Abstract
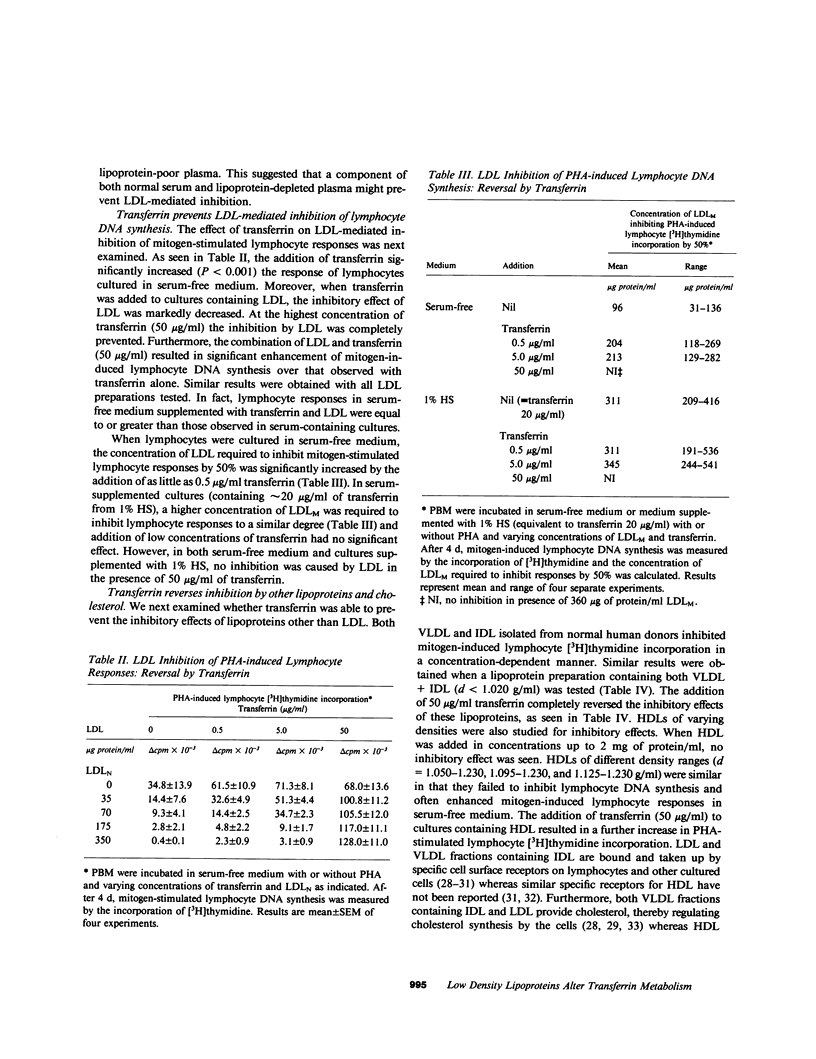
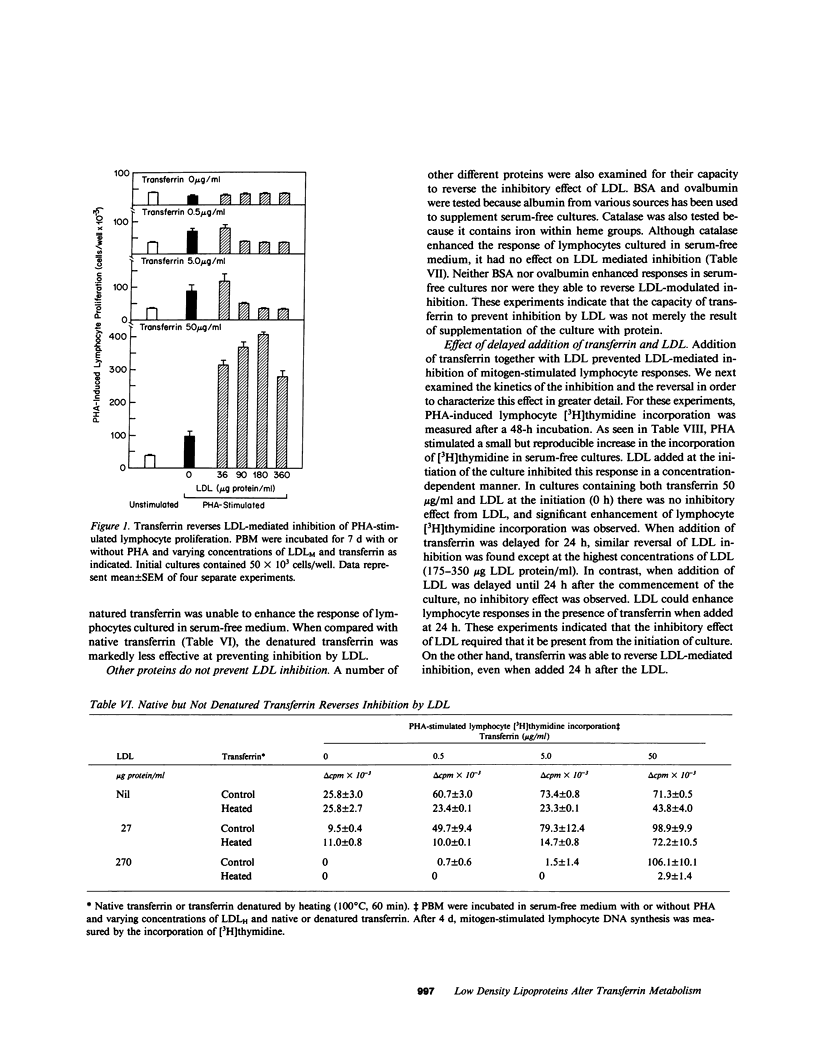
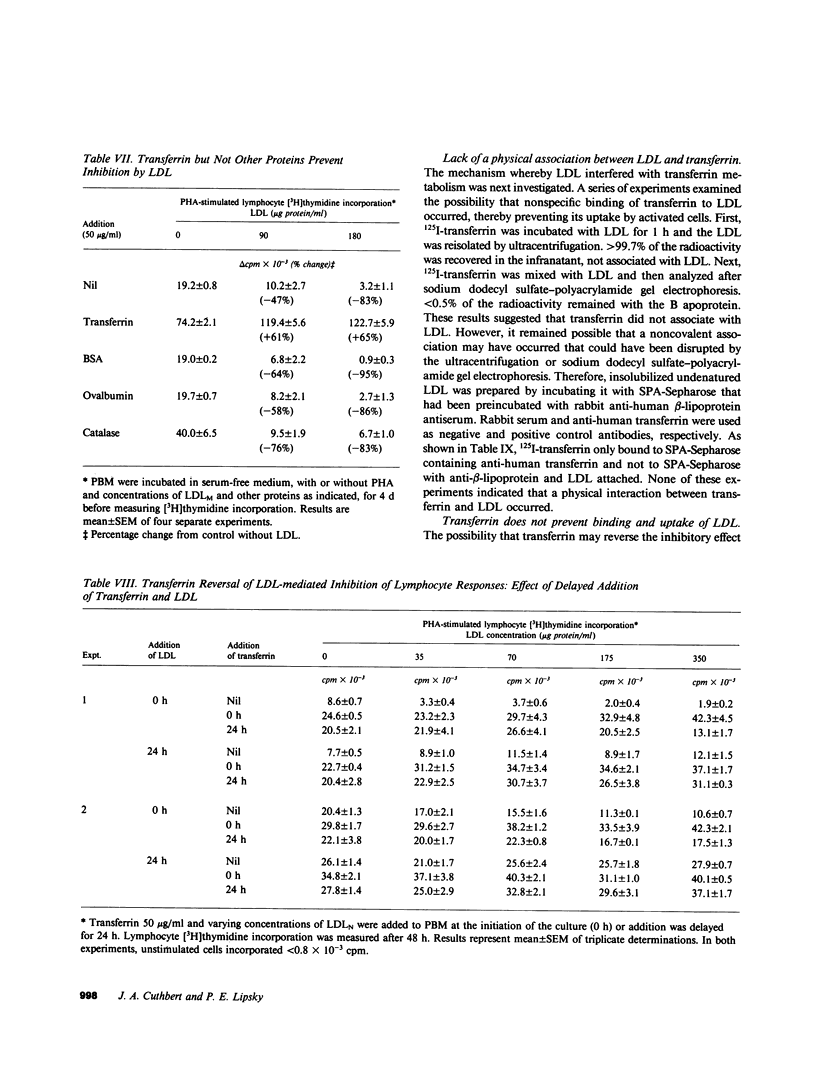
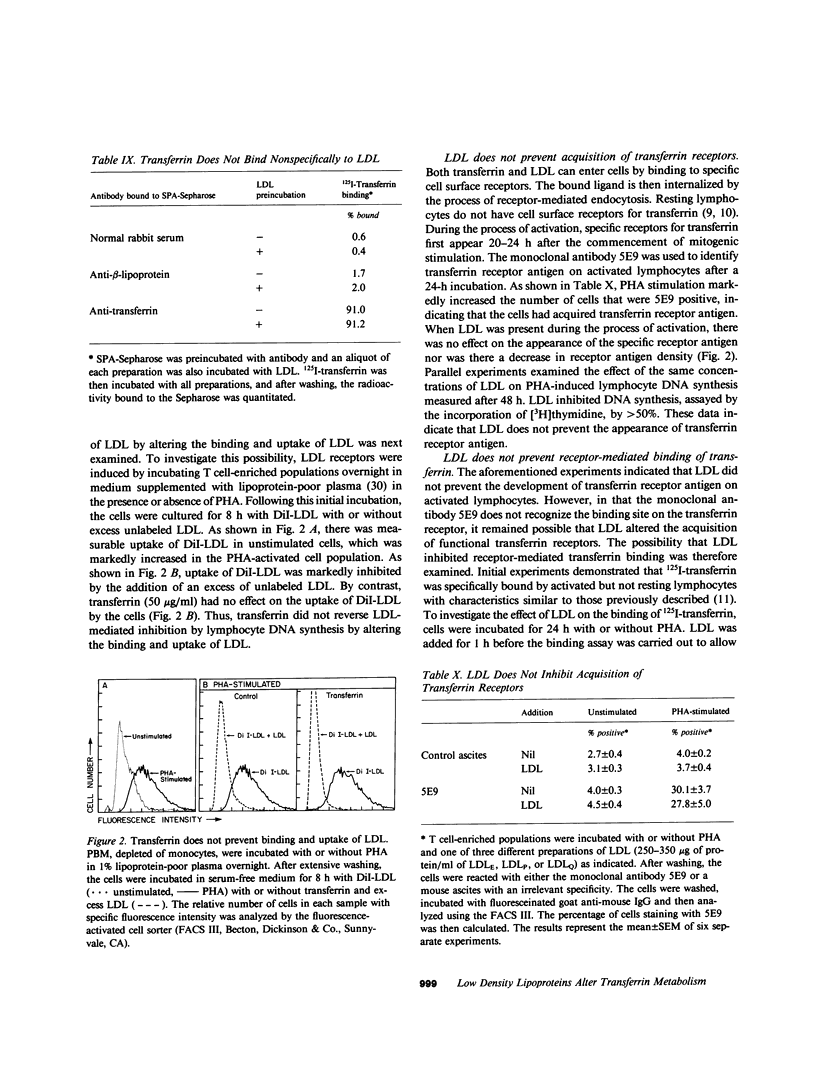
Human low density lipoprotein (LDL, d = 1.020-1.050 g/ml) inhibits mitogen-stimulated T lymphocyte DNA synthesis. Because both LDL and transferrin bind to specific cell surface receptors and enter cells by the similar means of receptor-mediated endocytosis, and because transferrin is necessary for lymphocyte DNA synthesis, we investigated the possibility that LDL may inhibit mitogen-stimulated lymphocyte responses by interfering with transferrin metabolism. LDL inhibited mitogen-stimulated lymphocyte [3H]thymidine incorporation in a concentration-dependent manner. The degree of inhibition was most marked in serum-free cultures, but was also observed in serum-containing cultures. The addition of transferrin not only augmented mitogen-induced lymphocyte [3H]thymidine incorporation in serum-free medium but also completely reversed the inhibitory effect of LDL in both serum-free and serum-containing media. Similar results were obtained when lymphocyte proliferation was assayed by counting the number of cells in culture. Transferrin also reversed the inhibition of lymphocyte responses caused by very low density lipoproteins and by cholesterol. The ability of transferrin to reverse the inhibitory effect of lipoproteins was specific, in that native but not denatured transferrin was effective whereas a variety of other proteins were ineffective. These results indicate that LDL inhibits mitogen-stimulated lymphocyte responses by interfering with transferrin metabolism. LDL only inhibited lymphocyte responses after a 48-h incubation if present from the initiation of the culture. By contrast, transferrin reversed inhibition when added after 24 h of the 48-h incubation. LDL did not inhibit lymphocyte responses by nonspecifically associating with transferrin. In addition, the acquisition of specific lymphocyte transferrin receptors was not blocked by LDL. Moreover, transferrin did not prevent the binding and uptake of fluorescent-labeled LDL by activated lymphocytes. Furthermore, LDL did not prevent the binding of transferrin