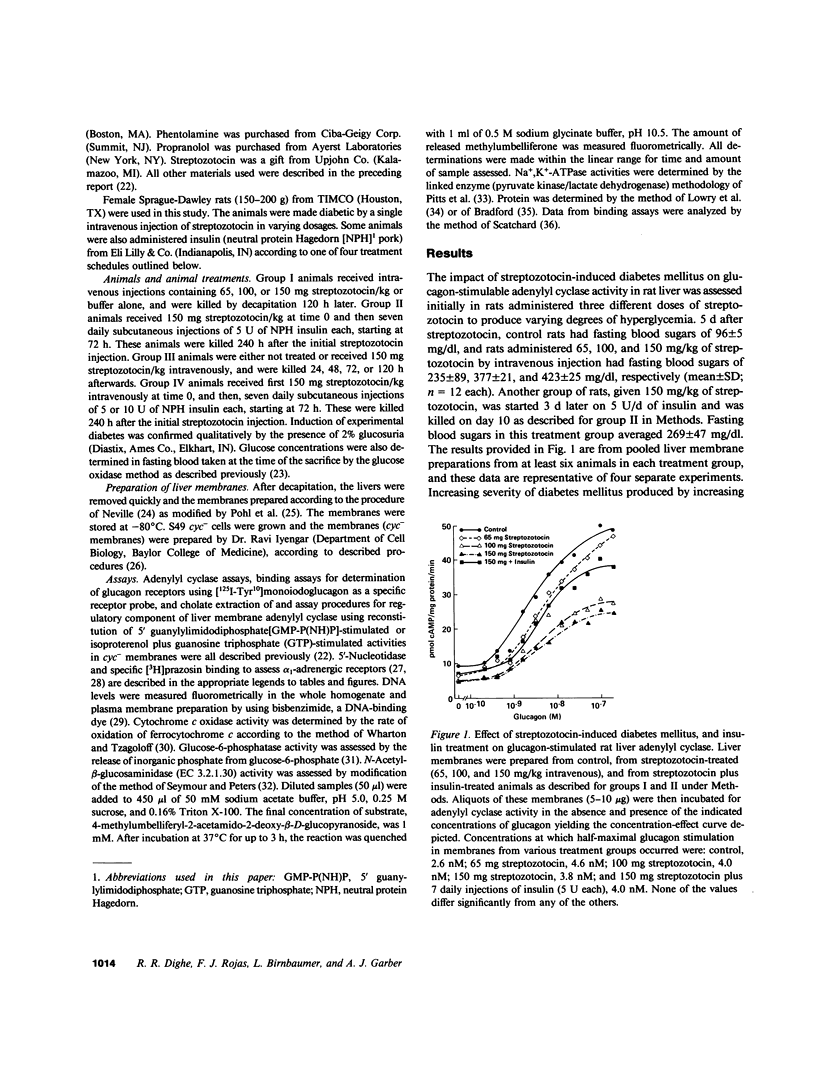
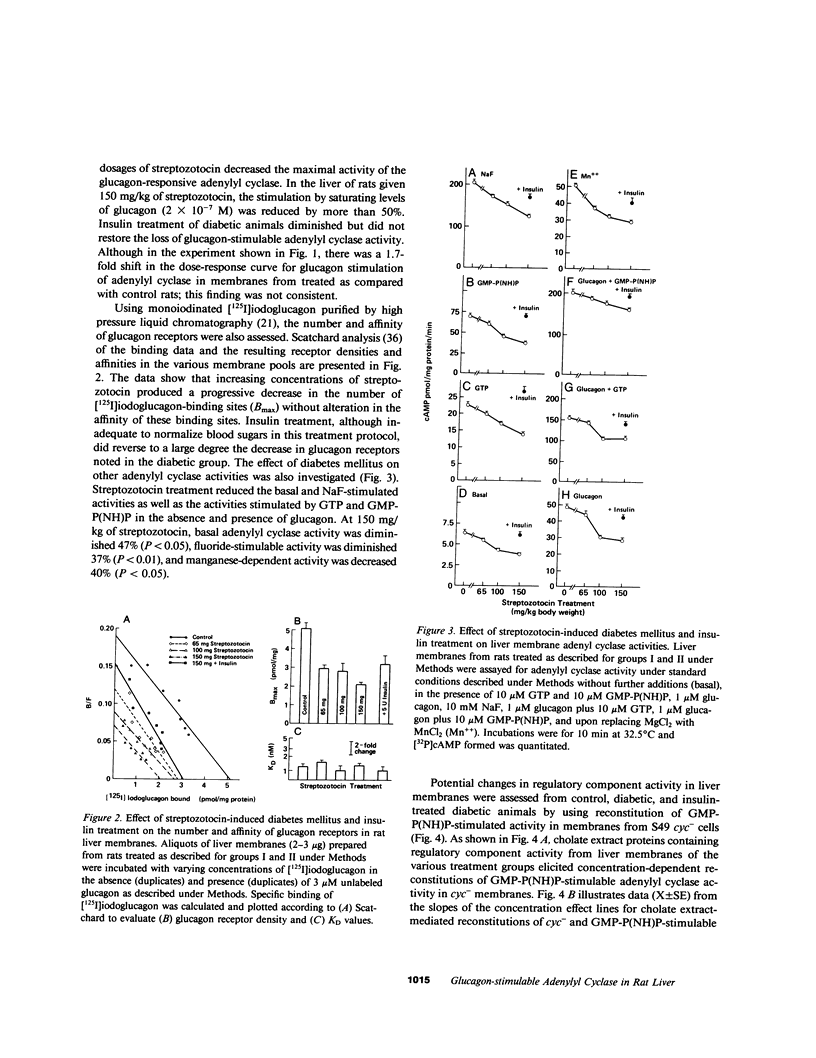
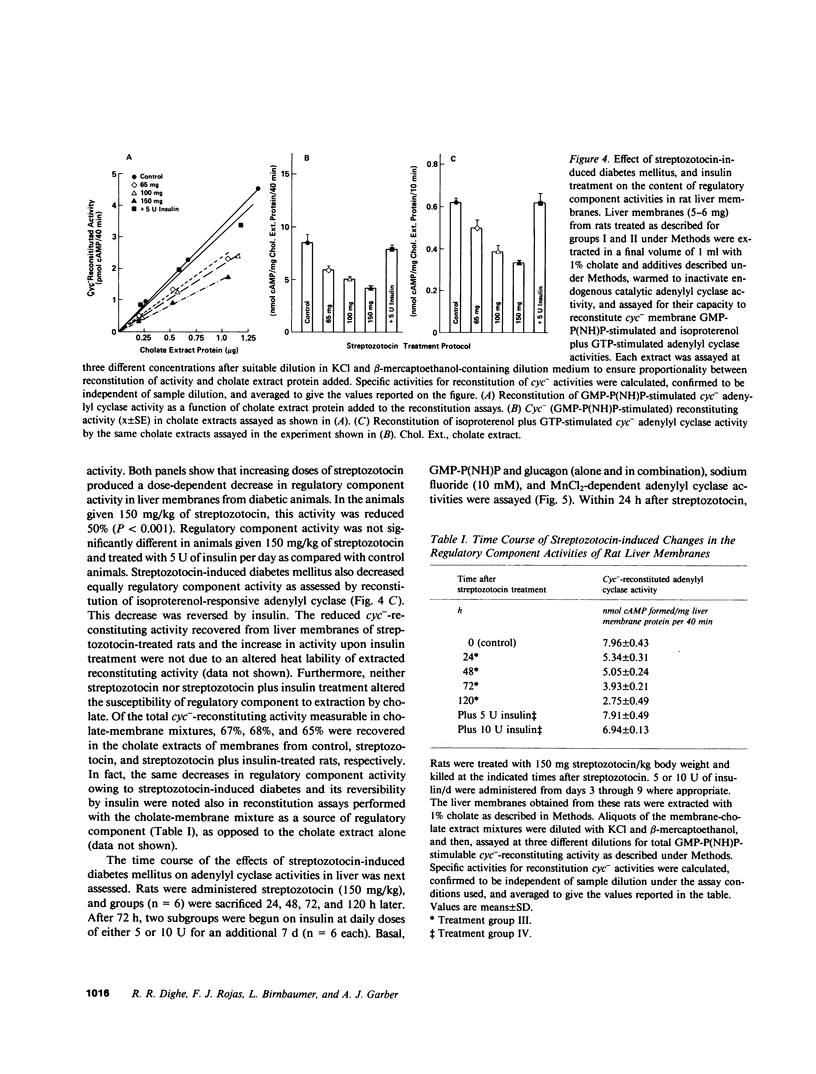
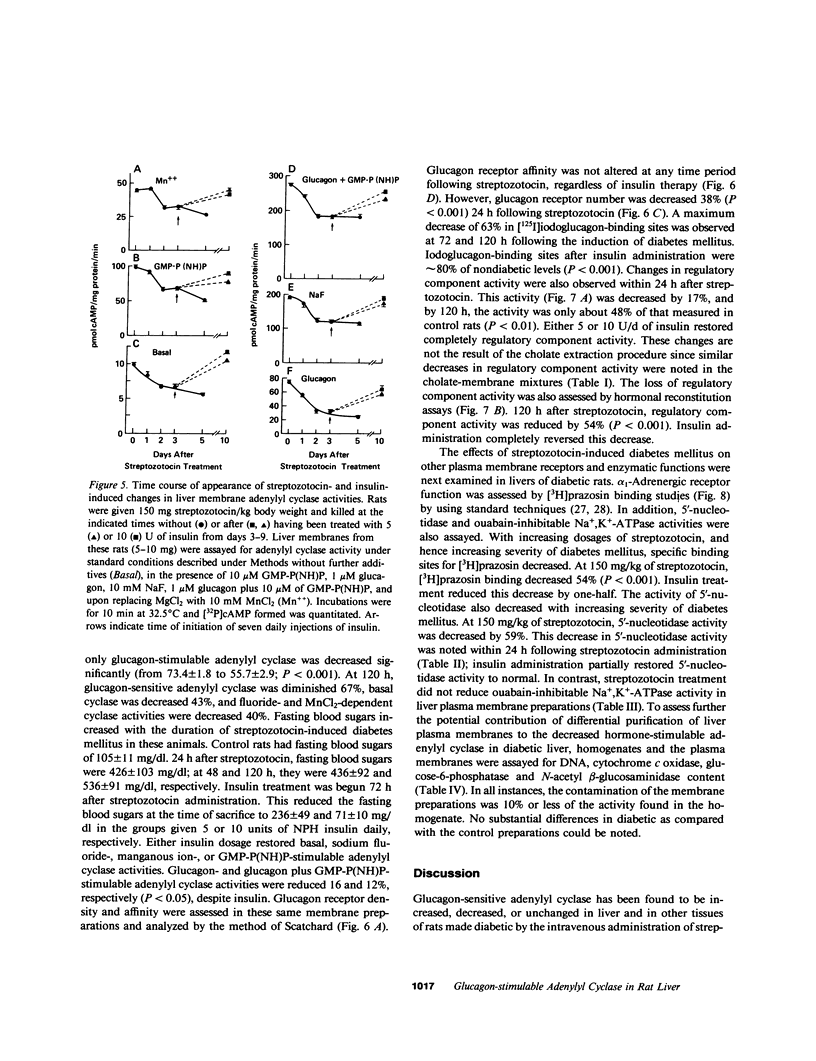
Abstract
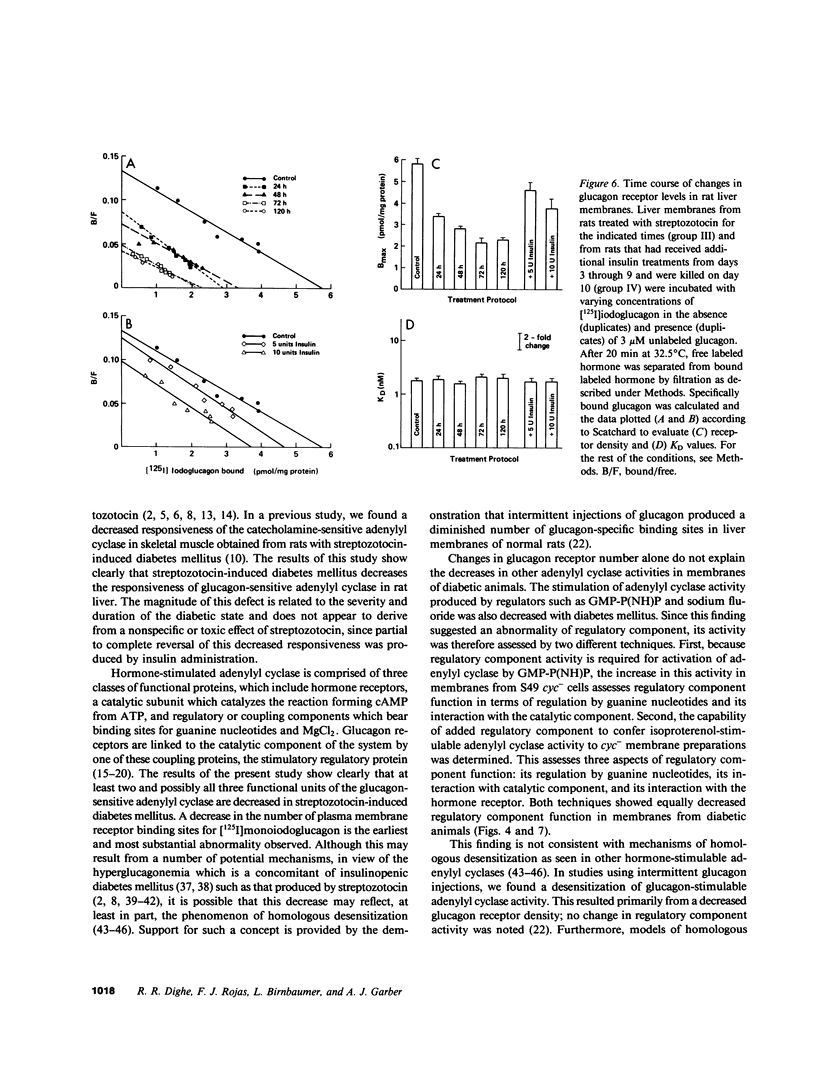
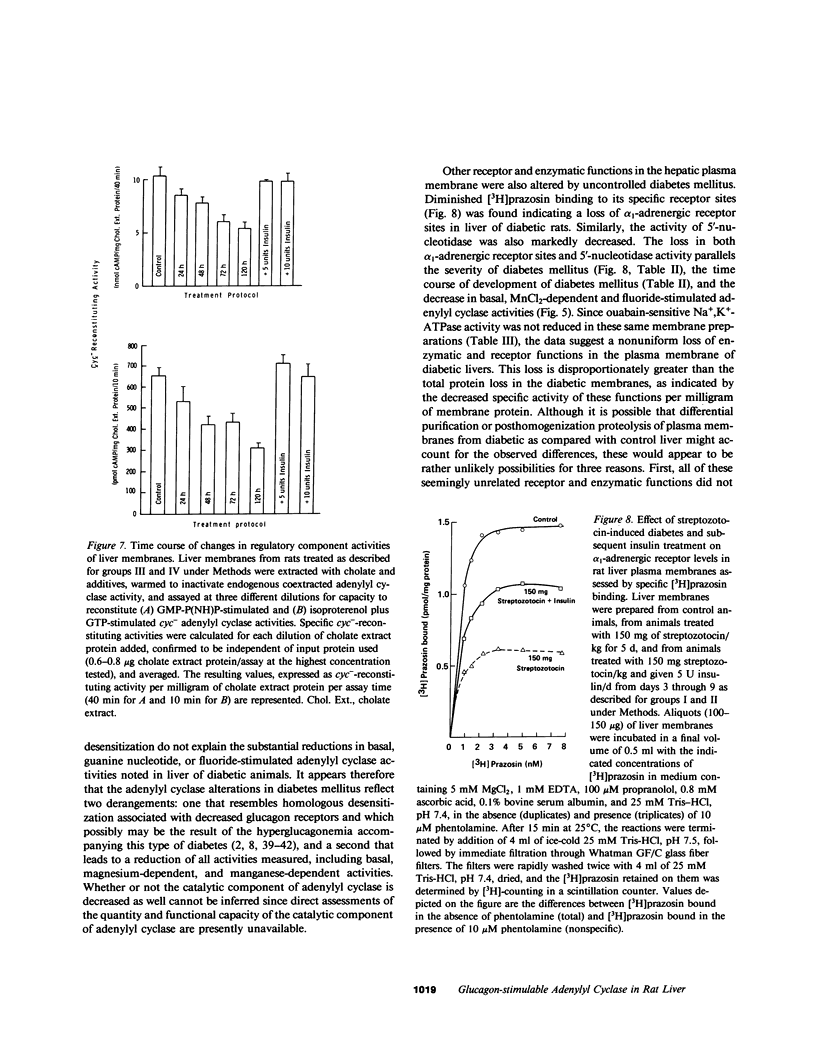
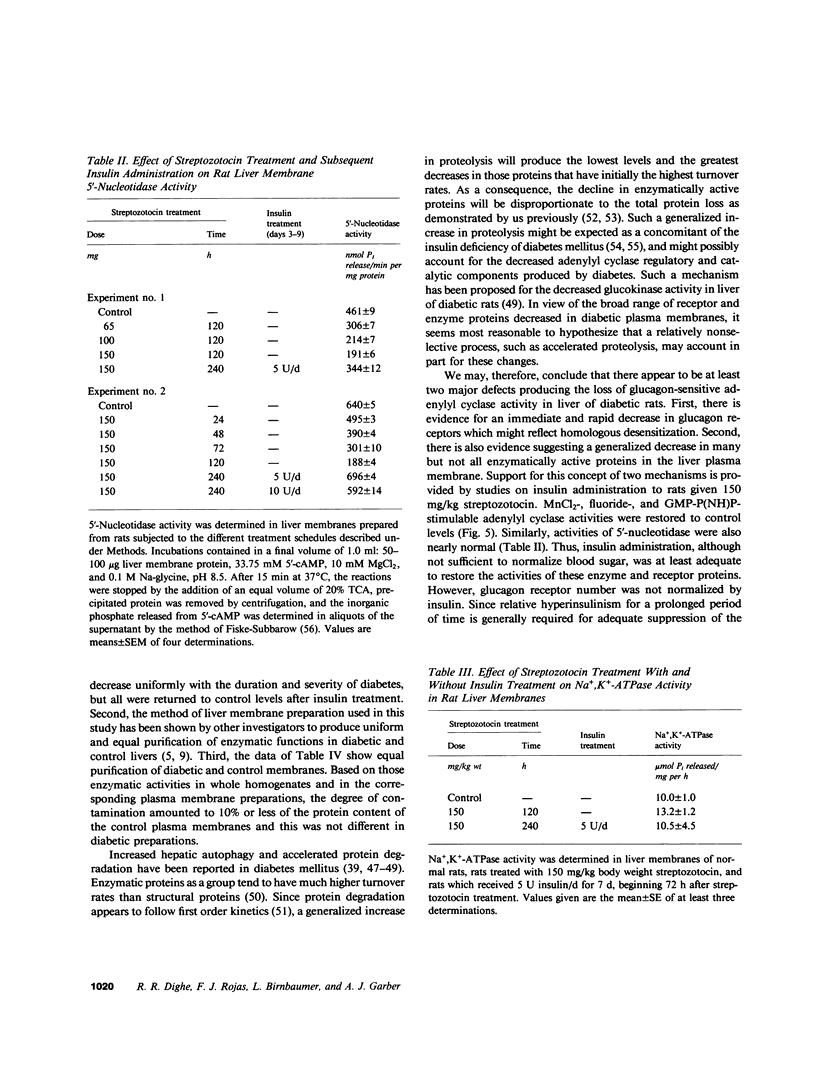
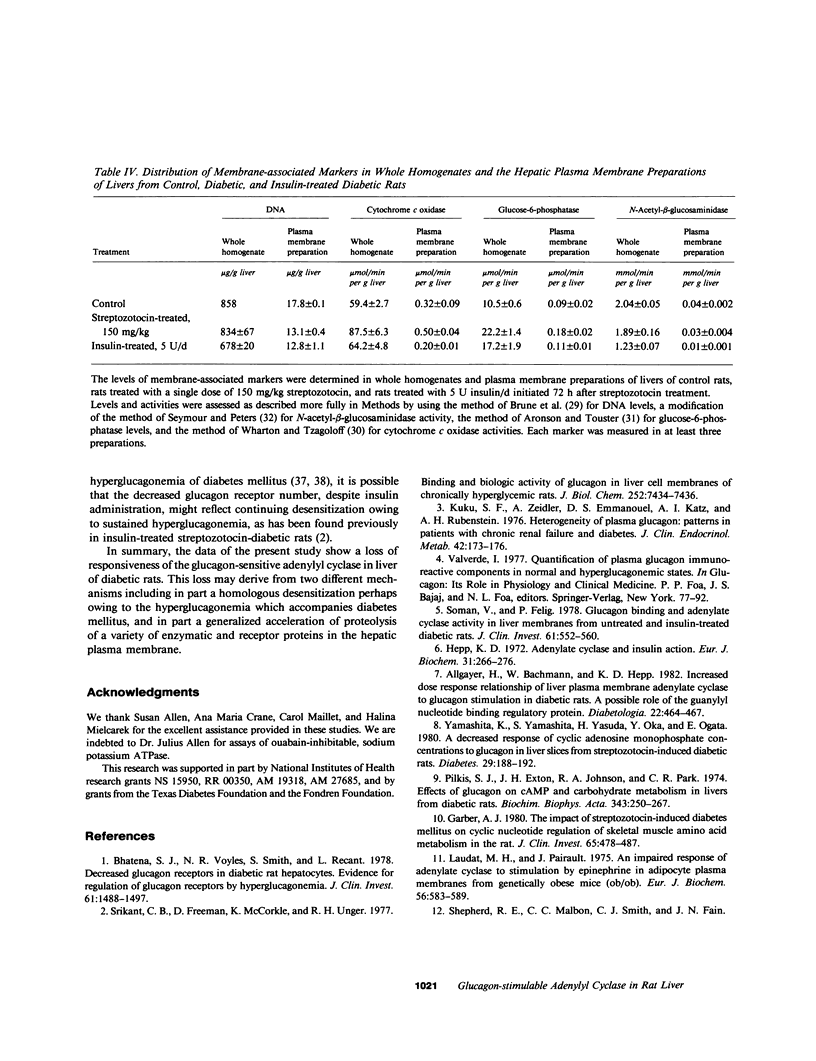
Glucagon receptor levels, glucagon-stimulated and other forms of adenylyl cyclase activity, and regulatory component activity of adenylyl cyclase were determined in hepatic plasma membranes of rats administered streptozotocin without and with insulin to produce varying degrees of hyperglycemia. Receptor levels were assayed by direct binding of the specific probe [125I-Tyr10]-iodoglucagon; regulatory component activity was assayed by the capacity to reconstitute stimulatory regulation in deficient membranes from cyc- S49 murine lymphoma cells. In rats given 150 mg streptozotocin, glucagon stimulation of adenylyl cyclase as well as basal, sodium fluoride, 5' guanylylimidodiphosphate [GMP-P(NH)P] and Mn-dependent activities were reduced 50%, glucagon receptor levels but not affinity were reduced 67%, and regulatory component activity was decreased 50%. In addition, alpha 1-adrenergic receptors and 5'-nucleotidase were similarly reduced in diabetes. However, specific ouabain-inhibitable Na+, K+, ATPase activity was not altered by streptozotocin treatment. The streptozotocin-induced changes were noted within 24 h and became maximal by 120 h after its administration. All of these decreases were partially reversed by in vivo insulin