Abstract
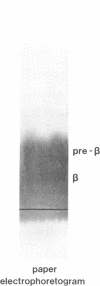
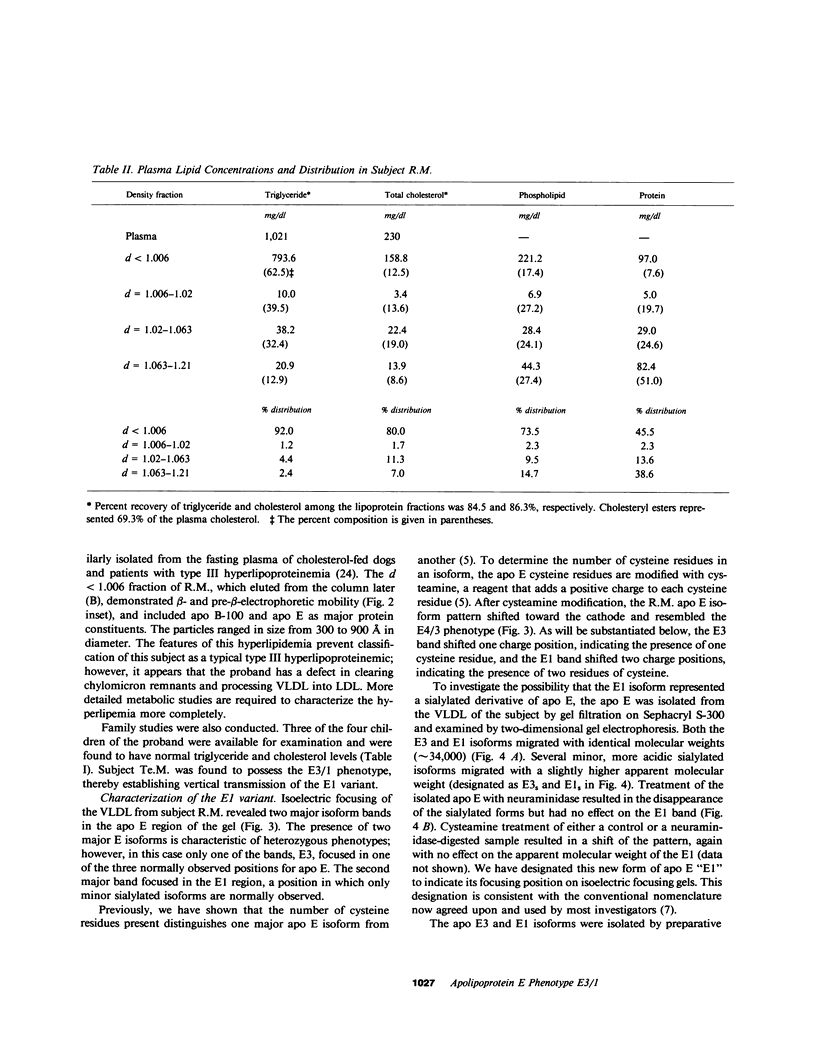
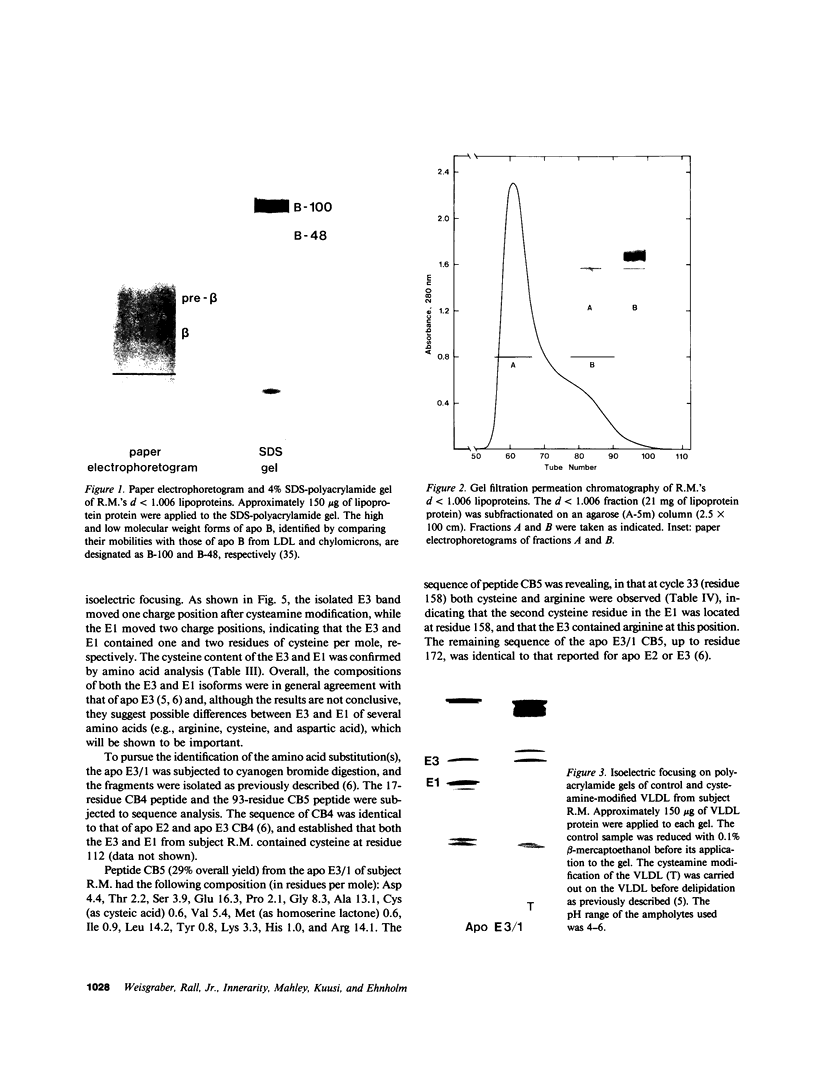
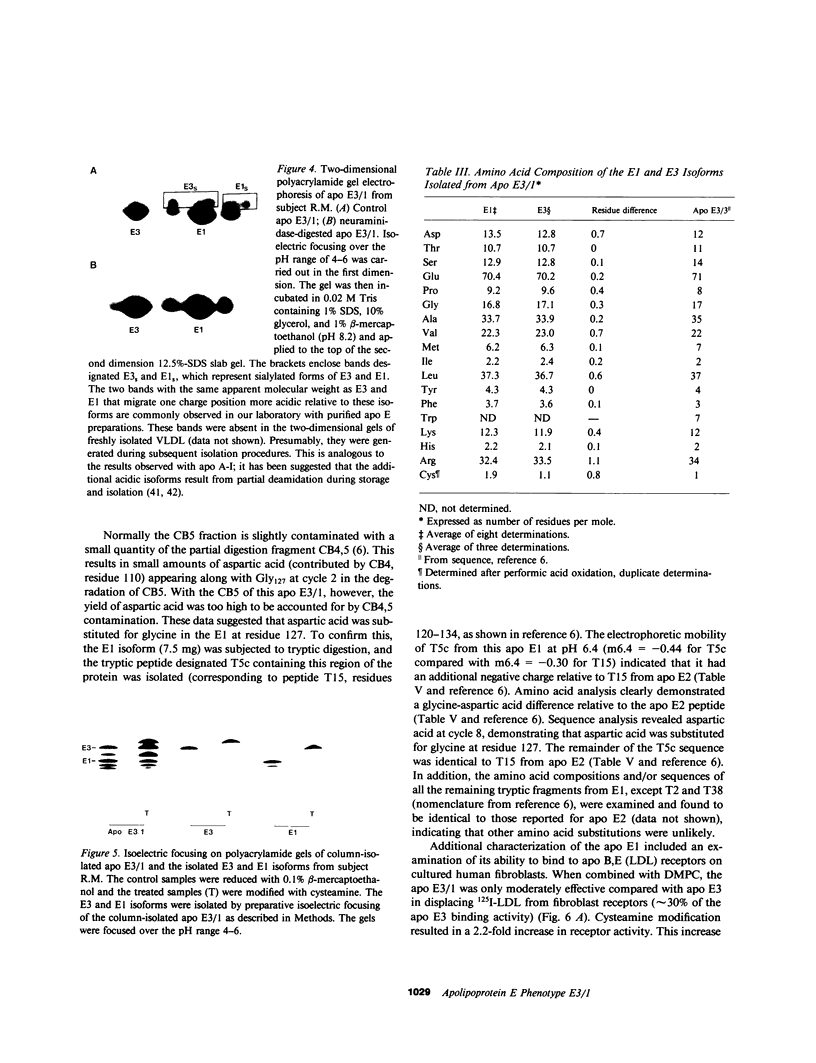
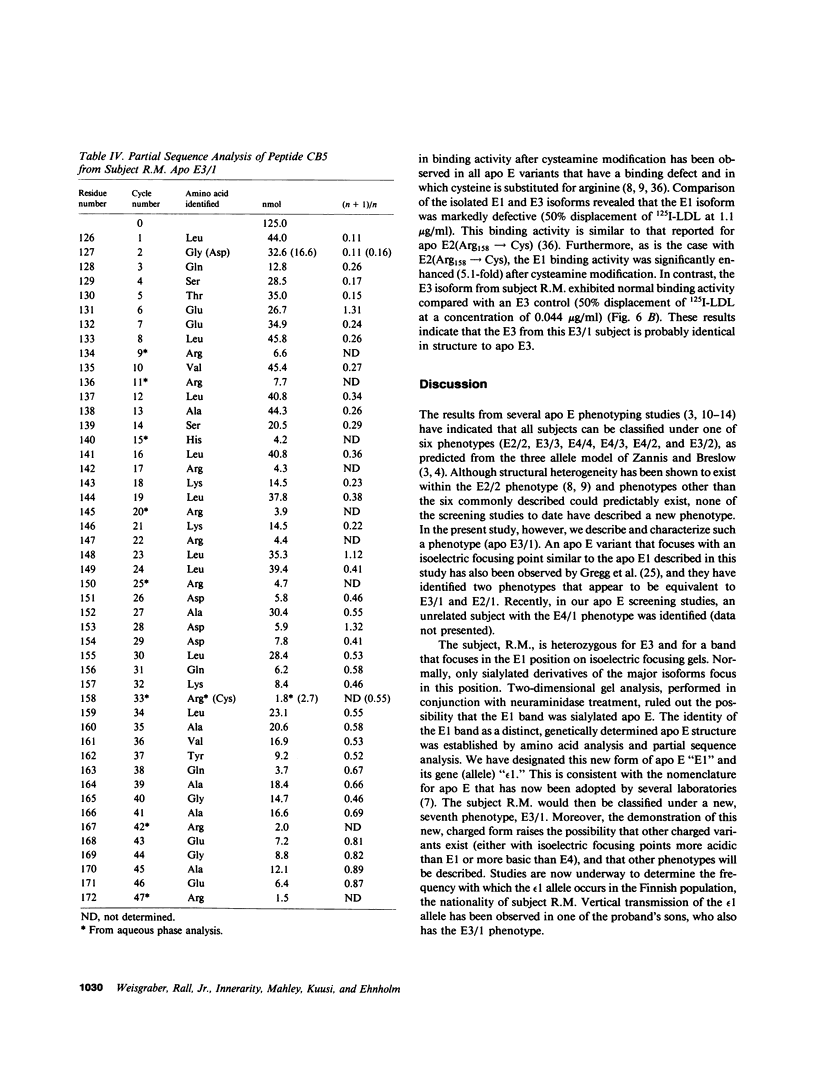
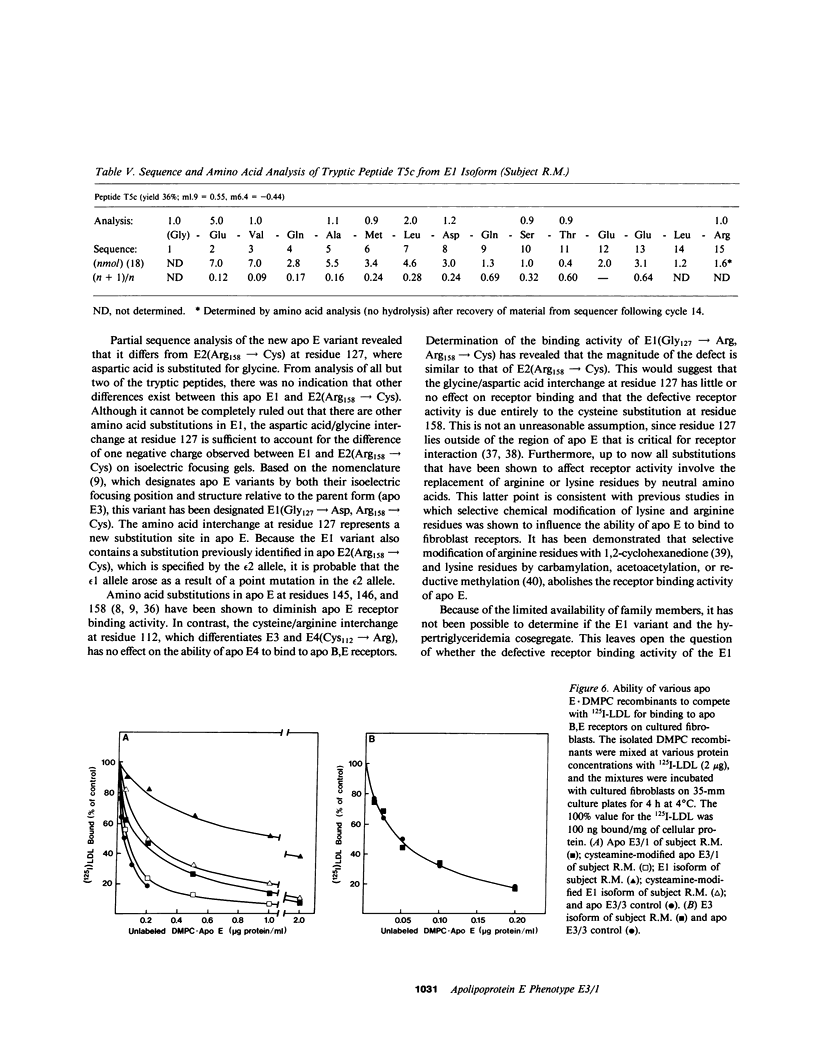
A new apolipoprotein E (apo E) phenotype has been demonstrated in a Finnish hypertriglyceridemic subject (R.M.). At the time of this study, R.M.'s plasma triglyceride and cholesterol levels were 1,021 and 230 mg/dl, respectively. The subject's apo E isoelectric focusing pattern was characterized by two major bands, one in the E3 position and the other in the E1 position. Normally the E1 position is occupied by sialylated derivatives of apo E4, E3, or E2. The E1 band of subject R.M. is not a sialylated form, however, because it was not affected by neuraminidase digestion. The identity of the E1 variant as a genetically determined structure was established by amino acid and partial sequence analyses, confirming that the variant is an example of a previously uncharacterized apo E phenotype, E3/1. Both cysteamine modification and amino acid analysis demonstrated that this variant contains two cysteine residues per mole. Sequence analysis of two cyanogen bromide fragments and one tryptic fragment of the apo E3/1 showed that it differs from E2(Arg158----Cys) at residue 127, where an aspartic acid residue is substituted for glycine. This single amino acid interchange is sufficient to account for the one-charge difference observed on isoelectric focusing gels between E2(Arg158----Cys) and the E1 variant. The variant has been designated E1 (Gly127----Asp, Arg158----Cys). When compared with apo E3, the E1 variant demonstrated reduced ability to compete with 125I-LDL for binding to LDL (apo B,E) receptors on cultured fibroblasts (approximately 4% of the amount of binding of apo E3). This