scholar]
- 10.Kuzmichev A, Margueron R, Vaquero A, Preissner TS, Scher M, Kirmizis A, Ouyang X, Brockdorff N, Abate-Shen C, Farnham P, Reinberg D. Composition and histone substrates of polycomb repressive group complexes change during cellular differentiation. Proc Natl Acad Sci USA. 2005;102:1859–1864. doi: 10.1073/pnas.0409875102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang KY, Kim KS, Hwang SH, Kwon KS, Kim KR, Park HS, Park BH, Chung MJ, Kang MJ, Lee DG, Moon WS. Expression and prognostic significance prognostic of SIRT1 in ovarian epithelial tumours. Pathology. 2009;41:366–371. doi: 10.1080/00313020902884451. [DOI] [PubMed] [Google Scholar]
- 12.Chen HC, Jeng YM, Yuan RH, Hsu HC, Chen YL. SIRT1 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma and its expression predicts poor prognosis. Ann Surg Oncol. 2012;19:2011–2019. doi: 10.1245/s10434-011-2159-4. [DOI] [PubMed] [Google Scholar]
- 13.Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 15.Lim CS. Human SIRT1: a potential biomarker for tumorigenesis? Cell Biol Int. 2007;31:636–637. doi: 10.1016/j.cellbi.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Japan Society of Gynecologic Oncology (eds), book Formulation Committee of the Treatment Guidelines for Cervical Cancer. Kanehara & Co.; Tokyo: 2011. (In Japanese) [Google Scholar]
- 17.National Comprehensive Cancer Network, corp-author. NCCN Clinical Practice Guidelines in Oncology - Cervical Cancer - Version II. 2013. [Google Scholar]
- 18.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, Rotman M, Gershenson DM, Mutch DG. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 19.Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, Rotman M, Gershenson D, Mutch DG. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90–01. J Clin Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 20.Ishiko O, Sumi T, Yasui T, Matsumoto Y, Kawamura N, Ogita S, Kamino T, Nakamura K, Yamada R. Balloon-occluded arterial infusion chemotherapy, simple total hysterectomy, and radiotherapy as a useful combination-therapy for advanced cancer of the uterine cervix. Oncol Rep. 2000;7:141–144. [PubMed] [Google Scholar]
- 21.Souhami L, Gil RA, Allan SE, Canary PC, Araújo CM, Pinto LH, Silveira TR. A randomized trial of chemotherapy followed by pelvic radiation therapy in stage IIIB carcinoma of the cervix. J Clin Oncol. 1991;9:970–977. doi: 10.1200/JCO.1991.9.6.970. [DOI] [PubMed] [Google Scholar]
- 22.Tattersall MH, Lorvidhaya V, Vootiprux V, Cheirsilpa A, Wong F, Azhar T, Lee HP, Kang SB, Manalo A, Yen MS. Randomized trial of epirubicin and cisplatin chemotherapy followed by pelvic radiation in locally advanced cervical cancer. Cervical Cancer Study Group of the Asian Oceanian Clinical Oncology Association. J Clin Oncol. 1995;13:444–451. doi: 10.1200/JCO.1995.13.2.444. [DOI] [PubMed] [Google Scholar]
- 23.Ishiko O, Sumi T, Yasui T, Matsumoto Y, Ogita S, Kaminou T, Nakamura K, Yamada R. Tumor marker and MR imaging criteria for evaluating the efficacy of cyclic balloon-occluded arterial infusion for advanced cancer of the uterine cervix. Oncol Rep. 2000;7:827–830. doi: 10.3892/or.7.4.827. [DOI] [PubMed] [Google Scholar]
