Abstract
A pulmonary lesion is an extremely common and clinically challenging disorder worldwide, and an accurate diagnosis of lung cancer is crucial for early treatment and management. The aim of the present study was to perform a comprehensive meta analysis to compare the diagnostic performance of 18F-fluorothymidine (18F-FLT) positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG) PET in evaluating patients with pulmonary lesions. Relevant studies were identified using the PubMed, EMBASE and Cochrane library databases. The pooled estimated sensitivity, specificity, positive-likelihood ratio, negative-likelihood ratio, and diagnostic odds ratio (DOR) for 18F-FLT PET versus 18F-FDG PET were calculated as the main outcome measures. Summary receiver operating characteristic curves were also constructed by Meta-Disk 1.4 software using a Mose's constant of linear model. The meta analysis showed that 18F-FLT PET had a higher specificity (0.70; 95% CI, 0.61–0.77), but lower sensitivity (0.81; 95% CI, 0.74–0.87) compared to 18F-FDG PET (0.50; 95% CI, 0.41–0.58 for specificity; 0.92; 95% CI 0.86–0.95 for sensitivity). For DOR, 18F-FLT PET (12.58; 95% CI, 6.81–23.24) was higher compared to 18F-FDG PET (10.72; 95% CI, 5.51–20.87). The area under the curve was 0.8592 and 0.9240 for 18F-FLT PET and 18F-FDG PET, respectively (Z=0.976, P>0.05). In conclusion, 18F-FLT PET and 18F-FDG PET had good diagnostic performance for the overall assessment of pulmonary lesions, and 18F-FLT PET had a higher specificity compared to 18F-FDG PET, but was less sensitive than 18F-FDG PET. Therefore, 18F-FLT and 18F-FDG together could add diagnostic confidence for pulmonary lesions.
Keywords: positron emission tomography, pulmonary lesions, meta analysis, 18F-fluorodeoxyglucose, 18F-fluorothymidine
Introduction
18F-fluorodeoxyglucose (FDG) with positron emission tomography (PET) or PET/computed tomography (CT) is a well-established functional imaging technique for diagnostic oncologic imaging of a variety of malignancies and has been applied for differentiation between benign and malignant lesions, particularly for patients with pulmonary lesions (1–3). However, FDG is not specific for malignant tumors and can also accumulate non-specifically in certain benign lesions, which potentially results in false-positive findings (4,5). Therefore, several alternative molecular probes have been developed to complement 18F-FDG (6–8). Among them, 18F-fluorothymidine (FLT) is an analog of thymidine and its uptake reflects cellular proliferation (9,10). High sensitivity and specificity of 18F-FLT PET has been reported (11,12) in lung cancer. The comparative diagnostic accuracy of 18F-FLT PET versus 18F-FDG PET for the differentiation of benign from malignant pulmonary lesions has been conducted by numerous studies (8,10,13–16), however, certain discrepancies existed and each study only had a limited sample size (13). Meta analysis is a method of combining data from eligible studies to reduce random error and to define the accuracy of diagnostic tests more precisely. To the best of our knowledge, there has been no meta analysis regarding the effect of 18F-FLT PET versus 18F-FDG PET on pulmonary lesions thus far, even though recent medical studies have shown a considerable growth in published systematic studies and meta-analyses in the field of nuclear medicine, specifically for PET or PET/CT in oncology (17–19). Therefore, the aim of the present study was to perform a comprehensive meta analysis to compare the diagnostic performance of 18F-FLT PET with that of 18F-FDG PET in evaluating patients with pulmonary lesions.
Materials and methods
Study identification
A comprehensive online literature search of studies was conducted by two investigators to identify the relevant studies regarding 18F-FLT versus 18F-FDG in the evaluation of pulmonary lesions in PubMed, EMBASE, Cochrane library using a search algorithm based on the following combination of terms: i) FLT or fluorothymidine or 3′-deoxy-3′-18F-fluorothymidine AND; ii) FDG or fluorodeoxyglucose or 2′-deoxy-2′-18F-fluoro-D-glucose AND; iii) PET or positron emission tomography AND; iv) pulmonary or lung or thoracic. There was no limit to the beginning date and the search was updated until February 2014. The final analysis was limited to studies published in English. Conference abstracts and letters to the journal editors were excluded due to the limitations in data presentation. The reference lists of the retrieved studies were also manually reviewed for additional studies.