defective binding is similar to that of E2-(Arg158----Cys). Therefore, the binding defect of the variant is probably due to the presence of cysteine at residue 158, rather than aspartic acid at residue 127. In contrast, the apo E3 isoform from this subject demonstrated normal binding activity, indicating that it has a normal structure. In family studies, the vertical transmission of the apo E1 variant has been established. It is not yet clear, however, if the hypertriglyceridemia observed in the proband is associated with the presence of the E1(Gly127----Asp, Arg158----Cys) variant.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Breslow J. L., Zannis V. I., SanGiacomo T. R., Third J. L., Tracy T., Glueck C. J. Studies of familial type III hyperlipoproteinemia using as a genetic marker the apoE phenotype E2/2. J Lipid Res. 1982 Nov;23(8):1224–1235. [PubMed] [Google Scholar]
- Cumming A. M., Robertson F. W. Genetics of the apolipoprotein-E isoprotein system in man. J Med Genet. 1982 Dec;19(6):417–423. doi: 10.1136/jmg.19.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainaru M., Havel R. J., Imaizumi K. Radioimmunoassay of arginine-rich apolipoprotein of rat serum. Biochim Biophys Acta. 1977 Jan 25;490(1):144–155. doi: 10.1016/0005-2795(77)90114-3. [DOI] [PubMed] [Google Scholar]
- Fainaru M., Mahley R. W., Hamilton R. L., Innerarity T. L. Structural and metabolic heterogeneity of beta-very low density lipoproteins from cholesterol-fed dogs and from humans with type III hyperlipoproteinemia. J Lipid Res. 1982 Jul;23(5):702–714. [PubMed] [Google Scholar]
