Abstract
Recent clinical trials, such as JCOG9912 and SPIRITS, excluded geriatric patients aged ≥75 years. The clinical significance of intensive chemotherapy for geriatric patients with advanced or recurrent gastric cancer remains unclear. Between 2002 and 2010, 54 consecutive advanced or recurrent gastric cancer patients aged ≥75 years were enrolled in this study. We analyzed the predictors of chemotherapy administration and evaluated the survival benefit of chemotherapy for geriatric patients with advanced or recurrent gastric cancer. A total of 23 geriatric patients received no chemotherapy (GP), whereas the remaining 31 patients were administered chemotherapy (GPC). Of the 54 patients, 20 had severe concomitant illnesses, such as cardiorespiratory disease. Lymph node involvement (P=0.044) and the absence of cardiorespiratory disease (P<0.001) were found to be independently associated with chemotherapy administration. The GPC group exhibited a significantly better prognosis compared to the GP group (median survival time, 19.4 vs. 13.6 months, respectively; P=0.043). GPC patients without cardiorespiratory disease tended to have a better prognosis compared to GP patients without cardiorespiratory disease (P=0.106), whereas there were no significant differences between GP and GPC patients with cardiorespiratory disease. However, administration of chemotherapy was identified as an independent prognostic factor by the Cox proportional hazards model (hazard ratio = 2.609; 95% confidence interval: 1.173–5.761; P=0.019). Therefore, chemotherapy appears to provide a survival benefit in geriatric patients with advanced or recurrent gastric cancer, particularly those without concomitant cardiorespiratory disease.
Keywords: chemotherapy, geriatric patients, advanced gastric cancer
Introduction
Despite the improvements in the early detection of gastric cancer (GC), it remains one of the leading causes of cancer-related mortality worldwide (1, 2). Surgical resection is generally recommended as standard treatment for curable GC. Furthermore, systemic chemotherapy is widely accepted as palliative treatment for patients with unresectable, advanced or recurrent GC and was shown to improve the quality of life and prolong survival time. Previous studies have indicated the superiority of systemic chemotherapy compared to best supportive care (BSC) in patients with advanced or recurrent GC (3–5) and several phase III trials investigating systemic chemotherapy in advanced GC patients have been conducted (6–11). Recently, several clinical trials assessed the tolerability or efficacy of systemic chemotherapy, including adjuvant chemotherapy, in geriatric patients with advanced or recurrent GC and demonstrated its feasibility (12–17). However, the effect of systemic chemotherapy in the prognosis of geriatric patients with advanced or recurrent GC remains unclear. In addition, standardized regimens have not been validated. It may be difficult to standardize cancer treatments for geriatric patients, as the ability to tolerate intensive chemotherapy is largely dependent on the patient's physical background.
In the present study, we investigated clinicopathological characteristics, including the presence of concomitant illnesses, such as cardiorespiratory disease, in geriatric patents with advanced or recurrent GC and evaluated the prognostic significance of intensive chemotherapy. The results of our study may affect decision making regarding treatment for geriatric patients with advanced or recurrent GC.
Patients and methods
Patients
A total of 54 geriatric patients (aged ≥75 years) with histologically confirmed advanced or recurrent adenocarcinoma of the stomach or gastroesophageal junction were enrolled in this study. The patients were treated at Kyoto Prefectural University of Medicine (Kyoto, Japan) between 2002 and 2010. We retrospectively reviewed the hospital data and evaluated the clinicopathological characteristics, such as age at diagnosis, gender, tumor stage, comorbidities and chemotherapeutic regimens. We also collected follow-up data on tumor recurrence and prognosis up to December 31, 2012.
Statistical analysis
A univariate analysis of the correlation between clinicopathological characteristics and the administration of chemotherapy was performed using the Chi-square and Fisher's exact probability tests. Multivariate logistic regression was used to assess the factors associated with the administration of chemotherapy. Kaplan-Meier survival curves were generated and compared with log-rank tests to assess the survival benefits between the patient treatment groups. The prognostic factors for overall survival were evaluated using the Cox proportional hazards regression. For all analyses, P-value <0.05 was considered to indicate statistically significant differences. Statistical analyses were conducted using JMP 10 software (SAS Institute Inc., Cary, NC).