treatment. DNA, cytochrome c oxidase, glucose-6-phosphatase, and N-acetyl-beta-glucosaminidase content in hepatic plasma membrane preparations were not substantially different in diabetic as compared with control animals. The data demonstrate that glucagon-mediated regulation of cyclic AMP formation is deranged in insulin deficiency owing to a combined decrease in receptors, derangement of the coupling mechanism intervening between receptor and adenylyl cyclase, and possibly, an altered basal effector system. Some of these changes appear to reflect a "desensitization-like" phenomenon which may or may not be attributable to the hyperglucagonemia of diabetes mellitus. There also appears to be a concurrent generalized decrease in several but not all plasma membrane receptor and enzymatic proteins. This may be the result of a number of processes among which is the accelerated proteolysis of uncontrolled diabetes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allgayer H., Bachmann W., Hepp K. D. Increased dose-response relationship of liver plasma membrane adenylate cyclase to glucagon stimulation in diabetic rats. A possible role of the guanyl nucleotide-binding regulatory protein. Diabetologia. 1982 Jun;22(6):464–467. doi: 10.1007/BF00282591. [DOI] [PubMed] [Google Scholar]
- Amherdt M., Harris V., Renold A. E., Orci L., Unger R. H. Hepatic autography in uncontrolled experimental diabetes and its relationships to insulin and glucagon. J Clin Invest. 