Abstract
Prostate cancer has become a leading cause of mortality in humans. Previous studies have shown the potential anticancer properties of kaempferol-3-O-rhamnoside in breast cancer cell lines. In the present study, the anticancer potential of kaempferol-3-O-rhamnoside was investigated in LNCaP human prostate cancer cell lines. The inhibition of cell proliferation was investigated using MTT assays, whereas its ability to induce the caspase-cascade pathway was investigated by western blotting. The results showed that kaempferol-3-O-rhamnoside inhibits the proliferation of LNCaP cells in a dose-dependent manner by upregulating the expression of caspase-8, caspase-9, caspase-3 and poly (ADP-ribose) polymerase proteins. Although further studies are required, the results of the present study indicate the potential application of kaempferol-3-O-rhamnoside in cancer treatment.
Keywords: kaempferol-3-O-rhamnoside, LNCaP, cancer, apoptosis, caspase
Introduction
Cancer has become a significant disease for humans. In the USA alone, there were ~1.6 million new cases and 577,190 predicted mortalities in 2012. Among all types of cancer, prostate cancer is one of the top causes of male cancer fatalities worldwide (1). It is predicted that 233,000 new cases of prostate cancer will occur in America during 2014 (2).
Several treatments are available for treatment of prostate cancer, by overcoming the aggressive tumor. These include surgery, radiation, radioactive implants and hormonal therapy. However, the treatment often impacts the quality of life due to side-effects or complications (3). Thus, numerous investigators have focused on discovering novel drugs or treatments. Among all the agents tested, natural products derived from medicinal plants are among the most favorable.
In our previous study, kaempferol-3-O-rhamnoside, the major compound found in the ethyl acetate fractions of the Schima wallichii (S. wallichii) Korth. leaves, was isolated and its properties were investigated against breast cancer cell lines. The results indicated that kaempferol-3-O-rhamnoside was favorable for further exploration of its anticancer therapeutic potential (4). Therefore, in the present study the anticancer properties and mechanism of kaempferol-3-O-rhamnoside were investigated in prostate cancer cell lines.
Materials and methods
Plant materials
S. wallichii Korth. leaves were collected from Lembang, West Java, Indonesia. The plant species was identified at the Department of Biology, Faculty of Mathematics and Natural Sciences, University of Padjadjaran (West Java, Indonesia).
Extraction and isolation
The S. wallichii leaves were dried and extracted with 70% ethanol at room temperature three times for 24 h each. A concentrated extract was obtained in vacuo at 50°C. The ethanol extract was partitioned into n-hexane, ethyl acetate and aqueous phases. Column chromatography on a Wakogel C 200 (Wako Pure Chemical Industries, Ltd., Osaka, Japan) column was performed to the ethyl acetate fraction, as it was previously reported as the most active fraction against cancer cell lines, using a mixture of n-hexane, ethyl acetate and methanol with increasing polarity. The major compound observed was purified using silica G 60 with sulfuric acid ethanol (1:9) and was found to be the most active fraction of S. wallichii, which was characterized and analyzed as described previously (4). The isolate was, however, identified by spectroscopic methods (ultraviolet, infrared and nuclear magnetic resonance) and liquid chromatography mass spectrometry (5).
Cell culture and treatment
The LNCaP human breast cancer cell line was purchased from Dainippon Pharmaceutical (Tokyo, Japan). The non-cancerous esophageal cell line (CHEK-1, an immortalized human esophageal cell line) was provided by Dr H. Matsubara. CHEK-1 was established by the transduction of the human papillomavirus type 16 E6/E7 into primary cultures of esophageal keratinocytes (6). The cell lines were cultured in RPMI-1640 medium (Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum and antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin). For the cell treatments, various concentrations of kaempferol-3-O-rhamnoside were added to the cell culture medium for 24 h, at which time the medium was replaced. The cells were subsequently collected at the indicated times.
