Abstract
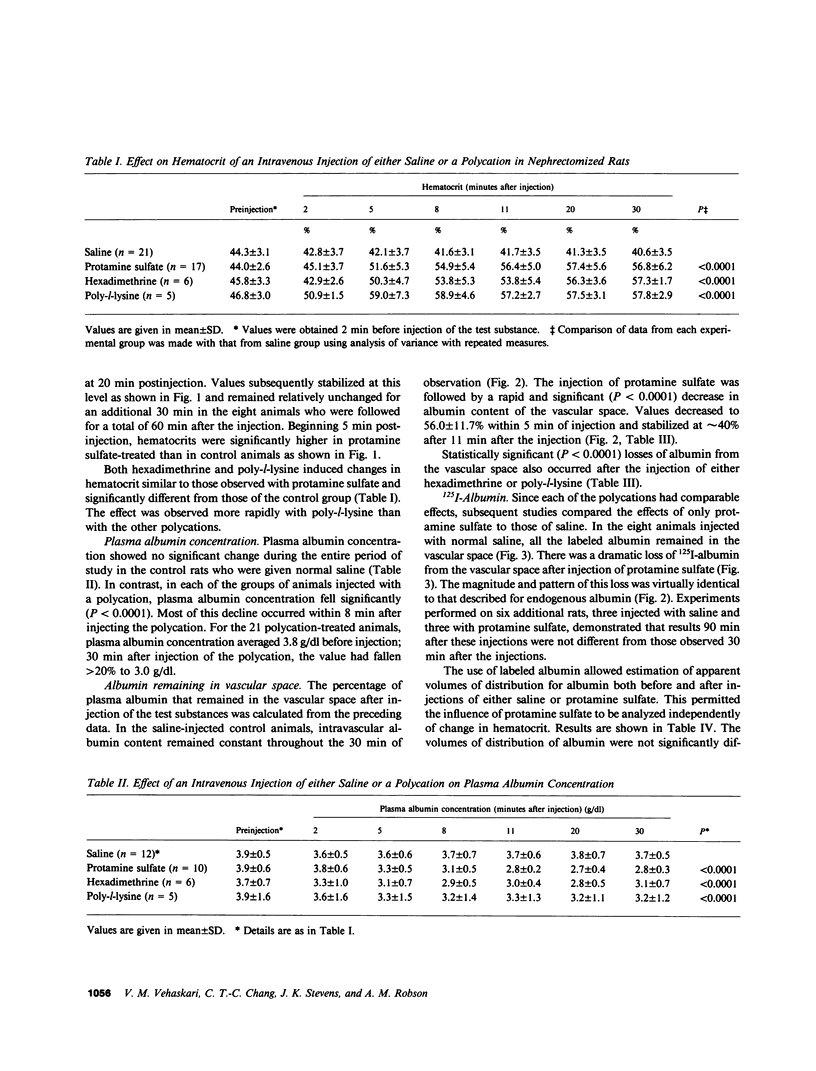
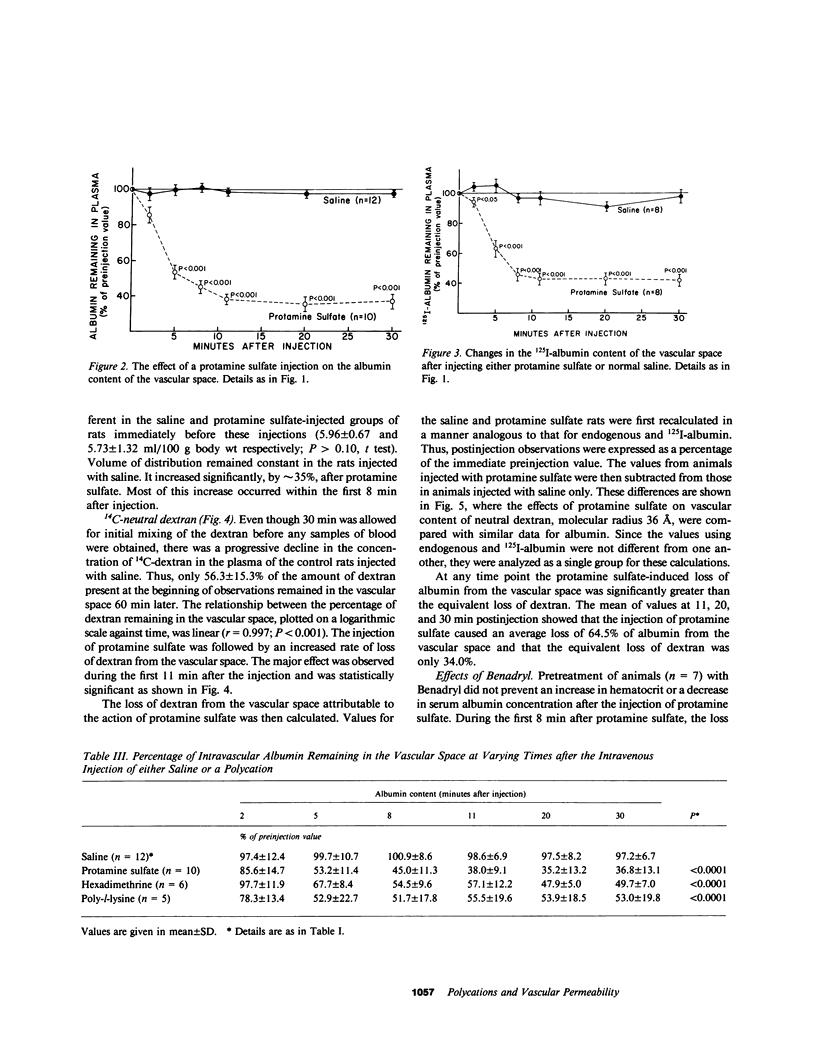
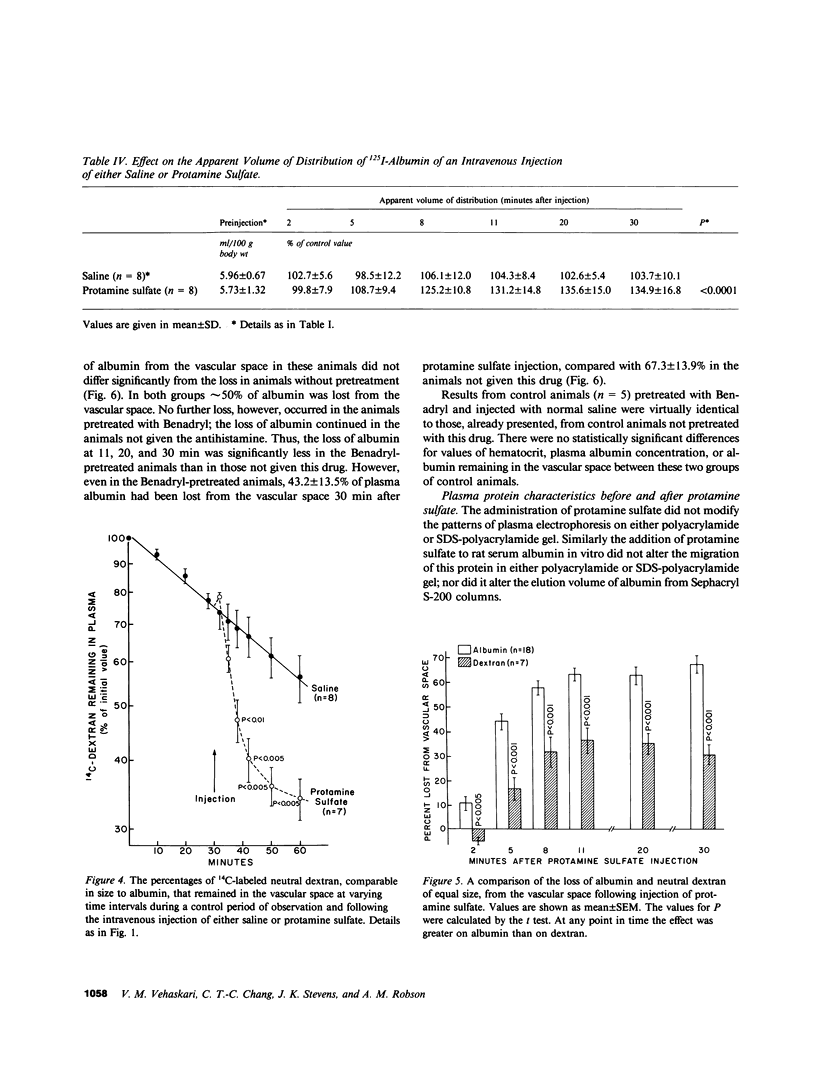
This study investigated whether charge sites in the walls of the microvasculature may play a role in maintaining the impermeability of the nonrenal capillaries to albumin. All experiments were performed in nephrectomized rats, studied in the awake state. The intravenous injection of protamine sulfate (4 mg/100 g body wt dissolved in 0.9% saline) was followed by a mean increase of 29.1% in hematocrit and a decrease of 28.4% in plasma albumin concentration over a 10-min period, indicating a significant 50-60% loss of albumin from the vascular space; a finding confirmed by studies using exogenous 125I-labeled albumin. Changes persisted for the remaining 80 min of observation, and could be reproduced by the injection of two other polycations, hexadimethrine and poly-l-lysine. These effects were not prevented by the antihistamine diphenhydramine hydrochloride. In contrast to 125I-labeled albumin, 14C-labeled neutral dextran of comparable size was not confined to the vascular space; its apparent volume of distribution progressively increased during the 90 min of observation. Intravenous injection of protamine sulfate was followed by a significantly smaller loss of 14C-dextran (36.5%) than albumin (59.1%) from the vascular space (P less than 0.01). Protamine sulfate could not be demonstrated to result in any changes in the physicochemical characteristics of albumin. These observations suggest that the negative charge sites present in nonglomerular capillary walls have functions similar to equivalent sites present in the glomerular capillaries. Thus, charge sites could contribute to the low permeability of the microvasculature to negatively charged macromolecules such as albumin. This may be an important mechanism for retaining albumin in the vascular space and preventing edema formation in health.