Results
Clinicopathological characteristics of gastric cancer patients
A total of 54 geriatric patients were included in this study. The patients were staged as follows: stage I, 1 patient; stage II, 17 patients; stage III, 22 patients; and stage IV, 14 patients, according to the 7th TNM classification (18). Tumor staging was performed at initial diagnosis. The mean age of the patients was 79.8 years (range: 75–89 years). Of the 54 patients, 47 (87%) underwent gastrectomy and regional lymphadenectomy, whereas 7 (13%) were ineligible for gastrectomy. A total of 31 patients (57%) received systemic chemotherapy and the remaining 23 (43%) did not receive chemotherapy (Table I).
Table I.
Characteristics of geriatric patients with advanced or recurrent gastric cancer.
| Characteristics | Patient no. (%) (n=54) | |
|---|---|---|
| Gender | ||
| Male | 37 | (69) |
| Female | 17 | (31) |
| Age, yearsa | ||
| 75–79 | 31 | (57) |
| > 80 | 23 | (43) |
| Tumor depthb | ||
| T1 | 5 | (9) |
| T2 | 3 | (6) |
| T3 | 12 | (22) |
| T4 | 34 | (63) |
| Lymph node metastasis | ||
| Negative | 42 | (78) |
| Positive | 12 | (22) |
| Distant metastasis | ||
| Negative | 40 | (74) |
| Positive | 14 | (26) |
| Stagea, b | ||
| I | 1 | (2) |
| II | 17 | (31) |
| III | 22 | (41) |
| IV | 14 | (26) |
| Resectability | ||
| Resectable | 47 | (87) |
| Non-resectable | 7 | (13) |
| Recurrence pattern | ||
| Peritoneal | 19 | (35) |
| Liver/lung | 10 | (19) |
| Lymph node | 11 | (20) |
| Local | 11 | (20) |
| Others | 3 | (6) |
| Chemotherapy | ||
| Absent | 23 | (43) |
| Present | 31 | (57) |
Recorded at the initial treatment.
Based on the 7th TNM staging system.
Details of chemotherapeutic regimens
Of the 31 patients who received chemotherapy, 27 (87%) were administered 5-fluorouracil (5-FU)-based regimens (S-1, S-1 plus cisplatin, uracil-tegafur and 5-FU) as first-line chemotherapy and the remaining 4 patients received taxane-based regimens (3 patients) or irinotecan plus cisplatin (1 patient). Furthermore, 12 patients received second-line chemotherapy, of whom 10 patients received 5-FU-based regimens (S-1 alone, 6 patients; S-1 plus cisplatin, 2 patients; uracil-tegafur, 1 patient; and 5-FU i.v., 1 patient).
Concomitant illnesses in geriatric patients prior to treatment
Of the 54 included patients, 20 (37%) did not experience any concomitant illness prior to treatment. However, the remaining 34 patients (63%) exhibited concomitant illnesses, including hypertension, cardiac disease, cerebrovascular disease, diabetes mellitus, pulmonary disease and liver disease. In particular, 20 patients (37%) had cardiorespiratory diseases, such as atrial fibrillation, myocardial infarction, brain infarction and pulmonary infarction. The patients with cardiorespiratory diseases were receiving treatment for their conditions (e.g., antithrombotic medication, antiarrhythmic agents, or home oxygen therapy) (Table II).
Table II.
Pretreatment classification of comorbidity in geriatric patients with advanced or recurrent gastric cancer.
| Comorbidities | Patient no. (%) (n=54) | |
|---|---|---|
| Hypertension | 15 | (27) |
| Cardiac disease | 10 | (15) |
| Cerebrovascular disease | 8 | (14) |
| Diabetes mellitus | 5 | (9) |
| Pulmonary disease | 3 | (5) |
| Liver disease | 2 | (3) |
| Cardiorespiratory diseasea | 20 | (37) |
The patients were receiving treatment for cardiorespiratory diseases (e.g., atrial fibrillation, myocardial infarction, brain infarction, pulmonary infarction).
Correlation of clinicopathological factors with chemotherapy administration and logistic regression analysis of independent factors associated with chemotherapy administration
Using the Chi-square test, the administration of chemotherapy was found to be significantly correlated with the absence of cardiorespiratory diseases (P=0.001). Moreover, using a multivariate logistic regression analysis, lymph node involvement [odds ratio (OR)=10.0; 95% confidence interval (CI): 1.07–139.05; P=0.044] and the absence of cardiorespiratory disease (OR=12.09; 95% CI: 2.66–74.94; P<0.001) were independently associated with the administration of chemotherapy (Table III).
Table III.