to its receptor. Finally, LDL inhibition did not require specific high affinity cell surface receptors for cholesterol transport by LDL because similar inhibition and reversal by transferrin were observed with lymphocytes from a patient with homozygous familial hypercholesterolemia. Thus, LDL alters lymphocyte responses in a non-LDL receptor-mediated way by interfering with transferrin metabolism after specific binding of transferrin to receptors on activated lymphocytes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. M., Dietschy J. M. Regulation of sterol synthesis in 15 tissues of rat. II. Role of rat and human high and low density plasma lipoproteins and of rat chylomicron remnants. J Biol Chem. 1977 Jun 10;252(11):3652–3659. [PubMed] [Google Scholar]
- Anderson W. L., Chase C. G., Tomasi T. B., Jr Transferrin support of stimulated lymphocytes. In Vitro. 1982 Sep;18(9):766–774. doi: 10.1007/BF02796500. [DOI] [PubMed] [Google Scholar]
- Curtiss L. K., Edgington T. S. Differences in the characteristics of inhibition of lymphocyte stimulation by 25-hydroxycholesterol and by the immunoregulatory serum lipoprotein LDL-In. J Immunol. 1980 Oct;125(4):1470–1474. [PubMed] [Google Scholar]
- Curtiss L. K., Edgington T. S. Identification of a lymphocyte surface receptor for low density lipoprotein inhibitor, an immunoregulatory species of normal human serum low density lipoprotein. J Clin Invest. 1978 May;61(5):1298–1308. doi: 10.1172/JCI109047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss L. K., Edgington T. S. Regulatory serum lipoproteins: regulation of lymphocyte stimulation by a species of low density lipoprotein. J Immunol. 1976 May;116(5):1452–1458. [PubMed] [Google Scholar]
- Cuthbert J. A., Lipsky P. E. Immunoregulation by low density lipoproteins in man: low density lipoprotein inhibits mitogen-stimulated human lymphocyte proliferation after initial activation. J Lipid Res. 1983 Nov;24(11):1512–1524. [PubMed] [Google Scholar]
- Cuthbert J. A., Lipsky P. E. Sterol metabolism and lymphocyte function: inhibition of endogenous sterol biosynthesis does not prevent mitogen-induced human T lymphocyte activation. J Immunol. 1980 May;124(5):2240–2246. [PubMed] [Google Scholar]