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Areekul S. Reflection coefficients of neutral and sulphate-substituted dextran molecules in the isolated perfused rabbit ear. Acta Soc Med Ups. 1969;74(3-4):129–138. [PubMed] [Google Scholar]
- Brenner B. M., Hostetter T. H., Humes H. D. Glomerular permselectivity: barrier function based on discrimination of molecular size and charge. Am J Physiol. 1978 Jun;234(6):F455–F460. doi: 10.1152/ajprenal.1978.234.6.F455. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Hostetter T. H., Humes H. D. Molecular basis of proteinuria of glomerular origin. N Engl J Med. 1978 Apr 13;298(15):826–833. doi: 10.1056/NEJM197804132981507. [DOI] [PubMed] [Google Scholar]
- Bridges C. R., Myers B. D., Brenner B. M., Deen W. M. Glomerular charge alterations in human minimal change nephropathy. Kidney Int. 1982 Dec;22(6):677–684. doi: 10.1038/ki.1982.229. [DOI] [PubMed] [Google Scholar]
- DE VRIES A., FELDMAN J. D., STEIN O., STEIN Y., KATCHALSKI E. Effects of intravenously administered poly-D L-lysine in rats. Proc Soc Exp Biol Med. 1953 Feb;82(2):237–240. doi: 10.3181/00379727-82-20078. [DOI] [PubMed] [Google Scholar]
- DE VRIES A., KATCHALSKI E., STEIN O. The effect of polyamino acids on the blood vessels of the rat. Arch Int Pharmacodyn Ther. 1956 Aug 1;107(2):243–253. [PubMed] [Google Scholar]
- Davies P. F., Rennke H. G., Cotran R. S. Influence of molecular charge upon the endocytosis and intracellular fate of peroxidase activity in cultured arterial endothelium. J Cell Sci. 1981 Jun;49:69–86. doi: 10.1242/jcs.49.1.69. [DOI] [PubMed] [Google Scholar]