Study selection
Two investigators independently evaluated the titles, abstracts and complete studies based on the following inclusion criteria: i) Studies with a comparison of 18F-FLT and 18F-FDG in evaluation of patients with pulmonary lesions. ii) The differentiation of malignancy from benign pulmonary lesions was confirmed with histopathological analysis and/or clinical and imaging follow-up. iii) The scan with two imaging tracers (18F-FLT or 18F-FDG) was performed within 2–4 weeks of one another. iv) The studies including >10 patients were selected for inclusion in the meta-analysis. Studies were excluded based on the following criteria: i) Only 18F-FLT or 18F-FDG was performed. ii) Complete data regarding the numbers of the true positive (TP), false positive (FP), true negative (TN) and false negative (FN) were not provided or could not be obtained from calculation. iii) 18F-FLT or 18F-FDG were used to determine the responses to chemotherapy (such as erlotinib) or radiotherapy for non-small cell lung cancer (NSCLC). Any disagreement was resolved by consensus.
Data extraction and quality assessment
Two investigators independently extracted the relevant data from each eligible study, including authors, year of publication, demographic characteristics, sample size, data type, study design, reference standard, PET technique, disease constitution and TP, FP, TN and FN. These data were reported by a form of tabulation. Any difference was resolved by consensus.
The methodological quality of the eligible studies was assessed using the quality assessment for studies of diagnostic accuracy (QUADAS) tool (20). The QUADAS tool includes 14 items and 12 methodological quality items were assessed for each study using the scores ‘yes’, ‘no’ or ‘unclear’.
Statistical analysis
Forest plots were performed to calculate the pooled sensitivity, specificity, positive-likelihood ratio (PLR), negative-likelihood ratio (NLR) and diagnostic odds ratio (DOR) with the corresponding 95% confidence interval (CI) for 18F-FLT PET versus 18F-FDG PET as the main outcome measures.
Heterogeneity due to the threshold effect was investigated using the Spearman correlation coefficient, and a positive correlation (P<0.05) suggested the threshold effect. The degree of heterogeneity due to the non-threshold effect among different studies was reported using the Cochran χ2 statistic and the inconsistency index (I2). P<0.05 or I2>50% indicated heterogeneity. According to the results of the heterogeneity tests, PLR, NLR and DOR were calculated by the Mantel-Haenszel method based on a fixed-effects model (FEM) when there was no heterogeneity observed (P>0.05 or I2<50%), or by the DerSimonian-Laird method based on a random-effects model when heterogeneity was observed (P<0.05 or I2>50%) (21). Each set of data regarding 18F-FLT PET or 18F-FDG PET was fit to a summary receiver operating characteristic (SROC) curve and the area under the curve (AUC) and Q* index, which represented the point at which the sensitivity and specificity were equal, were also calculated, measuring overall diagnostic accuracy.
Publication bias was assessed by Deek's funnel plots. Statistical analysis was performed using Meta-Disc version 1.4, which is a free statistical software package (21). P<0.05 was considered to indicate a statistically significant difference.
Results
Study selection and characteristics analysis
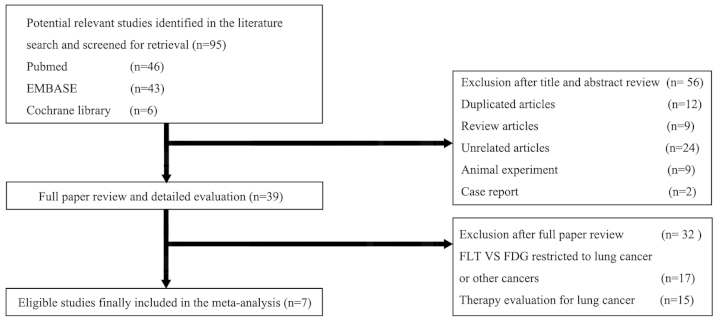
The initial online literature search identified a total of 95 potential studies (Fig. 1). A review of the titles and abstracts excluded 56 studies. These included 12 duplicated studies, nine reviews, two case studies, nine studies based on animal experiments and 24 studies not associated with FLT versus FDG in pulmonary lesions. The criteria excluded a further 32 of the remaining 39 studies: 15 studies focused on FLT and/or FDG PET in the therapy response evaluation for lung cancer, 17 studies were restricted to comparative diagnostic performance of FLT versus FDG in lung cancer or other cancers but not pulmonary lesions. Finally, seven studies (8,10,13,22–25) met the inclusion criteria and were selected.
Figure 1.
Study flow chart of the search for the eligible studies included in the meta analysis. FLT, fluorothymidine; FDG, fluorodeoxyglucose.