Factors associated with the administration of chemotherapy in geriatric patients determined by univariate and multivariate analysis.
| Univariate analysisa | Multivariate evaluation by logistic regression analysis | ||||||
|---|---|---|---|---|---|---|---|
| Factors, no. (%) | Total no. (n=54) | CT (n=31) | No CT (n=23) | P-value | Odds ratio | 95% CI | P-value |
| Gender | |||||||
| Male | 37 | 22 (71) | 15 (65) | 0.653 | 1.00 | 0.02–0.87 | 0.033 |
| Female | 17 | 9 (29) | 8 (35) | 0.17 | |||
| Age, years | |||||||
| 75–79 | 31 | 18 (58) | 13 (57) | 0.909 | 1.00 | 0.61–19.75 | 0.171 |
| ≥80 | 23 | 13 (42) | 10 (43) | 3.17 | |||
| Tumor depth | |||||||
| T1–3 | 20 | 12 (39) | 8 (35) | 0.767 | 1.00 | 0.55–24.24 | 0.191 |
| T4 | 34 | 19 (61) | 15 (65) | 3.38 | |||
| Nodal status | |||||||
| Positive | 42 | 26 (84) | 16 (70) | 0.213 | 1.00 | 1.07–139.05 | 0.044 |
| Negative | 12 | 5 (16) | 7 (30) | 10.00 | |||
| Distant metastasis | |||||||
| Positive | 14 | 7 (23) | 7 (30) | 0.516 | 1.00 | 0.12–3.66 | 0.658 |
| Negative | 40 | 24 (77) | 16 (70) | 0.68 | |||
| Stage | |||||||
| I/II | 18 | 12 (39) | 6 (26) | 0.327 | 1.00 | 0.01–1.26 | 0.081 |
| III/IV | 36 | 19 (61) | 17 (74) | 0.15 | |||
| Recurrence pattern | |||||||
| Peritoneal | 19 | 11 (35) | 8 (35) | 0.957 | 1.00 | 0.12–2.50 | 0.465 |
| Other | 35 | 20 (65) | 15 (65) | 0.58 | |||
| Cardiorespiratory disease | |||||||
| Present | 20 | 6 (19) | 14 (61) | 0.001 | 1.00 | 2.66–74.94 | < 0.001 |
| Absent | 34 | 25 (81) | 9 (39) | 12.09 | |||
Chi-square test. CT, chemotherapy; CI, confidence interval.
Survival analysis
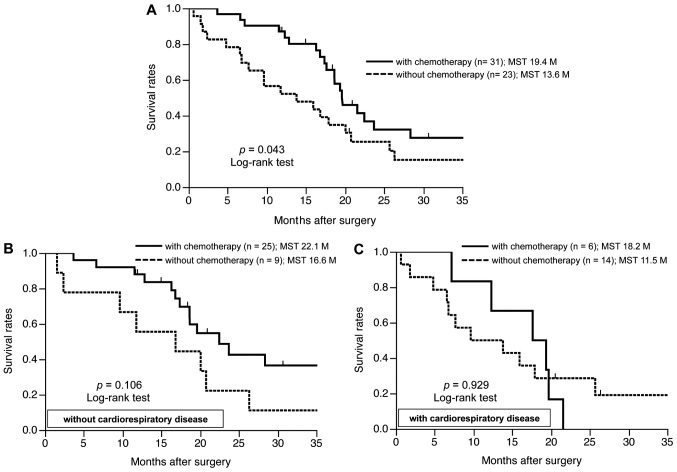
The geriatric patients treated with chemotherapy (GPC) had a significantly better prognosis compared to those without chemotherapy (GP) (median survival time, 19.4 vs. 13.6 months, respectively; P=0.043) (Fig. 1A). As regards cardiorespiratory disease as a concomitant illness, GPC without cardiorespiratory disease had a better prognosis compared to GP without cardiorespiratory disease (P=0.106) (Fig. 1B), whereas there were no significant differences between GP and GPC with cardiorespiratory disease (Fig. 1C). The prognostic evaluation using univariate analysis indicated a significantly better prognosis in patients without distant metastasis (P<0.001), patients without cardiorespiratory disease (P=0.020) and patients undergoing chemotherapy (P=0.043). The multivariate analysis using the Cox proportional hazards model indicated that the administration of chemotherapy [hazard ratio (HR)=2.609; 95% CI: 1.173–5.761; P=0.019) and the absence of distant metastasis (HR=5.169; 95% CI: 2.013–13.651; P=0.001) were independent factors for better prognosis in geriatric patients with advanced or recurrent GC, although the absence of cardiorespiratory disease was not identified as independent factor of a better prognosis (Table IV).
