Abstract
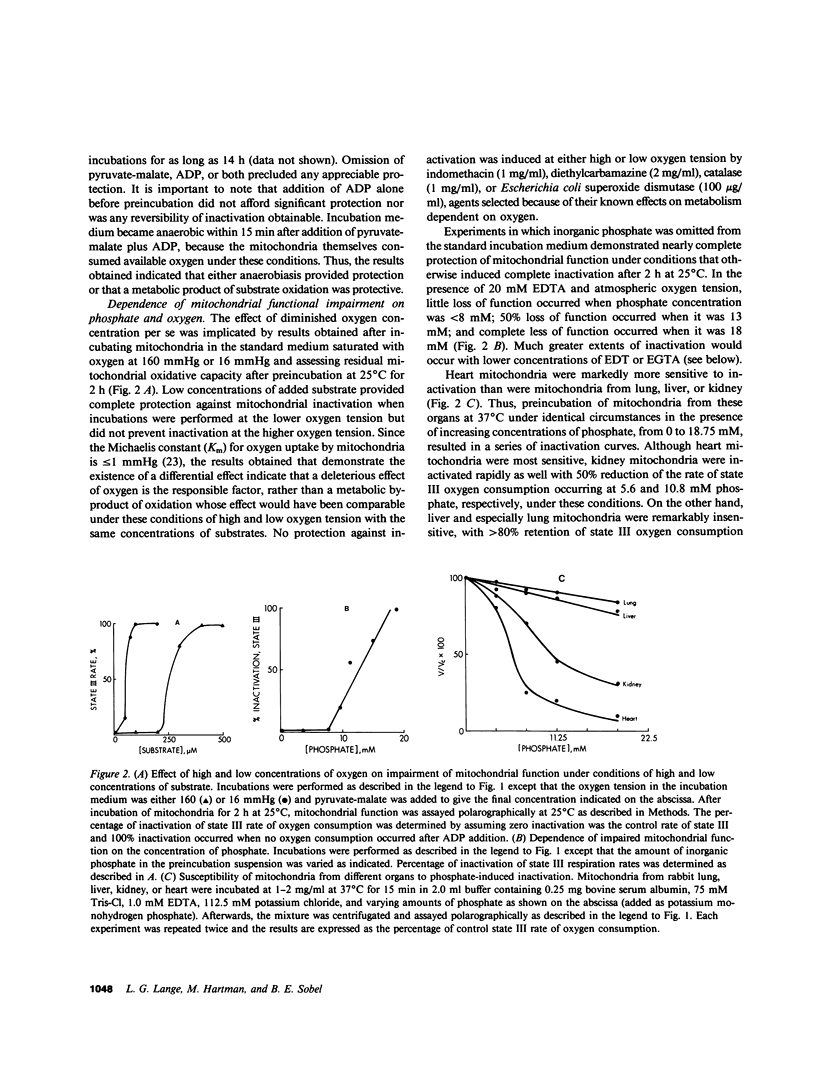
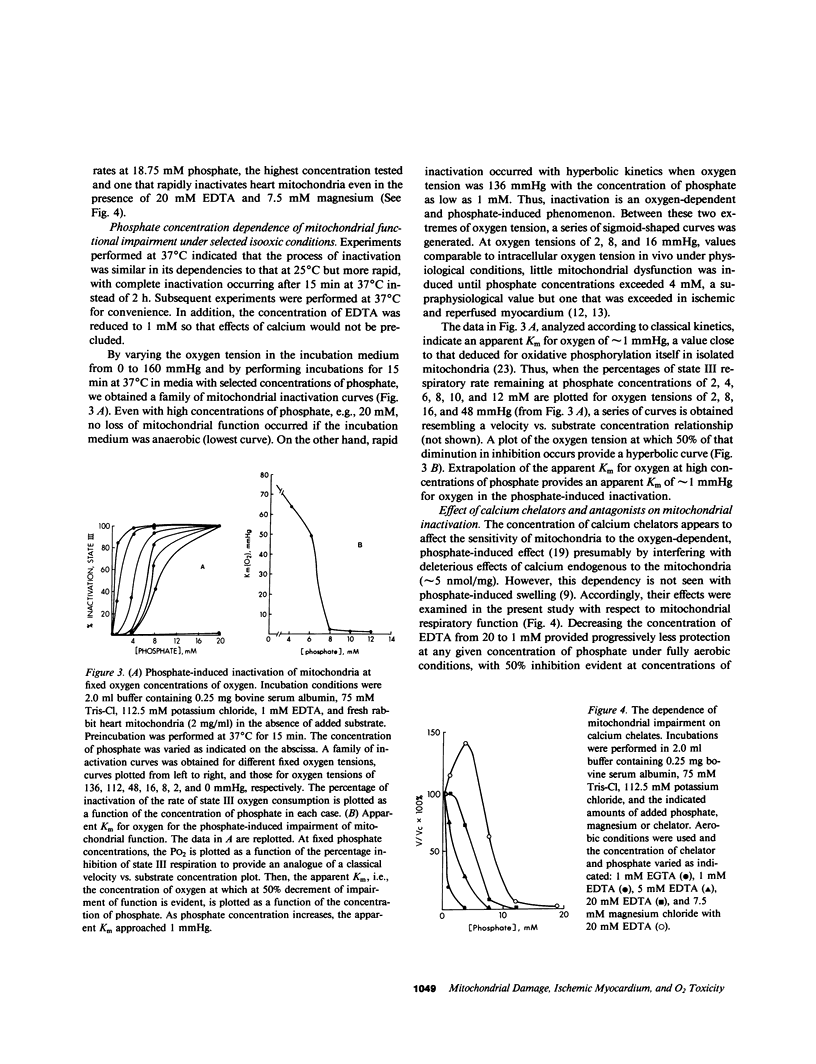
Cellular injury induced by reperfusion after myocardial ischemia is manifested by striking mitochondrial damage as well as other hallmarks such as contraction band necrosis. Calcium has been implicated as a mediator of irreversible cellular injury in several systems. To identify other potential mediators of the mitochondrial injury associated with reperfusion, interactions between inorganic phosphate, oxygen, and mitochondria harvested from rabbit hearts were evaluated in vitro. Mitochondria exhibited rapid inactivation of oxidative phosphorylation after preincubation at 25 degrees C when phosphate and oxygen were present. Inactivation was partially but not completely precluded by EDTA, EGTA, magnesium, diltiazem, or ruthenium red, results in concert with findings of others suggesting involvement of a deleterious influx of calcium into mitochondria; exogenous calcium enhanced inactivation. However, the present data indicate that inactivation is prevented by incubation of mitochondria in the absence of oxygen, and demonstrate for the first time that injury elicited by phosphate is dependent on oxygen at physiological concentrations either because calcium and/or phosphate influx is linked to aerobic metabolism or because oxygen exerts deleterious effects on mitochondria, which may render them particularly susceptible to calcium influx. Since intracellular inorganic phosphate concentration increases markedly with ischemia, reperfusion with oxygenated medium may paradoxically augment mitochondrial injury in this setting. Thus, in the presence of increased intracellular concentrations of calcium and phosphate induced by ischemia, subsequent reestablishment of physiological levels of intracellular oxygen tension may promote mitochondrial damage, which is known to increase with reperfusion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brierley G. P., Jurkowitz M., Scott K. M., Merola A. J. Ion transport by heart mitochondria. XX. Factors affecting passive osmotic swelling of isolated mitochondria. J Biol Chem. 1970 Oct 25;245(20):5404–5411. [PubMed] [Google Scholar]