Study characteristics
The characteristics of the eligible studies are summarized in Table I. These studies assessed a total of 301 patients with pulmonary lesions (n=166 for malignancy). All the seven studies included were prospective. In two studies, the study population underwent PET and PET/CT (13,25) and PET alone in the remaining studies (8,10,19–21). The dose of FDG or FLT ranged considerably across the studies. The results of the diagnostic performance were patient-based for all the studies except for a study by Yap et al (8). The totals of TP, FP, FN and TN for each included study are also presented in Table I.
Table I.
Characteristics of each study included.
| Study (ref) | Mean age, years (range) | Gender, F/M1 | n, M2/B | Reference standard | Design | Data type | Tracer | TP, n | FP, n | FN, n | TN, n | PET technique | Malignancy/benign types (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buck et al 2003 (10) | 62 | 9/17 | 26 | Histopathology | Prospective | Per patient | FLT | 15 | 0 | 3 | 8 | 334 MBq, 45 min | NSCLC (13), SCLC (1), metastases (4) / TB (1), IN (1), others (6) |
| (37–77) | 18/8 | FDG | 17 | 4 | 1 | 4 | 391 MBq, 45 min | ||||||
| Halter et al 2004 (21) | 63 | 7/21 | 28 | Histopathology | Prospective | Per patient | FLT | 18 | 0 | 3 | 7 | 265–370 MBq, 45 min | NSCLC (14), SCLC (6), others (1) / TB (1), IN (1), others (5), |
| (41–78) | 21/7 | FDG | 20 | 2 | 1 | 5 | 345–550 MBq, 45 min | ||||||
| Buck et al 2005 (20) | 62 | 16/27 | 43 | Histopathology | Prospective | Per patient | FLT | 22 | 0 | 6 | 15 | 265–370 MBq, 60 min | NSCLC (19), SCLC (1), metastases (8) / TB (1), others (14) |
| (36–80) | 28/15 | FDG | 27 | 6 | 1 | 9 | 345–550 MBq, 60 min | ||||||
| Yap et al 2006 (8) | 66 | 11/11 | 22 | Histopathology | Prospective | Per lesion | FLT | 16 | 1 | 6 | 6 | 185.2 MBq, 60 min | NSCLC (17), metastases (2) / IN (1), fibrosis (1), pneumonia (1) |
| 19/3 | FDG | 20 | 3 | 2 | 4 | 0.21mCi/Kg, 60 min | |||||||
| Tian et al 2008 (22) | 62 | 22/33 | 55 | Histopathology/follow-up | Multi-center | Per patient | FLT | 11 | 9 | 5 | 30 | 300–400 MBq, 60 min | Lung cancer (16) / TB (16), IN (7), others (16) |
| (17–82) | 16/39 | Prospective | FDG | 14 | 16 | 2 | 23 | 300–400 MBq, 60 min | |||||
| Yamamoto et al 2008 (19) | 70 | 17/37 | 54 | Histopathology/follow-up | Prospective | Per patient | FLT | 31 | 5 | 5 | 13 | 101–238 MBq, 60 min | NSCLC (34), SCLC (2) / TB (1), IN (11), others (6) |
| (52–88) | 36/18 | FDG | 32 | 6 | 4 | 12 | 111–161 MBq, 60 min | ||||||
| Xu et al 2011 (13) | Unclear | 27/46 | 73 | Histopathology/follow-up | Prospective | Per patient | FLT | 24 | 27 | 4 | 18 | 300–400 MBq, 60 min | Lung cancer (28) / TB (18), IN (27) |
| (17–85) | 28/45 | FDG | 25 | 33 | 3 | 12 | 300–400 MBq, 60 min |
B, benign; M1, male; M2, malignancy; PET, positron emission tomography; F, female; FLT, fluorothymidine; FDG, fluorodeoxyglucose; TP, true positive; FP, false positive; FN, false negative; FP, false positive; MBq, Megabecquerel; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; TB, tuberculosis, IN, inflammation.