Drug sensitivity assay
A cell proliferation analysis was performed in the presence of various concentrations of kaempferol-3-O-rhamnoside using a colorimetric MTT assay, as described in a previous study (7). Briefly, the cells were plated in 96-well plates (2×104 in 50 µl/well). Following the initial cell seeding, the indicated concentrations of extract were applied and incubated for 24 h. WST-8 assay cell-counting solution (10 µl) (Dojindo Lab., Tokyo, Japan) was added to each well and incubated at 37°C for 3 h. After the addition of 1 M HCl (100 µl/well), the cell proliferation rates were determined by measuring the absorbance at a wavelength of 450 nm with a reference wavelength of 650 nm using a microtiter plate reader (Becton-Dickinson, Franklin Lakes, NJ, USA). The results were derived from triplicate experiments.
Cell extraction and western blot analysis
Protein concentrations were determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA). Proteins (40 µg) were electrophoresed on 5–20% Tris-Tricine ReadyGel (Bio-Rad, Tokyo, Japan) and electro-transferred to a Hybond-enhanced chemiluminescence membrane (Amersham, Buckinghamshire, UK). Apoptosis-related proteins were analyzed by immunoblot analysis using caspase-3 (cat no. 9668), caspase-8 (cat no. 9746), caspase-9 (cat no. 9508) and poly (ADP-ribose) polymerase (PARP) antibodies (cat no. 9542) at a 1:1,000 dilution (Cell Signaling Technology, Beverly, MA, USA). β-actin (cat no. A1978; Sigma) served as the loading control.
Results
Isolation and identification of kaempferol-3-O-rhamnoside
Based on a previous study (4), it was found that the major compound of the ethyl acetate fraction of the S. wallichii extract was kaempferol-3-O-rhamnoside. The compound was purified, isolated and identified as kaempferol-3-O-rhamnoside (C21H20O10 or 3,4′,5,7-tetrahydroxyflavone-3-O-rhamnoside) with a molecular weight of 432 (Fig. 1).
Figure 1.
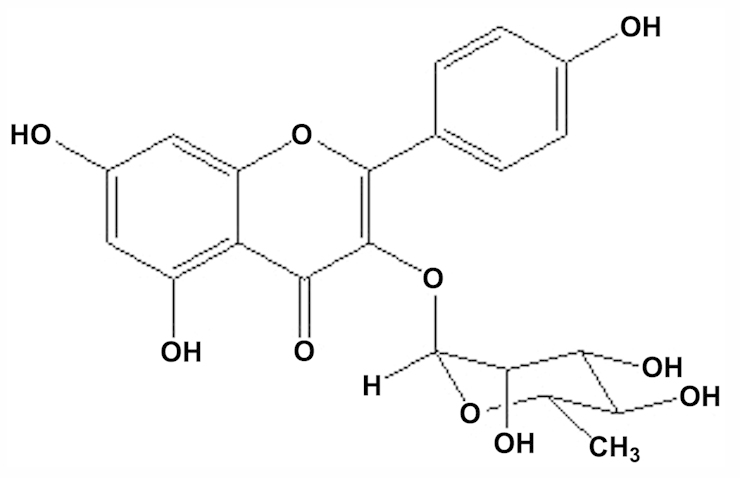
Structure of kaempferol-3-O-rhamnoside isolated from Schima wallichii Korth. leaves.
Kaempferol-3-O-rhamnoside inhibits LNCaP cell proliferation
The effect of kaempferol-3-O-rhamnoside on the viability of LNCaP and CHEK-1 cells was evaluated. The result of the MTT assay indicated that the treatment of cancer (LNCaP) and non-cancer (CHEK-1) cell lines with kaempferol-3-O-rhamnoside resulted in dose-dependent inhibition of cell growth (Fig. 2). Twenty-four hours of treatment with kaempferol-3-O-rhamnoside inhibited the proliferation of the LNCaP cells with a half maximal inhibitory concentration (IC50) value of 218 µM. However, the inhibition of the CHEK-1 cell proliferation with kaempferol-3-O-rhamnoside demonstrated an IC50 of 386 µM, which was higher than that of the LNCaP cells. This may indicate that kaempferol-3-O-rhamnoside has fewer cytotoxic effects on the non-cancerous cells.
Figure 2.