- Ghiselli G., Schaefer E. J., Zech L. A., Gregg R. E., Brewer H. B., Jr Increased prevalence of apolipoprotein E4 in type V hyperlipoproteinemia. J Clin Invest. 1982 Aug;70(2):474–477. doi: 10.1172/JCI110638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg R. E., Ghiselli G., Brewer H. B., Jr Apolipoprotein EBethesda: a new variant of apolipoprotein E associated with type III hyperlipoproteinemia. J Clin Endocrinol Metab. 1983 Nov;57(5):969–974. doi: 10.1210/jcem-57-5-969. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. L., Regen D. M., Gray M. E., LeQuire V. S. Lipid transport in liver. I. Electron microscopic identification of very low density lipoproteins in perfused rat liver. Lab Invest. 1967 Feb;16(2):305–319. [PubMed] [Google Scholar]
- Havel R. J., Kotite L., Kane J. P., Tun P., Bersot T. Atypical familial dysbetalipoproteinemia associated with apolipoprotein phenotype E3/3. J Clin Invest. 1983 Jul;72(1):379–387. doi: 10.1172/JCI110978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazzard W. R., Warnick G. R., Utermann G., Albers J. J. Genetic transmission of isoapolipoprotein E phenotypes in a large kindred: relationship to dysbetalipoproteinemia and hyperlipidemia. Metabolism. 1981 Jan;30(1):79–88. doi: 10.1016/0026-0495(81)90223-7. [DOI] [PubMed] [Google Scholar]
- Innerarity T. L., Friedlander E. J., Rall S. C., Jr, Weisgraber K. H., Mahley R. W. The receptor-binding domain of human apolipoprotein E. Binding of apolipoprotein E fragments. J Biol Chem. 1983 Oct 25;258(20):12341–12347. [PubMed] [Google Scholar]
- Innerarity T. L., Pitas R. E., Mahley R. W. Binding of arginine-rich (E) apoprotein after recombination with phospholipid vesicles to the low density lipoprotein receptors of fibroblasts. J Biol Chem. 1979 May 25;254(10):4186–4190. [PubMed] [Google Scholar]
- Jackson R. L., Morrisett J. D., Gotto A. M., Jr Lipoprotein structure and metabolism. Physiol Rev. 1976 Apr;56(2):259–316. doi: 10.1152/physrev.1976.56.2.259. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Hardman D. A., Paulus H. E. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci U S A. 1980 May;77(5):2465–2469. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahley R. W., Angelin B. Type III hyperlipoproteinemia: recent insights into the genetic defect of familial dysbetalipoproteinemia. Adv Intern Med. 1984;29:385–411. [PubMed] [Google Scholar]