1974 Jul;54(1):188–193. doi: 10.1172/JCI107742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P., Ostman J. Abnormalities in the adrenergic control and the rate of lipolysis in isolated human subcutaneous adipose tissue in diabetes mellitus. Diabetologia. 1976 Dec;12(6):593–599. doi: 10.1007/BF01220636. [DOI] [PubMed] [Google Scholar]
- Aronson N. N., Jr, Touster O. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 1974;31:90–102. doi: 10.1016/0076-6879(74)31009-9. [DOI] [PubMed] [Google Scholar]
- Bhathena S. J., Voyles N. R., Smith S., Recant L. Decreased glucagon receptors in diabetic rat hepatocytes. Evidence for regulation of glucagon receptors by hyperglucagonemia. J Clin Invest. 1978 Jun;61(6):1488–1497. doi: 10.1172/JCI109069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J., Hunzicker-Dunn M., Birnbaumer L. Hormone-stimulated desensitization of hormone-dependent adenylyl cyclase. Dual action of luteninizing hormone on pig graafian follicle membranes. J Biol Chem. 1976 May 10;251(9):2653–2663. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Jones K. C., James T. W. Assay for nanogram quantities of DNA in cellular homogenates. Anal Biochem. 1979 Jan 15;92(2):497–500. doi: 10.1016/0003-2697(79)90690-0. [DOI] [PubMed] [Google Scholar]
- Carr F. P., Pogson C. I. Phenylalanine metabolism in isolated rat liver cells. Effects of glucagon and diabetes. Biochem J. 1981 Sep 15;198(3):655–660. doi: 10.1042/bj1980655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim Biophys Acta. 1976 Dec 8;452(2):538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezra E., Salomon Y. Mechanism of desensitization of adenylate cyclase by lutropin. Impaired introduction of GTP into the regulatory site. J Biol Chem. 1981 Jun 10;256(11):5377–5382. [PubMed] [Google Scholar]