Quality assessment
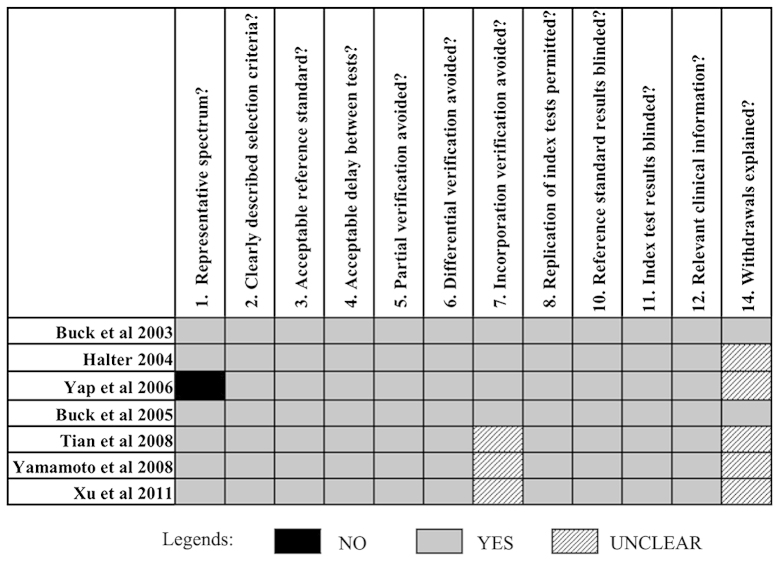
The methodological quality of all the eligible studies were assessed according to the 14-item QUADAS assessment tool (20). A total of 12 of the 14 items (without item 9 and 13) could be scored for all the included studies (Fig. 2).
Figure 2.
Assessment of the methodological quality of the eligible studies using the quality assessment for studies of diagnostic accuracy (QUADAS) tool.
All the studies had well-described selection criteria (item 2) and proper spectrum of patients (item 1), except for Yap et al (8). Therefore, histopathological analysis and clinical or imaging follow-up were considered as reference standards in this study (item 3,5–7), so it was not clear whether the FLT and/or FDG PET (index tests) formed part of the imaging follow-up (reference standard) for three studies (13,22,25), and none of the included studies provided sufficient detail of execution of the reference standard (item 9) to permit its replication due to its universality in clinical practice. The reference standard was considered to be blind to the index tests results and vice versa as the index tests (18F-FLT PET and 18F-FDG PET) were performed ahead of the reference standard and they were performed at a different department (item 10,11). The scan with two imaging tracers (18F-FLT or 18F-FDG) was performed within two weeks of one another for all the included studies, except for a study by Yap et al (within 1 month) (item 4). All the studies described the 18F-FLT PET and 18F-FDG PET technique in detail (item 8). No study reported whether there were any results that could not be interpreted (item 13). Two studies explained the reasons for patient withdrawal (item 14).
Heterogeneity tests and diagnostic performance
Table II summarizes the results for sensitivity, specificity, PLR, NLR, DOR, P-values for heterogeneity and I2 values for 18F-FLT PET and 18F-FDG PET, respectively, indicating heterogeneity for specificity and PLR (I2>50% and P<0.05), but homogeneity for sensitivity, NLR and DOR between studies (I2<50% and P>0.05). According to the results of the heterogeneity tests, the pooled estimates were calculated by the Mantel-Haenszel method based on an FEM (P>0.05) or by the DerSimonian-Laird method based on a random-effects model (P<0.05).
Table II.
Diagnostic performance of 18F-FLT PET and 18F-FDG PET for the evaluation of pulmonary lesions.
| Sensitivity | Specificity | PLR | NLR | DOR | Threshold effect | |
|---|---|---|---|---|---|---|
| 18F-FLT PET | ||||||
| Pooled estimates | 0.81 | 0.7 | 4.01 | 0.27 | 12.58 | P=0.383 |
| 95% CI | 0.74–0.87 | 0.61–0.77 | 1.62–9.88 | 0.20–0.37 | 6.81–23.24 | |
| Cochran-Q (P-value) | 3.85 (0.6973) | 40.61 (0.0000) | 32.15 (0.0000) | 3.74 (0.7114) | 8.95 (0.1766) | |
| I2 value, % | 0.00 | 85.20 | 81.30 | 0.00 | 32.90 | |
| 18F-FDG PET | ||||||
| Pooled estimates | 0.92 | 0.5 | 2.01 | 0.17 | 10.72 | P=0.645 |
| 95% CI | 0.86–0.95 | 0.41–0.58 | 1.38–2.93 | 0.10–0.29 | 5.51–20.87 | |
| Cochran-Q (P-value) | 2.51 (0.8670) | 15.56 (0.0163) | 18.92 (0.0043) | 4.30 (0.6360) | 6.46 (0.3734) | |
| I2 value, % | 0.00 | 67.50 | 68.30 | 0.00 | 7.20 |
PET, positron emission tomography; FLT, fluorothymidine; FDG, fluorodeoxyglucose; PLR, positive-likelihood ratio; NLR, negative-likelihood ratio; DOR, diagnostic odds ratio.