- Mahley R. W. Apolipoprotein E and cholesterol metabolism. Klin Wochenschr. 1983 Mar 1;61(5):225–232. doi: 10.1007/BF01496128. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L. Lipoprotein receptors and cholesterol homeostasis. Biochim Biophys Acta. 1983 May 24;737(2):197–222. doi: 10.1016/0304-4157(83)90001-1. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Pitas R. E., Weisgraber K. H., Brown J. H., Gross E. Inhibition of lipoprotein binding to cell surface receptors of fibroblasts following selective modification of arginyl residues in arginine-rich and B apoproteins. J Biol Chem. 1977 Oct 25;252(20):7279–7287. [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H. Canine lipoproteins and atherosclerosis. I. Isolation and characterization of plasma lipoproteins from control dogs. Circ Res. 1974 Nov;35(5):713–721. doi: 10.1161/01.res.35.5.713. [DOI] [PubMed] [Google Scholar]
- Menzel H. J., Kladetzky R. G., Assmann G. Apolipoprotein E polymorphism and coronary artery disease. Arteriosclerosis. 1983 Jul-Aug;3(4):310–315. doi: 10.1161/01.atv.3.4.310. [DOI] [PubMed] [Google Scholar]
- Osborne J. C., Jr, Brewer H. B., Jr The plasma lipoproteins. Adv Protein Chem. 1977;31:253–337. doi: 10.1016/s0065-3233(08)60220-x. [DOI] [PubMed] [Google Scholar]
- Rall S. C., Jr, Weisgraber K. H., Innerarity T. L., Bersot T. P., Mahley R. W., Blum C. B. Identification of a new structural variant of human apolipoprotein E, E2(Lys146 leads to Gln), in a type III hyperlipoproteinemic subject with the E3/2 phenotype. J Clin Invest. 