- Ezra E., Salomon Y. Mechanism of desensitization of adenylate cyclase in lutropin. GTP-dependent uncoupling of the receptor. J Biol Chem. 1980 Jan 25;255(2):653–658. [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Garber A. J., Birnbaumer L., Bornet E. P., Thompson W. J., Entman M. L. Skeletal muscle protein and amino acid metabolism in hereditary mouse muscular dystrophy. The role of disordered cyclic nucleotide metabolism in the accelerated alanine and glutamine formation and release. J Biol Chem. 1980 Sep 10;255(17):8325–8333. [PubMed] [Google Scholar]
- Garber A. J., Schwartz R. J., Seidel C. L., Silvers A., Entman M. L. Skeletal muscle protein and amino acid metabolism in hereditary mouse muscular dystrophy. Accelerated protein turnover and increased alanine and glutamine formation and release. J Biol Chem. 1980 Sep 10;255(17):8315–8324. [PubMed] [Google Scholar]
- Garber A. J. The impact of streptozotocin-induced diabetes mellitus on cyclic nucleotide regulation of skeletal muscle amino acid metabolism in the rat. J Clin Invest. 1980 Feb;65(2):478–487. doi: 10.1172/JCI109691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodhardt M., Ferry N., Geynet P., Hanoune J. Hepatic alpha 1-adrenergic receptors show agonist-specific regulation by guanine nucleotides. Loss of nucleotide effect after adrenalectomy. J Biol Chem. 1982 Oct 10;257(19):11577–11583. [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Hepp K. D. Adenylate cyclase and insulin action. Effect of insulin, nonsuppressible insulin-like material, and diabetes on adenylate-cyclase activity in mouse liver. Eur J Biochem. 