- CAULFIELD J., KLIONSKY B. Myocardial ischemia and early infarction: an electron microscopic study. Am J Pathol. 1959 May-Jun;35(3):489–523. [PMC free article] [PubMed] [Google Scholar]
- CHAPPELL J. B., CROFTS A. R. CALCIUM ION ACCUMULATION AND VOLUME CHANGES OF ISOLATED LIVER MITOCHONDRIA. CALCIUM ION-INDUCED SWELLING. Biochem J. 1965 May;95:378–386. doi: 10.1042/bj0950378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND K. W., SLATER E. C. Respiratory granules of heart muscle. Biochem J. 1953 Mar;53(4):547–556. doi: 10.1042/bj0530547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonin P., Di Gennaro M., Valle R., Weisz A. M. Inhibitory effect of anoxia on reperfusion- and digitalis-induced ventricular tachyarrhythmias. Am J Physiol. 1981 May;240(5):H730–H737. doi: 10.1152/ajpheart.1981.240.5.H730. [DOI] [PubMed] [Google Scholar]
- Flaherty J. T., Weisfeldt M. L., Bulkley B. H., Gardner T. J., Gott V. L., Jacobus W. E. Mechanisms of ischemic myocardial cell damage assessed by phosphorus-31 nuclear magnetic resonance. Circulation. 1982 Mar;65(3):561–570. doi: 10.1161/01.cir.65.3.561. [DOI] [PubMed] [Google Scholar]
- Garlick P. B., Radda G. K., Seeley P. J. Studies of acidosis in the ischaemic heart by phosphorus nuclear magnetic resonance. Biochem J. 1979 Dec 15;184(3):547–554. doi: 10.1042/bj1840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER F. E., Jr, DAVIS J., CARLAT L. The stability of oxidative and phosphorylative systems in mitochondria under anaerobic conditions. Biochim Biophys Acta. 1956 Apr;20(1):237–242. doi: 10.1016/0006-3002(56)90282-7. [DOI] [PubMed] [Google Scholar]
- HUNTER F. E., Jr, LEVY J. F., FINK J., SCHUTZ B., GUERRA F., HURWITZ A. Studies on the mechanism by which anaerobiosis prevents swelling of mitochondria in vitro: effect of electron transport chain inhibitors. J Biol Chem. 1959 Aug;234(8):2176–2186. [PubMed] [Google Scholar]