1983 Oct;72(4):1288–1297. doi: 10.1172/JCI111085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall S. C., Jr, Weisgraber K. H., Innerarity T. L., Mahley R. W. Structural basis for receptor binding heterogeneity of apolipoprotein E from type III hyperlipoproteinemic subjects. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4696–4700. doi: 10.1073/pnas.79.15.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall S. C., Jr, Weisgraber K. H., Mahley R. W. Human apolipoprotein E. The complete amino acid sequence. J Biol Chem. 1982 Apr 25;257(8):4171–4178. [PubMed] [Google Scholar]
- Utermann G. Apolipoprotein E (role in lipoprotein metabolism and pathophysiology of hyperlipoproteinemia type III). Ric Clin Lab. 1982 Jan-Mar;12(1):23–30. [PubMed] [Google Scholar]
- Utermann G., Hees M., Steinmetz A. Polymorphism of apolipoprotein E and occurrence of dysbetalipoproteinaemia in man. Nature. 1977 Oct 13;269(5629):604–607. doi: 10.1038/269604a0. [DOI] [PubMed] [Google Scholar]
- Utermann G., Jaeschke M., Menzel J. Familial hyperlipoproteinemia type III: deficiency of a specific apolipoprotein (apo E-III) in the very-low-density lipoproteins. FEBS Lett. 1975 Aug 15;56(2):352–355. doi: 10.1016/0014-5793(75)81125-2. [DOI] [PubMed] [Google Scholar]
- Utermann G., Langenbeck U., Beisiegel U., Weber W. Genetics of the apolipoprotein E system in man. Am J Hum Genet. 1980 May;32(3):339–347. [PMC free article] [PubMed] [Google Scholar]
- Utermann G., Pruin N., Steinmetz A. Polymorphism of apolipoprotein E. III. Effect of a single polymorphic gene locus on plasma lipid levels in man. Clin Genet. 1979 Jan;15(1):63–72. [PubMed] [Google Scholar]
- Utermann G., Steinmetz A., Weber W. Genetic control of human apolipoprotein E polymorphism: comparison of one- and two-dimensional techniques of isoprotein analysis. Hum Genet. 1982;60(4):344–351. doi: 10.1007/BF00569216. [DOI] [PubMed] [Google Scholar]