The overall pooled estimates of sensitivity and specificity for 18F-FLT PET versus 18F-FDG PET in the differentiation between benign and malignant pulmonary lesions were 0.81 (95% CI, 0.74–0.87) and 0.70 (95% CI, 0.60–0.77) versus 0.92 (95% CI, 0.86–0.95) and 0.50 (95% CI, 0.41–0.58), respectively. The DOR is a single indicator of test accuracy that combines the data from sensitivity and specificity into a single number (23), and the estimated DORs based on FEM were 12.58 (95% CI, 6.81–23.24) and 10.72 (95% CI, 5.51–20.87) for 18F-FLT PET and 18F-FDG PET, respectively.
The SROC curves for 18F-FLT PET and 18F-FDG PET are shown in Fig. 3. The SROC plot presents a global summary of test performance and shows the adjustment between sensitivity and specificity. The AUC was 0.8592 and 0.9240 for 18F-FLT PET and 18F-FDG PET, respectively, indicating good diagnostic accuracy and no statistically significant difference (Z=0.976, P>0.05).
Figure 3.
Summary receiver operating characteristic (SROC) curves for (A) 18F-FLT PET and (B) 18F-FDG PET. The middle line was the summary ROC curve and the two beside are 95% confidence intervals. Each black circle represents an individual study included in the meta-analysis, with the size of the circle directly proportional to the sample size of the study. AUC, area under the ROC curve; SE (AUC), standard error of the AUC; Q*, the maximum joint sensitivity and specificity on a symmetric ROC curve; SE(Q*): standard error of Q*; FLT, fluorothymidine; PET, positron emission tomography; FDG, fluorodeoxyglucose.
To explore the possible explanations for the heterogeneity, threshold effect analysis was applied. Spearman correlation coefficient was determined to be 0.393 (P=0.383) and −0.214 (P=0.645) for 18F-FLT PET and 18F-FDG PET, respectively, which indicated an absence of threshold effect in studies included. Meta-regression analysis and subgroup analysis were not conducted in the study owing to the limitations in total sample size and the clinical characteristics information of the included studies.
Assessment of publication bias
The results of Deek's funnel plots (Fig. 4) showed an absence of publication bias for the included studies for 18F-FLT PET (P=0.109) and 18F-FDG PET (P=0.101).
Figure 4.
Deek's funnel plots for the evaluation of asymmetry between the included studies regarding the diagnostic performance of (A) 18F-FLT PET and (B) 18F-FDG PET for patients with pulmonary lesions. P>0.05 represents no asymmetry between the DORs of larger and smaller studies. Publication bias was not indicated by the Deek's funnel plots for 18F-FLT PET (P=0.109) and 18F-FDG PET (P=0.101). ESS, effective sample size; FLT, fluorothymidine; PET, positron emission tomography; FDG, fluorodeoxyglucose; DOR, diagnostic odds ratio.
Discussion
A pulmonary lesion is an extremely common and clinically challenging disorder in China, as well as in a number of other countries (27,28), and lung cancer is the first leading cause of tumor-related mortality. The differentiation of lung cancer from benign pulmonary lesions is a well-known diagnostic problem in daily clinical practice. Despite high sensitivity for staging lung cancer with 18F-FDG PET compared to conventional imaging modalities, false-positive findings can occur, particularly in inflammatory lesions (4,5,10). Non-specific uptake by these non-malignant tissues resulted in a positive predictive value of 18F-FDG PET in the pulmonary lesions as low as 44.6% (27). 18F-FLT is an analog of thymidine and its uptake has been found to correlate significantly better with the proliferative activity, as indicated by the Ki-67 index in pulmonary lesions (12). In the majority of cases, 18F-FLT PET showed higher specificity compared to 18F-FDG PET for the characterization of pulmonary lesions as benign or malignant. As reported by Buck et al (10,11), the specificity of 18F-FLT PET was ≤100%. However, the specificity in the study by Xu et al (13) was significantly lower than that of the other investigations.
As it is known that the result of a single study may be affected by numerous factors, a meta analysis regarding the performance of 18F-FLT PET versus 18F-FDG PET in evaluating patients with pulmonary lesions was performed for the first time to reduce the bias and increase the statistical power of the small sample study. The final analysis indicated that 18F-FLT PET and 18F-FDG PET had good diagnostic performance for the overall assessment of pulmonary lesions, and 18F-FLT PET had a higher specificity compared to 18F-FDG, but was less sensitive compared to 18F-FDG PET.