- Henry P. D., Shuchleib R., Borda L. J., Roberts R., Williamson J. R., Sobel B. E. Effects of nifedipine on myocardial perfusion and ischemic injury in dogs. Circ Res. 1978 Sep;43(3):372–380. doi: 10.1161/01.res.43.3.372. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A., Southard J. H. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem. 1976 Aug 25;251(16):5069–5077. [PubMed] [Google Scholar]
- JENNINGS R. B., BAUM J. H., HERDSON P. B. FINE STRUCTURAL CHANGES IN MYOCARDIAL ISCHEMIC INJURY. Arch Pathol. 1965 Feb;79:135–143. [PubMed] [Google Scholar]
- Jacobus W. E., Tiozzo R., Lugli G., Lehninger A. L., Carafoli E. Aspects of energy-linked calcium accumulation by rat heart mitochondria. J Biol Chem. 1975 Oct 10;250(19):7863–7870. [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E. Structural changes in myocardium during acute ischemia. Circ Res. 1974 Sep;35 (Suppl 3):156–172. [PubMed] [Google Scholar]
- Jennings R. B., Hawkins H. K., Lowe J. E., Hill M. L., Klotman S., Reimer K. A. Relation between high energy phosphate and lethal injury in myocardial ischemia in the dog. Am J Pathol. 1978 Jul;92(1):187–214. [PMC free article] [PubMed] [Google Scholar]
- Jennings R. B., Reimer K. A., Hill M. L., Mayer S. E. Total ischemia in dog hearts, in vitro. 1. Comparison of high energy phosphate production, utilization, and depletion, and of adenine nucleotide catabolism in total ischemia in vitro vs. severe ischemia in vivo. Circ Res. 1981 Oct;49(4):892–900. doi: 10.1161/01.res.49.4.892. [DOI] [PubMed] [Google Scholar]
- LEHNINGER A. L. Reversal of various types of mitochondrial swelling by adenosine triphosphate. J Biol Chem. 1959 Sep;234:2465–2471. [PubMed] [Google Scholar]
- Lange L. G., Sobel B. E. Myocardial metabolites of ethanol. Circ Res. 1983 Apr;52(4):479–482. doi: 10.1161/01.res.52.4.479. [DOI] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Nagao T., Matlib M. A., Franklin D., Millard R. W., Schwartz A. Effects of diltiazem, a calcium antagonist, on regional myocardial function and mitochondria after brief coronary occlusion. J Mol Cell Cardiol. 1980 Jan;12(1):29–43. doi: 10.1016/0022-2828(80)90109-1. [DOI] [PubMed] [Google Scholar]
- ROSSI C. S., LEHNINGER A. L. STOICHIOMETRY OF RESPIRATORY STIMULATION, ACCUMULATION OF CA++ AND PHOSPHATE, AND OXIDATIVE PHOSPHORYLATION IN RAT LIVER MITOCHONDRIA. J Biol Chem. 1964 Nov;239:3971–3980. [PubMed] [Google Scholar]