- 24.Ishiko O, Sumi T, Yoshida H, Ogita S, Yamada R. Expression of apoptosis regulatory proteins in advanced cancer of the uterine cervix after cyclic balloon-occluded arterial infusion chemotherapy. Int J Oncol. 2001;18:1151–1155. doi: 10.3892/ijo.18.6.1151. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto E, Sumi T, Misugi F, Nobeyama H, Hattori K, Yoshida H, Matsumoto Y, Yasui T, Honda K, Ishiko O. Expression of apoptosis-related proteins in advanced uterine cervical cancer after balloon-occluded arterial infusion chemotherapy as an indicator of the efficiency of this therapy. Int J Mol Med. 2005;15:41–47. [PubMed] [Google Scholar]
- 26.Nobeyama H, Sumi T, Misugi F, Okamoto E, Hattori K, Matsumoto Y, Yasui T, Honda K, Iwai K, Ishiko O. Association of HPV infection with prognosis after neoadjuvant chemotherapy in advanced uterine cervical cancer. Int J Mol Med. 2004;14:101–105. [PubMed] [Google Scholar]
- 27.Benedetti Panici P, Bellati F, Manci N, Pernice M, Plotti F, Di Donato V, Calcagno M, Zullo MA, Muzii L, Angioli R. Neoadjuvant chemotherapy followed by radical surgery in patients affected by FIGO stage IVA cervical cancer. Ann Surg Oncol. 2007;14:2643–2648. doi: 10.1245/s10434-007-9408-6. [DOI] [PubMed] [Google Scholar]
- 28.Tsuji K, Yamada R, Kawabata M, Mitsuzane K, Sato M, Iwahashi M, Kitayama S, Nakano R. Effect of balloon occluded arterial infusion of anticancer drugs on the prognosis of cervical cancer treated with radiation therapy. Int J Radiat Oncol Biol Phys. 1995;32:1337–1345. doi: 10.1016/0360-3016(94)00651-z. [DOI] [PubMed] [Google Scholar]
- 29.Sironi S, Belloni C, Taccagni G, DelMaschio A. Invasive cervical carcinoma: MR imaging after preoperative chemotherapy. Radiology. 1991;180:719–722. doi: 10.1148/radiology.180.3.1871283. [DOI] [PubMed] [Google Scholar]
- 30.Kim KH, Lee BH, Do YS, Chin SY, Park SY, Kim BG, Jang JJ. Stage IIb cervical carcinoma: MR evaluation of effect of intraarterial chemotherapy. Radiology. 1994;192:61–65. doi: 10.1148/radiology.192.1.8208967. [DOI] [PubMed] [Google Scholar]
- 31.Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995;55:237–241. [PubMed] [Google Scholar]
- 32.Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, Hahn WC, Guarente LP, Sinclair DA. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang RH, Zheng Y, Kim HS, Xu X, Cao L, Luhasen T, Lee MH, Xiao C, Vassilopoulos A, Chen W, Gardner K, Man YG, Hung MC, Finkel T, Deng CX. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol Cell. 2008;32:11–20. doi: 10.1016/j.molcel.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000;92:690–698. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- 35.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 36.Bremer GL, Tieboschb AT, van der Putten HW, de Haan J, Arends JW. p53 tumor suppressor gene protein expression in cervical cancer: relationship to prognosis. Eur J Obstet Gynecol Reprod Biol. 1995;63:55–59. doi: 10.1016/0301-2115(95)02225-v. [DOI] [PubMed] [Google Scholar]
- 37.Allison SJ, Jiang M, Milner J. Oncogenic viral protein HPV E7 up-regulates the SIRT1 longevity protein in human cervical cancer cells. Aging (Albany NY) 2009;1:316–327. doi: 10.18632/aging.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morishita M, Sumi T, Nakano Y, Teramae M, Fukuda T, Nobeyama H, Yoshida H, Matsumoto Y, Yasui T, Ishiko O. Expression of mitotic-arrest deficiency 2 predicts the efficacy of neoadjuvant chemotherapy for locally advanced uterine cervical cancer. Exp Ther Med. 2011;3:341–346. doi: 10.3892/etm.2011.407. [DOI] [PMC free article] [PubMed] [Google Scholar]