Figure 1.
Survival analysis of geriatric patients with advanced or recurrent gastric cancer. (A) The geriatric patients treated with chemotherapy (GPC) had a significantly better prognosis compared to those without chemotherapy (GP). (B) GPC without cardiorespiratory disease had a better prognosis compared to GP without cardiorespiratory disease. (C) There were no significant differences between GP and GPC with cardiorespiratory disease. MST, mean survival time.
Table IV.
Prognostic factors in geriatric patients with advanced or recurrent gastric cancer using the Cox proportional hazards model.
| Univariate analysisa | Multivariate analysisb | |||
|---|---|---|---|---|
| Factors | P-value | HR | 95% CI | P-value |
| Gender | ||||
| Male vs. female | 0.221 | - | ||
| Age, years | ||||
| 75–79 vs. ≥80 | 0.276 | - | ||
| Tumor depth | ||||
| T4 vs. T1–3 | 0.228 | - | ||
| Nodal status | ||||
| Positive vs. negative | 0.211 | - | ||
| Distant metastasis | ||||
| Positive vs. negative | <0.001 | 5.169 | 2.013–13.651 | 0.001 |
| Cardiorespiratory disease | ||||
| Present vs. absent | 0.020 | 1.368 | 0.627–2.907 | 0.424 |
| Chemotherapy | ||||
| Absent vs. present | 0.043 | 2.609 | 1.173–5.761 | 0.019 |
Log-rank test.
Cox proportional hazards model; HR, hazard ratio; CI, confidence interval.
Discussion
The overall elderly population is currently increasing, in Japan as well as other Asian and Western countries (1, 2). As a result, the number of geriatric patients with malignant neoplasms is also increasing. Therefore, there is a need for the development and establishment of a therapeutic strategy for malignant neoplasms in geriatric patients. However, the decision to administer chemotherapy to geriatric patients with malignant neoplasms may be difficult, as the therapeutic strategy applied to this population is identical to that for younger patients. The difficulty in standardizing therapeutic regimens may be due to the fact that the therapeutic strategies for geriatric cancer patients depend on their physical and/or social background. Numerous retrospective and prospective cohort studies regarding the therapeutic indications for geriatric GC patients are currently performed in Japan; however, the criteria for selecting surgery or chemotherapy for such patients remain unclear. It is important to consider clinical characteristics, such as underlying or concomitant illness, when deciding on the therapeutic strategy, particularly in geriatric patients. Therefore, we focused on concomitant illness in geriatric patients with GC and evaluated its effect on chemotherapeutic indications.
A significant survival benefit for first- and second-line chemotherapy compared to BSC in advanced or recurrent GC was previously reported (19, 20). Therefore, a shift from first- to second-line chemotherapy may exert a significant effect on survival benefit. By contrast, although the precise indications for the administration of first- or second-line chemotherapy for geriatric GC remains unclear, the non-administration of second-line chemotherapy was significantly correlated with the presence of cardiorespiratory disease in the present study (data not shown).
As regards the survival analysis, the presence of cardiorespiratory disease was not identified as an independent factor for poor prognosis. However, the administration of chemotherapy was an independent factor for better prognosis, suggesting that chemotherapy safely administered to geriatric GC patients, even those with cardiorespiratory disease, may contribute to a better prognosis. Moreover, these findings suggested that the use of chemotherapy for geriatric GC may be of prognostic significance regardless of concomitant illness. However, chemotherapy may exert a beneficial effect on geriatric patients without critical concomitant illness; thus, the chemotherapeutic regimen should be adapted for such patients. Therefore, an accurate assessment of the physical ability of geriatric patients with GC is required to ensure adequate and safe treatment.
The Comprehensive Geriatric Assessment (CGA) is a multidimensional tool used to evaluate comorbidities, nutrition, cognition, functional status and geriatric syndromes. CGA is also used to identify patients at increased risk of adverse outcomes and guide management (21). In previous studies, the CGA tool and the frailty index for geriatric cancer patients were shown to provide valuable information through the prediction of complications from chemotherapy and tolerance to treatment (22, 23).