- Shen A. C., Jennings R. B. Kinetics of calcium accumulation in acute myocardial ischemic injury. Am J Pathol. 1972 Jun;67(3):441–452. [PMC free article] [PubMed] [Google Scholar]
- Sobel B. E., Corr P. B., Robison A. K., Goldstein R. A., Witkowski F. X., Klein M. S. Accumulation of lysophosphoglycerides with arrhythmogenic properties in ischemic myocardium. J Clin Invest. 1978 Sep;62(3):546–553. doi: 10.1172/JCI109159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel B., Jequier E., Sjoerdsma A., Lovenberg W. Effect of catecholamines and adrenergic blocking agents on oxidative phosphorylation in rat heart mitochondria. Circ Res. 1966 Dec;19(6):1050–1061. doi: 10.1161/01.res.19.6.1050. [DOI] [PubMed] [Google Scholar]
- Vághy P. L., Johnson J. D., Matlib M. A., Wang T., Schwartz A. Selective inhibition of Na+-induced Ca2+ release from heart mitochondria by diltiazem and certain other Ca2+ antagonist drugs. J Biol Chem. 1982 Jun 10;257(11):6000–6002. [PubMed] [Google Scholar]
- Vághy P. L., Matlib M. A., Schwartz A. Phosphate induced swelling, inhibition and partial uncoupling of oxidative phosphorylation in heart mitochondria in the absence of external calcium and the presence of EGTA. Biochem Biophys Res Commun. 1981 May 15;100(1):37–44. doi: 10.1016/s0006-291x(81)80059-9. [DOI] [PubMed] [Google Scholar]
- Vághy P. L., Matlib M. A., Szekeres L., Schwartz A. Protective effects on verapamil and diltiazem against inorganic phosphate induced impairment of oxidative phosphorylation of isolated heart mitochondria. Biochem Pharmacol. 1981 Sep 15;30(18):2603–2610. doi: 10.1016/0006-2952(81)90588-8. [DOI] [PubMed] [Google Scholar]
- Whitmer J. T., Idell-Wenger J. A., Rovetto M. J., Neely J. R. Control of fatty acid metabolism in ischemic and hypoxic hearts. J Biol Chem. 1978 Jun 25;253(12):4305–4309. [PubMed] [Google Scholar]
- Wittenberg J. B. Myoglobin-facilitated oxygen diffusion: role of myoglobin in oxygen entry into muscle. Physiol Rev. 1970 Oct;50(4):559–636. doi: 10.1152/physrev.1970.50.4.559. [DOI] [PubMed] [Google Scholar]