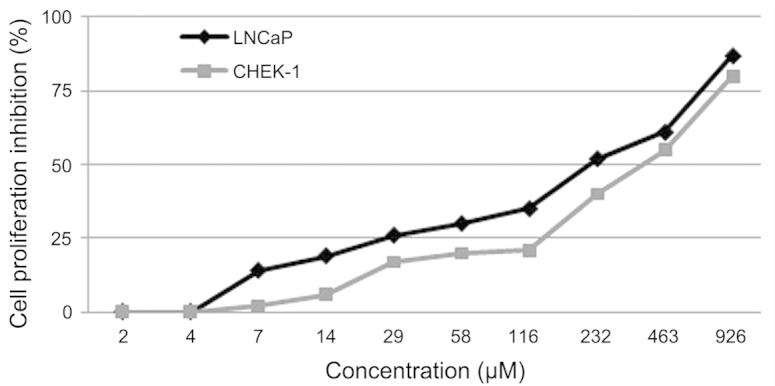
Effect of the 24-h kaempferol-3-O-rhamnoside treatment on prostate cancer (LNCaP) and non-cancer (CHEK-1) cells.
Kaempferol-3-O-rhamnoside induces caspase cascade protein expression in LNCaP cells
Kaempferol-3-O-rhamnoside treatment of the LNCaP cells induced the upregulation of caspase-8, caspase-9 and caspase-3 in a time-dependent manner (Fig. 3). Furthermore, the changes in the expression levels of PARP, a hallmark biomarker of apoptosis, suggested that kaempferol-3-O-rhamnoside may induce apoptosis on LNCaP cells through the activation of caspase-dependent signaling pathways after 24 h of treatment.
Figure 3.
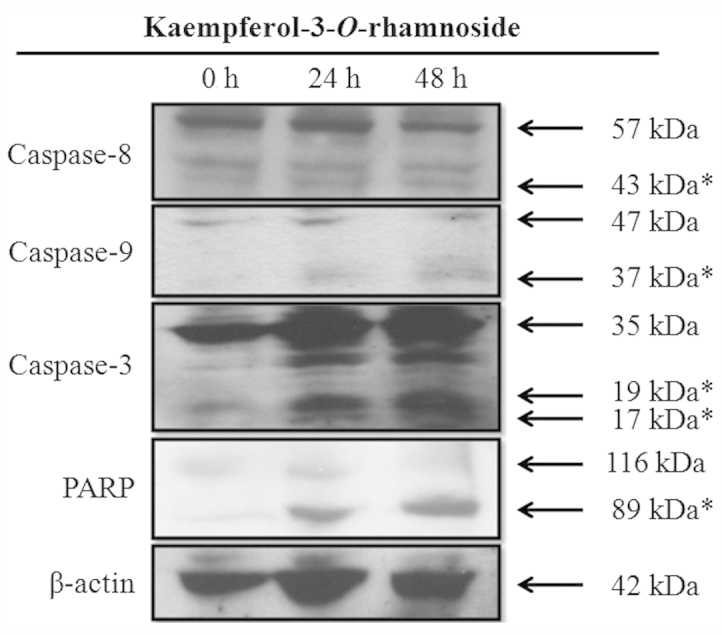
Western blot analysis of apoptotic proteins that are upregulated in LNCaP cells treated with kaempferol-3-O-rhamnoside for 24 h and collected at the indicated times. *, cleaved form.
Discussion
S. wallichii plants are found in Asia, from Indochina to Papua New Guinea. Several of the compounds found in S. wallichii, including alkaloids and tannins, are ethnobotanically used for snake and insect bites (8). A previous study reported the antimicrobial activity of the hydroalcoholic extract from the S. wallichii bark against Escherichia coli, Pseudomonas aeruginosa and Shigella species (9). Furthermore, our previous study also reported the antiplasmodial properties of S. wallichii and its component, kaempferol-3-O-rhamnoside, against chloroquine-resistant Plasmodium falciparum (10).
Our previous study also reported the anticancer properties of kaempferol-3-O-rhamnoside on MCF-7 human breast cancer cell lines. The results indicated that kaempferol-3-O-rhamnoside inhibits the proliferation of MCF-7 cells through the activation of caspase-9 and caspase-3 proteins and that it induced apoptosis (4).