- Wardell M. R., Suckling P. A., Janus E. D. Genetic variation in human apolipoprotein E. J Lipid Res. 1982 Nov;23(8):1174–1182. [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Harder K. J., Mahley R. W., Milne R. W., Marcel Y. L., Sparrow J. T. The receptor-binding domain of human apolipoprotein E. Monoclonal antibody inhibition of binding. J Biol Chem. 1983 Oct 25;258(20):12348–12354. [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982 Mar 10;257(5):2518–2521. [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978 Dec 25;253(24):9053–9062. [PubMed] [Google Scholar]
- Weisgraber K. H., Mahley R. W. Apoprotein (E--A-II) complex of human plasma lipoproteins. I. Characterization of this mixed disulfide and its identification in a high density lipoprotein subfraction. J Biol Chem. 1978 Sep 10;253(17):6281–6288. [PubMed] [Google Scholar]
- Weisgraber K. H., Rall S. C., Jr, Mahley R. W. Human E apoprotein heterogeneity. Cysteine-arginine interchanges in the amino acid sequence of the apo-E isoforms. J Biol Chem. 1981 Sep 10;256(17):9077–9083. [PubMed] [Google Scholar]
- Zannis V. I., Breslow J. L. Human very low density lipoprotein apolipoprotein E isoprotein polymorphism is explained by genetic variation and posttranslational modification. Biochemistry. 1981 Feb 17;20(4):1033–1041. doi: 10.1021/bi00507a059. [DOI] [PubMed] [Google Scholar]
- Zannis V. I., Breslow J. L., Utermann G., Mahley R. W., Weisgraber K. H., Havel R. J., Goldstein J. L., Brown M. S., Schonfeld G., Hazzard W. R. Proposed nomenclature of apoE isoproteins, apoE genotypes, and phenotypes. J Lipid Res. 1982 Aug;23(6):911–914. [PubMed] [Google Scholar]
- Zannis V. I., Just P. W., Breslow J. L. Human apolipoprotein E isoprotein subclasses are genetically determined. Am J Hum Genet. 1981 Jan;33(1):11–24. [PMC free article] [PubMed] [Google Scholar]