1972 Dec 4;31(2):266–276. doi: 10.1111/j.1432-1033.1972.tb02529.x. [DOI] [PubMed] [Google Scholar]
- Hoffman B. B., Mullikin-Kilpatrick D., Lefkowitz R. J. Heterogeneity of radioligand binding to alpha-adrenergic receptors. Analysis of guanine nucleotide regulation of agonist binding in relation to receptor subtypes. J Biol Chem. 1980 May 25;255(10):4645–4652. [PubMed] [Google Scholar]
- Iyengar R., Bhat M. K., Riser M. E., Birnbaumer L. Receptor-specific desensitization of the S49 lymphoma cell adenylyl cyclase. Unaltered behavior of the regulatory component. J Biol Chem. 1981 May 25;256(10):4810–4815. [PubMed] [Google Scholar]
- Iyengar R. Hysteretic activation of adenylyl cyclases. II. Mg ion regulation of the activation of the regulatory component as analyzed by reconstitution. J Biol Chem. 1981 Nov 10;256(21):11042–11050. [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Katsilambros N., Rahman Y. A., Hinz M., Fussgänger R., Schröder K. E., Straub K., Pfeiffer E. F. Action of streptozotocin on insulin and glucagon responses of rat islets. Horm Metab Res. 1970 Sep;2(5):268–270. doi: 10.1055/s-0028-1095056. [DOI] [PubMed] [Google Scholar]
- Kuku S. F., Zeidler A., Emmanouel D. S., Katz A. I., Rubenstein A. H. Heterogeneity of plasma glucagon: patterns in patients with chronic renal failure and diabetes. J Clin Endocrinol Metab. 1976 Jan;42(1):173–176. doi: 10.1210/jcem-42-1-173. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laudat M. H., Pairault J. An impaired response of adenylate cyclase to stimulation by epinephrine in adipocyte plasma membranes from genetically obese mice (ob/ob). Eur J Biochem. 1975 Aug 15;56(2):583–589. doi: 10.1111/j.1432-1033.1975.tb02265.x. [DOI] [PubMed] [Google Scholar]
- Levilliers J., Pairault J., Lecot F., Tournemolle A., Laudat M. H. Adenosine 3':5'-monophosphate and guanosine 3':5'-monophosphate: levels and cyclase activities in liver and adipose tissue from diabetic mice (db/db). Eur J Biochem. 1978 Aug 1;88(2):323–330. doi: 10.1111/j.1432-1033.1978.tb12453.x. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Pagliara A. S., Hover B. A., Pace C. S., Ferrendelli J. A., Williams A. Hormone secretion and glucose metabolism in islets of Langerhans of the isolated perfused pancreas from normal and streptozotocin diabetic rats. J Biol Chem. 1976 Oct 10;251(19):6053–6061. [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Pfeuffer T. GTP-binding proteins in membranes and the control of adenylate cyclase activity. J Biol Chem. 1977 Oct 25;252(20):7224–7234. [PubMed] [Google Scholar]
- Pilkis S. J., Exton J. H., Johnson R. A., Park C. R. Effects of glucagon on cyclic AMP and carbohydrate metabolism in livers from diabetic rats. Biochim Biophys Acta. 1974 Mar 20;343(1):250–267. doi: 10.1016/0304-4165(74)90258-x. [DOI] [PubMed] [Google Scholar]