In the present study, we demonstrated a survival benefit in geriatric patients with advanced or recurrent gastric cancer receiving chemotherapy. We also demonstrated that chemotherapy was safer and better tolerated in geriatric GC patients without cardiorespiratory diseases. Moreover, geriatric patients with malignancies commonly exhibit variations in physical status, cognitive function and social environment. Thus, assessments of the general condition of geriatric gastric cancer patients, including various physical, cognitive and social factors, using methods such as CGA or other modified assessment tools, must be established as a standard clinical routine. These issues are currently under evaluation. The clinical application of such criteria may aid in the decision to administer intensive chemotherapy to geriatric patients with advanced or recurrent gastric cancer. Further studies are required to investigate the reproducibility of the present results in a larger cohort study or prospective trials and to establish acceptable criteria for cancer therapy in geriatric gastric cancer patients.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Horner MJ, Howlader N, Eisner MP, Reichman M, Edwards BK. SEE. cancer statistics review. 1975–2004. National Cancer Institute; Bethesda, MD: 2013. [Google Scholar]
- 3.Glimelius B, Hoffman K, Haglund U, Nyren O, Sjoden PO. Initial or delayed chemotherapy with best supportive care in advanced gastric cancer. Ann Oncol. 1994;5:189–190. doi: 10.1093/oxfordjournals.annonc.a058778. [DOI] [PubMed] [Google Scholar]
- 4.Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37–41. doi: 10.1002/1097-0142(19930701)72:1<37::aid-cncr2820720109>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 5.Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995;71:587–591. doi: 10.1038/bjc.1995.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Batran SE, Hartmann JT, Probst S, et al. (Arbeitsgemeinschaft Internistische Onkologie). Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–1442. doi: 10.1200/JCO.2007.13.9378. [DOI] [PubMed] [Google Scholar]
- 7.Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063–1069. doi: 10.1016/S1470-2045(09)70259-1. [DOI] [PubMed] [Google Scholar]
- 8.Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–673. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 9.Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 10.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. (V325 Study Group). Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 study group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 11.Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–267. doi: 10.1200/JCO.1997.15.1.261. [DOI] [PubMed] [Google Scholar]
- 12.Catalano V, Bisonni R, Graziano F, et al. A phase II study of modified FOLFOX as first-line chemotherapy for metastatic gastric cancer in elderly patients with associated diseases. Gastric Cancer. 2013;16:411–419. doi: 10.1007/s10120-012-0204-z. [DOI] [PubMed] [Google Scholar]
- 13.Jin Y, Qiu MZ, Wang DS, et al. Adjuvant chemotherapy for elderly patients with gastric cancer after D2 gastrectomy. PLoS One. 2013;8:e53149. doi: 10.1371/journal.pone.0053149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JH, Kim HS, Han AR, et al. Irinotecan, leucovorin and 5-fluorouracil (modified FOLFIRI) as salvage chemotherapy for frail or elderly patients with advanced gastric cancer. Oncol Lett. 2012;4:751–754. doi: 10.3892/ol.2012.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koizumi W, Akiya T, Sato A, et al. Phase II study of S-1 as first-line treatment for elderly patients over 75 years of age with advanced gastric cancer: the Tokyo Cooperative Oncology Group study. Cancer Chemother Pharmacol. 2010;65:1093–1099. doi: 10.1007/s00280-009-1114-6. [DOI] [PubMed] [Google Scholar]
- 16.Lee JL, Kang YK, Kang HJ, et al. A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer. 2008;99:584–590. doi: 10.1038/sj.bjc.6604536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trumper M, Ross PJ, Cunningham D, et al. Efficacy and tolerability of chemotherapy in elderly patients with advanced oesophago-gastric cancer: A pooled analysis of three clinical trials. Eur J Cancer. 2006;42:827–834. doi: 10.1016/j.ejca.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 18.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TN. classification of malignant tumours. 7th. Wiley-Blackwell; New York, NY: 2009. [Google Scholar]
- 19.Kang JH, Lee SI, Lim do H, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513–1518. doi: 10.1200/JCO.2011.39.4585. [DOI] [PubMed] [Google Scholar]
- 20.Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer - a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Eur J Cancer. 2011;47:2306–2314. doi: 10.1016/j.ejca.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Caillet P, Canoui-Poitrine F, Vouriot J, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer. ELCAPA study: J Clin Oncol. 2011;29:3636–3642. doi: 10.1200/JCO.2010.31.0664. [DOI] [PubMed] [Google Scholar]
- 22.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 23.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA J Clin. 2010;60:120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]