- Eisenbach G. M., Liew J. B., Boylan J. W., Manz N., Muir P. Effect of angiotensin on the filtration of protein in the rat kidney: a micropuncture study. Kidney Int. 1975 Aug;8(2):80–87. doi: 10.1038/ki.1975.83. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Haraldsson B., Moxham B. J., Rippe B. Capillary permeability of sulphate-substituted and neutral dextran fractions in the rat hindquarter vascular bed. Acta Physiol Scand. 1982 Aug;115(4):397–404. doi: 10.1111/j.1748-1716.1982.tb07097.x. [DOI] [PubMed] [Google Scholar]
- Hunsicker L. G., Shearer T. P., Shaffer S. J. Acute reversible proteinuria induced by infusion of the polycation hexadimethrine. Kidney Int. 1981 Jul;20(1):7–17. doi: 10.1038/ki.1981.98. [DOI] [PubMed] [Google Scholar]
- KEDEM O., KATCHALSKY A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta. 1958 Feb;27(2):229–246. doi: 10.1016/0006-3002(58)90330-5. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Linker A., Farquhar M. G. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol. 1980 Aug;86(2):688–693. doi: 10.1083/jcb.86.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M. A. Changes in transcapillary protein flux by permeative and convective mechanisms as functions of increasing transcapillary water flux. Microvasc Res. 1981 Nov;22(3):271–295. doi: 10.1016/0026-2862(81)90097-2. [DOI] [PubMed] [Google Scholar]
- Larsen B. Increased permeability to albumin induced with protamine in modified gelatine membranes. Nature. 1967 Aug 5;215(5101):641–642. doi: 10.1038/215641a0. [DOI] [PubMed] [Google Scholar]
- Linker A., Hovingh P., Kanwar Y. S., Farquhar M. G. Characterization of heparan sulfate isolated from drug glomerular basement membranes. Lab Invest. 1981 Jun;44(6):560–565. [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Patlak C. S., Goldstein D. A., Hoffman J. F. The flow of solute and solvent across a two-membrane system. J Theor Biol. 1963 Nov;5(3):426–442. doi: 10.1016/0022-5193(63)90088-2. [DOI] [PubMed] [Google Scholar]