Even though it was previously widely accepted that 18F-FLT correlates significantly better with the proliferative activity of lung tumors compared to 18F-FDG, 18F-FLT is clearly limited by its low sensitivity with respect to evaluation of pulmonary lesions. This low sensitivity is due to the low 18F-FLT uptake in malignant lesions, which was only half that of 18F-FDG (8,10). Due to its low sensitivity, 18F-FLT PET or PET/CT does not appear to be able to replace 18F-FDG PET or PET/CT for the characterization of pulmonary lesions as benign or malignant. However, the significant correlation between FLT uptake and tumor cell proliferation indicates the importance of 18F-FLT PET or PET/CT for the assessment of therapy response and outcome (12). To a certain extent, this was one of the reasons for the increasing studies regarding the assessment of the responses to erlotinib therapy for NSCLC using 18F-FLT versus 18F-FDG PET and/or PET/CT (25–30).
Several published meta analyses only reported pooled data from statistically heterogeneous studies and possibly provided the misleading information. For example, data from studies using PET and those using PET/CT are often pooled together for the meta analysis on the diagnostic performance of PET or PET/CT in oncology. This could lead to a possible bias on the final results as the overall diagnostic accuracy of PET/CT is usually higher than that of PET alone, as it is known that the CT scanner in PET/CT has allowed the possibility of acquiring metabolic and anatomical imaging data and provides precise anatomical localization. The comparison of the diagnostic performance of 18F-FLT PET was focused on with regards to that of 18F-FDG PET in evaluating pulmonary lesions in the present meta analysis study, but did not utilize the analysis results regarding 18F-FLT PET/CT versus 18F-FDG PET/CT as the relevant studies were only published in Chinese, except for two English studies included in the meta analysis (13,25). The present meta analysis will be updated in the future with the development of relevant studies, particularly for the studies regarding the integration of 18F-FLT PET/CT versus 18F-FDG PET/CT and PET/CT with a combination of 18F-FLT and 18F-FDG in the evaluation of patients with pulmonary lesions.
A notable phenomenon was that the reported specificities in the only two studies included in the present meta analysis that were performed in China were significantly lower compared to the other included studies [particularly for the Xu et al (13) study], and the pooled specificity had improved to 0.84 (95% CI, 0.75–0.91) for 18F-FLT PET and 0.61 (95% CI, 0.50–0.71) for 18F-FDG PET, respectively, when the study was excluded. The regional difference (including the different diagnostic criteria and the inclusion of patients with different disease constitution) possibly contributed to the heterogeneity in the specificity value to a certain extent. In general, the final results with regards to the comparison of the overall diagnostic performance of 18F-FLT PET with that of 18F-FDG PET were not markedly influenced with the exclusion of the Xu et al (13) study.
Even though 18F-FLT PET and 18F-FDG PET had a good diagnostic performance for the overall assessment of pulmonary lesions, the effectiveness of each tracer alone was limited. Multi-tracer imaging has received attention in recent years (28,29), and a multi-center clinical trial included in the present meta analysis had conducted a dual-tracer (the combination of 18F-FLT and 18F-FDG) study for assessment of pulmonary lesions (25). The 18F-FLT/18F-FDG ratio has been indicated to be more accurate in revealing the nature of the pulmonary lesions and a ratio between 0.4 and 0.90 is the most recommended (25). Cost effectiveness and an increased radiation dose should also be considered as a major difficulty that requires investigating in the future with dual-tracer imaging.
There are numerous potential limitations in the present meta analysis. Firstly, the number of evaluated studies included in the study was relatively small, and therefore, subgroup analyses according to clinical characteristics of patients with pulmonary lesion were not performed. The limited number of available studies and the clinical heterogeneity among them may have affected the generalizability of the results and impaired the strength of the present meta-analysis study. Secondly, for practical reasons only studies written in English were included in the study, which may have incurred a bias as other studies with positive results that are reported in a language other than English may have been published in international journals (30). However, the Deek's funnel plots did not indicate any publication bias, even though the influence of bias in the present analysis was not completely excluded, particularly for the relatively small number of studies. Thirdly, there was no well-accepted reference standard in the meta analysis that ranged from histopathological analysis to clinical or imaging follow-up, and not all the follow-up were performed in the same manner in all the studies. Fourthly, the variability in the quality of the evaluated studies may introduce significant limitations for the interpretation of the meta analysis study. Finally, the present study did not compare 18F-FLT or 18F-FDG PET with other imaging modalities, such as magnetic resonance imaging or diagnostic CT scan, as systematic deviation exists among modalities using different imaging mechanisms. Due to the limitations mentioned above, the results of the meta-analysis should be interpreted with care.
In conclusion, although a large-scale prospective multi-center randomized-controlled trial is required, the evidence in the present study shows that 18F-FLT PET had a higher specificity compared to 18F-FDG PET, but was less sensitive for the evaluation of pulmonary lesions. 18F-FDG and 18F-FLT, which provide information regarding the different aspects of tumor biology, are complementary to each other and may add diagnostic confidence in pulmonary lesions when the combination of 18F-FLT and 18F-FDG is available.