- Pitts B. J., Wallick E. T., Van Winkle W. B., Allen J. C., Schwartz A. On the lack of inotropy of cardiac glycosides on skeletal muscle: a comparison of Na+, K+-ATPases from skeletal and cardiac muscle. Arch Biochem Biophys. 1977 Dec;184(2):431–440. doi: 10.1016/0003-9861(77)90453-2. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. IV. Effects of guanylnucleotides on binding of 125I-glucagon. J Biol Chem. 1971 Mar 25;246(6):1872–1876. [PubMed] [Google Scholar]
- Rojas F. J., Swartz T. L., Iyengar R., Garber A. J., Birnbaumer L. Monoiodoglucagon: synthesis, purification by high pressure liquid chromatography, and characteristics as a receptor probe. Endocrinology. 1983 Aug;113(2):711–719. doi: 10.1210/endo-113-2-711. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Maguire M. E., Sturgill T. W., Biltonen R. L., Gilman A. G. Relationship between the beta-adrenergic receptor and adenylate cyclase. J Biol Chem. 1977 Aug 25;252(16):5761–5775. [PubMed] [Google Scholar]
- Seymour C. A., Peters T. J. Enzyme activities in human liver biopsies: assay methods and activities of some lysosomal and membrane-bound enzymes in control tissue and serum. Clin Sci Mol Med. 1977 Mar;52(3):229–239. doi: 10.1042/cs0520229. [DOI] [PubMed] [Google Scholar]
- Sibrowski W., Staegemann U., Seitz H. J. Accelerated turnover of hepatic glucokinase in starved and streptozotocin-diabetic rat. Eur J Biochem. 1982 Oct;127(3):571–574. doi: 10.1111/j.1432-1033.1982.tb06910.x. [DOI] [PubMed] [Google Scholar]