- Cuthbert J. A., Lipsky P. E. Sterol metabolism and lymphocyte responsiveness: inhibition of endogenous sterol synthesis prevents mitogen-induced human T cell proliferation. J Immunol. 1981 Jun;126(6):2093–2099. [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillner-Centerlind M. L., Hammarström S., Perlmann P. Transferrin can replace serum for in vitro growth of mitogen-stimulated T lymphocytes. Eur J Immunol. 1979 Dec;9(12):942–948. doi: 10.1002/eji.1830091207. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Galbraith G. M., Goust J. M., Mercurio S. M., Galbraith R. M. Transferrin binding by mitogen-activated human peripheral blood lymphocytes. Clin Immunol Immunopathol. 1980 Aug;16(4):387–395. doi: 10.1016/0090-1229(80)90180-4. [DOI] [PubMed] [Google Scholar]
- Galbraith R. M., Galbraith G. M. Expression of transferrin receptors on mitogen-stimulated human peripheral blood lymphocytes: relation to cellular activation and related metabolic events. Immunology. 1981 Dec;44(4):703–710. [PMC free article] [PubMed] [Google Scholar]
- Galbraith R. M., Werner P., Arnaud P., Galbraith G. M. Transferrin binding to peripheral blood lymphocytes activated by phytohemagglutinin involves a specific receptor. Ligand interaction. J Clin Invest. 1980 Nov;66(5):1135–1143. doi: 10.1172/JCI109943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianturco S. H., Gotto A. M., Jr, Jackson R. L., Patsch J. R., Sybers H. D., Taunton O. D., Yeshurun D. L., Smith L. C. Control of 3-hydroxy-3-methylglutaryl-CoA reductase activity in cultured human fibroblasts by very low density lipoproteins of subjects with hypertriglyceridemia. J Clin Invest. 1978 Feb;61(2):320–328. doi: 10.1172/JCI108942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Harmony J. A., Hui D. Y. Inhibition by membrane-bound low-density lipoproteins of the primary inductive events of mitogen-stimulated lymphocyte activation. Cancer Res. 1981 Sep;41(9 Pt 2):3799–3802. [PubMed] [Google Scholar]
- Haynes B. F., Hemler M., Cotner T., Mann D. L., Eisenbarth G. S., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (5E9) that defines a human cell surface antigen of cell activation. J Immunol. 1981 Jul;127(1):347–351. [PubMed] [Google Scholar]