Acknowledgements
The study was supported in part by grants from the study project of the Tianjin Medical University Cancer Institute and Hospital (no. B1220), the Tianjin Medical University Science Fund (no. 2013KYQ07) and the Tianjin Municipal Bureau of Health Science & Technology Fund (no. 2013KZ088).
References
- 1.Demura Y, Tsuchida T, Ishizaki T, et al. 18F-FDG accumulation with PET for differentiation between benign and malignant nodules in the thorax. J Nucl Med. 2003;44:540–548. [PubMed] [Google Scholar]
- 2.Changlai SP, Tsai SC, Chou MC, Ho YJ, Kao CH. Whole body 18F-2-deoxyglucose positron emission tomography to restage non-small cell lung cancer. Oncol Rep. 2001;8:337–339. [PubMed] [Google Scholar]
- 3.Kaira K, Yamamoto N, Endo M, et al. 18F-FDG uptake on PET is a predictive marker of thymidylate synthase expression in patients with thoracic neoplasms. Oncol Rep. 2014;31:209–215. doi: 10.3892/or.2013.2816. [DOI] [PubMed] [Google Scholar]
- 4.Shreve PD, Anzai Y, Wahl RL. Pitfalls in oncologic diagnosis with FDG PET imaging: physiologic and benign variants. Radiographics. 1999;19:61–77. doi: 10.1148/radiographics.19.1.g99ja0761. [DOI] [PubMed] [Google Scholar]
- 5.Lee TS, Ahn SH, Moon BS, et al. Comparison of 18F-FDG, 18F-FET and 18F-FLT for differentiation between tumor and inflammation in rats. Nucl Med Biol. 2009;36:681–686. doi: 10.1016/j.nucmedbio.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Dittmann H, Dohmen BM, Paulsen F, et al. [18F] FLT PET for diagnosis and staging of thoracic tumours. Eur J Nucl Med Mol Imaging. 2003;30:1407–1412. doi: 10.1007/s00259-003-1257-3. [DOI] [PubMed] [Google Scholar]
- 7.Cobben DC, Elsinga PH, Hoekstra HJ, et al. Is 18F-3-Fluoro-3′-deoxy-L-thymidine useful for the staging and restaging of non-small cell lung cancer? J Nucl Med. 2004;45:1677–1682. [PubMed] [Google Scholar]
- 8.Yap CS, Czernin J, Fishbein MC, et al. Evaluation of thoracic tumors with 18F-fluorothymidine and 18F-fluorodeoxyglucose-positron emission tomography. Chest. 2006;129:393–401. doi: 10.1378/chest.129.2.393. [DOI] [PubMed] [Google Scholar]
- 9.Grierson JR, Shields AF. Radiosynthesis of 3′-deoxy-3′-[(18)F]fluorothymidine: [(18)F]FLT for imaging of cellular proliferation in vivo. Nucl Med Biol. 2000;27:143–156. doi: 10.1016/s0969-8051(99)00104-3. [DOI] [PubMed] [Google Scholar]
- 10.Buck AK, Halter G, Schirrmeister H, et al. Imaging proliferation in lung tumors with PET: 18 F-FLT versus 18 F-FDG. J Nucl Med. 2003;44:1426–1431. [PubMed] [Google Scholar]
- 11.Buck AK, Schirrmeister H, Hetzel M, et al. 3-deoxy-3-[18F] fluorothymidine-positron emission tomography for noninvasive assessment of proliferation in pulmonary nodules. Cancer Res. 2002;62:3331–3334. [PubMed] [Google Scholar]
- 12.Vesselle H, Grierson J, Muzi M, et al. In vivo validation of 3-deoxy-31-[18F] fluorothymidine ([18F]FLT) as a proliferation imaging tracer in humans: correlation of [18F] FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res. 2002;8:3315–3332. [PubMed] [Google Scholar]
- 13.Xu B, Guan Z, Liu C, et al. Can multimodality imaging using 18F-FDG/18F-FLT PET/CT benefit the diagnosis and management of patients with pulmonary nodules? Eur J Nucl Med Mol Imaging. 2011;38:285–292. doi: 10.1007/s00259-010-1625-8. [DOI] [PubMed] [Google Scholar]
- 14.Buck AK, Hetzel M, Schirrmeister H, et al. Clinical relevance of imaging proliferative activity in lung nodules. Eur J Nucl Med Mol Imaging. 2005;32:525–533. doi: 10.1007/s00259-004-1706-7. [DOI] [PubMed] [Google Scholar]