- Soman V., Felig P. Glucagon binding and adenylate cyclase activity in liver membranes from untreated and insulin-treated diabetic rats. J Clin Invest. 1978 Mar;61(3):552–560. doi: 10.1172/JCI108966. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Srikant C. B., Freeman D., McCorkle K., Unger R. H. Binding and biologic activity of glucagon in liver cell membranes of chronically hyperglucagonemic rats. J Biol Chem. 1977 Nov 10;252(21):7434–7438. [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Reconstitution of catecholamine-sensitive adenylate cyclase. Reconstitution of the uncoupled variant of the S40 lymphoma cell. J Biol Chem. 1979 May 10;254(9):3333–3340. [PubMed] [Google Scholar]
- Unger R. H., Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975 Jan 4;1(7897):14–16. doi: 10.1016/s0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- Unger R. H. The Banting Memorial Lecture 1975. Diabetes and the alpha cell. Diabetes. 1976 Feb;25(2):136–151. doi: 10.2337/diab.25.2.136. [DOI] [PubMed] [Google Scholar]
- Williams I. H., Chua B. H., Sahms R. H., Siehl D., Morgan H. E. Effects of diabetes on protein turnover in cardiac muscle. Am J Physiol. 1980 Sep;239(3):E178–E185. doi: 10.1152/ajpendo.1980.239.3.E178. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Yamashita S., Yasuda H., Oka Y., Ogata E. A decreased response of cyclic adenosine monophosphate concentrations to glucagon in liver slices from streptozotocin-induced diabetic rats. Diabetes. 1980 Mar;29(3):188–192. doi: 10.2337/diab.29.3.188. [DOI] [PubMed] [Google Scholar]
- Yoshino G., Kazumi T., Morita S., Baba S. Insulin and glucagon in rats with islet cell tumors induced by small doses of streptozotocin. Can J Physiol Pharmacol. 1981 Aug;59(8):818–823. doi: 10.1139/y81-121. [DOI] [PubMed] [Google Scholar]