- Helderman J. H., Strom T. B. Emergence of insulin receptors upon alloimmune T cells in the rat. J Clin Invest. 1977 Feb;59(2):338–344. doi: 10.1172/JCI108646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. K., Brown S., Bilheimer D. W., Goldstein J. L. Regulation of low density lipoprotein receptor activity in freshly isolated human lymphocytes. J Clin Invest. 1976 Dec;58(6):1465–1474. doi: 10.1172/JCI108603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. K., Faust J. R., Bilheimer D. W., Brown M. S., Goldstein J. L. Regulation of cholesterol synthesis by low density lipoprotein in isolated human lymphocytes. Comparison of cells from normal subjects and patients with homozygous familial hypercholesterolemia and abetalipoproteinemia. J Exp Med. 1977 Jun 1;145(6):1531–1549. doi: 10.1084/jem.145.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Mintz B. Receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. J Biol Chem. 1981 Apr 10;256(7):3245–3252. [PubMed] [Google Scholar]
- Kovanen P. T., Schneider W. J., Hillman G. M., Goldstein J. L., Brown M. S. Separate mechanisms for the uptake of high and low density lipoproteins by mouse adrenal gland in vivo. J Biol Chem. 1979 Jun 25;254(12):5498–5505. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Modulation of cell surface iron transferrin receptors by cellular density and state of activation. J Supramol Struct. 1979;11(4):579–586. doi: 10.1002/jss.400110415. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E., Ziff M. The effect of D-penicillamine on mitogen-induced human lymphocyte proliferation: synergistic inhibition by D-penicillamine and copper salts. J Immunol. 1978 Mar;120(3):1006–1013. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L. Lipoprotein receptors and cholesterol homeostasis. Biochim Biophys Acta. 1983 May 24;737(2):197–222. doi: 10.1016/0304-4157(83)90001-1. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse J. H., Witte L. D., Goodman D. S. Inhibition of lymphocyte proliferation stimulated by lectins and allogeneic cells by normal plasma lipoproteins. J Exp Med. 1977 Dec 1;146(6):1791–1803. doi: 10.1084/jem.146.