- 15.Halter G, Buck AK, Schirrmeister H, et al. [18F] 3-deoxy-3′ fluorothymidine positron emission tomography: alternative or diagnostic adjunct to 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography in the workup of suspicious central focal nodules? J Thorac Cardiovasc Surg. 2004;127:1093–1099. doi: 10.1016/j.jtcvs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Tian J, Yang X, Yu L, et al. A multicenter clinical trial on the diagnostic value of dual-tracer PET/CT in pulmonary nodules using 3′-deoxy-3′-18F fluorothymidine and 18F-FDG. J Nucl Med. 2008;49:186–194. doi: 10.2967/jnumed.107.044966. [DOI] [PubMed] [Google Scholar]
- 17.Treglia G, Sadeghi R. Meta-analyses and systematic reviews on PET and PET-CT in oncology: the state of the art. Clin Transl Imaging. 2013;1:73–75. [Google Scholar]
- 18.Xu G, Zhao L, He Z. Performance of whole-body PET/CT for the detection of distant malignancies in various cancers: a systematic review and meta-analysis. J Nucl Med. 2012;53:1847–1854. doi: 10.2967/jnumed.112.105049. [DOI] [PubMed] [Google Scholar]
- 19.He YQ, Gong HL, Deng YF, Li WM. Diagnostic efficacy of PET and PET/CT for recurrent lung cancer: a meta-analysis. Acta Radiol. 2014;55:309–317. doi: 10.1177/0284185113498536. [DOI] [PubMed] [Google Scholar]
- 20.Kwee TC, Kwee RM. Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Radiol. 2009;19:731–744. doi: 10.1007/s00330-008-1194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto Y, Nishiyama Y, Ishikawa S, et al. 3′-Deoxy-3′-18F-fluorothymidine as a proliferation imaging tracer for diagnosis of lung tumors: comparison with 2-deoxy-2-18F-fluoro-D-glucose. J Comput Assist Tomogr. 2008;32:432–437. doi: 10.1097/RCT.0b013e3180980db9. [DOI] [PubMed] [Google Scholar]
- 23.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–1135. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 24.Bunyaviroch T, Coleman RE. PET evaluation of lung cancer. J Nucl Med. 2006;47:451–469. [PubMed] [Google Scholar]
- 25.Mileshkin L, Hicks RJ, Hughes BG, et al. Changes in 18F-fluorodeoxyglucose and 18F-fluorodeoxythymidine positron emission tomography imaging in patients with non-small cell lung cancer treated with erlotinib. Clin Cancer Res. 2011;17:3304–3315. doi: 10.1158/1078-0432.CCR-10-2763. [DOI] [PubMed] [Google Scholar]
- 26.Kahraman D, Holstein A, Scheffler M, et al. Tumor lesion glycolysis and tumor lesion proliferation for response prediction and prognostic differentiation in patients with advanced non small cell lung cancer treated with erlotinib. Clin Nucl Med. 2012;37:1058–1064. doi: 10.1097/RLU.0b013e3182639747. [DOI] [PubMed] [Google Scholar]
- 27.Kobe C, Scheffler M, Holstein A, et al. Predictive value of early and late residual 18F-fluorodeoxyglucose and 18F-fluorothymidine uptake using different SUV measurements in patients with non-small-cell lung cancer treated with erlotinib. Eur J Nucl Med Mol Imaging. 2012;39:1117–1127. doi: 10.1007/s00259-012-2118-8. [DOI] [PubMed] [Google Scholar]
- 28.Li XF, Huang T, Jiang HJ, et al. Combined injection of 18F-fluorodeoxyglucose and 3′-deoxy-3′-[18F]-fluorothymidine PET achieves more complete identification of viable lung cancer cells in mice and patients than individual radiopharmaceutical: A proof-of-concept study. Transl Oncol. 2013;6:775–783. doi: 10.1593/tlo.13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu BX, Liu CB, Wang RM, et al. The influence of interpreters' professional background and experience on the interpretation of multimodality imaging of pulmonary lesions using 18F-3′-deoxy-fluorothymidine and 18F-fluorodeoxyglucose PET/CT. PLoS One. 2013;8:e60104. doi: 10.1371/journal.pone.0060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grégoire G, Derderian F, Le Lorier J. Selecting the language of the publications included in a meta-analysis: is there a tower of babel bias? J Clin Epidemiol. 1995;48:159–163. doi: 10.1016/0895-4356(94)00098-b. [DOI] [PubMed] [Google Scholar]