In the present study study, kaempferol-3-O-rhamnoside inhibited the proliferation of LNCaP cells in a dose-dependent manner. Notably, the cell proliferation inhibition by kaempferol-3-O-rhamnoside was lower on the non-cancerous CHEK-1 cells. Kaempferol-3-O-rhamnoside may possibly be considered less harmful to non-cancerous cells. Furthermore, the study also showed that the anticancer properties of kaempferol-3-O-rhamnoside occurred via the upregulation of caspase-8, caspase-9, caspase-3 and finally PARP, the marker of apoptosis. Caspases are synthesized as inactive precursors. There are numerous caspase-activation pathways that promote apoptosis, such as mitochondrial stress by apoptosome pathways, death receptor engagement and granzyme B-induced caspase activation (11). Caspase-9 is activated by mitochondrial stress. Divergent cellular stresses, such as DNA damage, heat shock and oxidative stress, may result in caspase-9 activation through the release of cytochrome c to the cytoplasm. Efflux of cytochrome c stimulates several high molecular weight caspase-activating complexes in the cytoplasm. Cytochrome c is a major component of the apoptosome and it activates caspase-9. Caspase-8 and caspase-3, however, are activated by granzyme B. This pathway shows that there are several cytotoxic granules from cytotoxic T cells, natural killer cells and granzyme B. Granzyme B will activate caspase-3 and caspase-8 to facilitate the destruction of the target cells (11). Therefore, these results showed that the potential anticancer properties of kaempferol-3-O-rhamnoside trigger death extrinsically via caspase-8 activation and intrinsically via caspase-9 activation in the LNCaP cells.
In conclusion, although further toxicity studies and activity enhancing structure modifications are required, the results of the present study indicate the potential application of kaempferol-3-O-rhamnoside in cancer treatment.
Acknowledgements
The authors would like to thank Dr Yudi Padmadisastra for his valuable guidance during this research.
References
- 1.Siegel R, Naishadam D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society, author. Cancer Facts and Figures 2014. American Cancer Society; Atlanta, GA: 2014. [Google Scholar]
- 3.Stangelberger A, Waldert M, Djavan B. Prostate cancer in elderly men. Rev Urol. 2008;10:111–119. [PMC free article] [PubMed] [Google Scholar]
- 4.Diantini A, Subarnas A, Lestari K, et al. Kaempferol-3-O-rhamnoside isolated from the leaves of Schima wallichii Korth. inhibits MCF-7 breast cancer cell proliferation through activation of the caspase cascade pathway. Oncol Lett. 2012;3:1069–1072. doi: 10.3892/ol.2012.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverstein RM, Webster FX, Kiemle DJ, editors. Spectrometric Identification of Organic Compounds. 7th. John Wiley & Sons; New Jersey, NJ: 2005. pp. 72–229. [Google Scholar]
- 6.Sashiyama H, Shino Y, Sakao S, Shimada H, Kobayashi S, Ochiai T, Shirasawa H. Alteration of integrin expression relates to malignant progression of human papillomavirus-immortalized esophageal keratinocytes. Cancer Lett. 2002;177:21–28. doi: 10.1016/s0304-3835(01)00771-6. [DOI] [PubMed] [Google Scholar]
- 7.Abdulah R, Faried A, Kobayashi K, Yamazaki C, Suradji EW, Ito K, Suzuki K, Murakami M, Kuwano H, Koyama H. Selenium enrichment of broccoli sprout extract increases chemosensitivity and apoptosis of LNCaP prostate cancer cells. BMC Cancer. 2009;9:414. doi: 10.1186/1471-2407-9-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lalfakzuala R, Lalramnghinglova H, Kayang H. Ethnobotanical usages of plants in western Mizoram. Indian J Tradit Knowl. 2007;6:486–493. [Google Scholar]
- 9.Dewanjee S, Maiti A, Majumdar R, Majumdar A, Mandal SC. Evaluation of antimicrobial activity of hydroalcoholic extract Schima wallichii bark. Pharmacologyonline. 2008;1:523–528. [Google Scholar]
- 10.Barliana MI, Suradji EW, Abdulah R, Diantini A, Hatabu T, Shimada JN, Subarnas A, Koyama H. Antiplasmodial properties of kaempferol-3-O-rhamnoside isolated from the leaves of Schima wallichii against chloroquine-resistant Plasmodium falciparum. Biomed Rep. 2014;2:579–583. doi: 10.3892/br.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2005;37:719–727. doi: 10.1111/j.1745-7270.2005.00108.x. [DOI] [PubMed] [Google Scholar]