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C., Shinitzky M. Modulation of transferrin receptors in bone marrow cells by changes in lipid fluidity. Br J Haematol. 1979 Jul;42(3):355–362. doi: 10.1111/j.1365-2141.1979.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Nunez M. T., Glass J. Reconstitution of the transferrin receptor in lipid vesicles. Effect of cholesterol on the binding of transferrin. Biochemistry. 1982 Aug 17;21(17):4139–4143. doi: 10.1021/bi00260a034. [DOI] [PubMed] [Google Scholar]
- Pitas R. E., Innerarity T. L., Weinstein J. N., Mahley R. W. Acetoacetylated lipoproteins used to distinguish fibroblasts from macrophages in vitro by fluorescence microscopy. Arteriosclerosis. 1981 May-Jun;1(3):177–185. doi: 10.1161/01.atv.1.3.177. [DOI] [PubMed] [Google Scholar]
- Roeschlau P., Bernt E., Gruber W. Enzymatic determination of total cholesterol in serum. Z Klin Chem Klin Biochem. 1974 May;12(5):226–226. [PubMed] [Google Scholar]
- Rosenberg S. A., Ligler F. S., Ugolini V., Lipsky P. E. A monoclonal antibody that identifies human peripheral blood monocytes recognizes the accessory- cells required for mitogen-induced T lymphocyte proliferation. J Immunol. 1981 Apr;126(4):1473–1477. [PubMed] [Google Scholar]
- Rudland P. S., Durbin H., Clingan D., de Asua L. J. Iron salts and transferrin are specifically required for cell division of cultured 3T6 cells. Biochem Biophys Res Commun. 1977 Apr 11;75(3):556–562. doi: 10.1016/0006-291x(77)91508-x. [DOI] [PubMed] [Google Scholar]
- Scanu A. M., Byrne R. E., Mihovilovic M. Functional roles of plasma high density lipoproteins. CRC Crit Rev Biochem. 1982;13(2):109–140. doi: 10.3109/10409238209108711. [DOI] [PubMed] [Google Scholar]
- Tormey D. C., Imrie R. C., Mueller G. C. Identification of transferrin as a lymphocyte growth promoter in human serum. Exp Cell Res. 1972 Sep;74(1):163–169. doi: 10.1016/0014-4827(72)90492-2. [DOI] [PubMed] [Google Scholar]
- Ugai K., Ziff M., Lipsky P. E. Gold-induced changes in the morphology and functional capabilities of human monocytes. Arthritis Rheum. 1979 Dec;22(12):1352–1360. doi: 10.1002/art.1780221206. [DOI] [PubMed] [Google Scholar]
- Wedner H. J., Parker C. W. Lymphocyte activation. Prog Allergy. 1976;20:195–300. [PubMed] [Google Scholar]