- Robson A. M., Giangiacomo J., Kienstra R. A., Naqvi S. T., Ingelfinger J. R. Normal glomerular permeability and its modification by minimal change nephrotic syndrmone. J Clin Invest. 1974 Nov;54(5):1190–1199. doi: 10.1172/JCI107862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson A. M., Mor J., Root E. R., Jager B. V., Shankel S. W., Ingelfinger J. R., Kienstra R. A., Bricker N. S. Mechanism of proteinuria in nonglomerular renal disease. Kidney Int. 1979 Sep;16(3):416–429. doi: 10.1038/ki.1979.145. [DOI] [PubMed] [Google Scholar]
- Shankel S. W., Robson A. M., Bricker N. S. On the mechanism of the splay in the glucose titration curve in advanced experimental renal disease in the rat. J Clin Invest. 1967 Feb;46(2):164–172. doi: 10.1172/JCI105519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skutelsky E., Danon D. Redistribution of surface anionic sites on the luminal front of blood vessel endothelium after interaction with polycationic ligand. J Cell Biol. 1976 Oct;71(1):232–241. doi: 10.1083/jcb.71.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skutelsky E., Rudich Z., Danon D. Surface charge properties of the luminal front of blood vessel walls: an electron microscopical analysis. Thromb Res. 1975 Oct;7(4):623–634. doi: 10.1016/0049-3848(75)90108-5. [DOI] [PubMed] [Google Scholar]
- Sobel A., Heslan J. M., Branellec A., Lagrue G. Vascular permeability factor produced by lymphocytes of patients with nephrotic syndrome. Adv Nephrol Necker Hosp. 1981;10:315–332. [PubMed] [Google Scholar]
- Starling E. H. On the Absorption of Fluids from the Connective Tissue Spaces. J Physiol. 1896 May 5;19(4):312–326. doi: 10.1113/jphysiol.1896.sp000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehaskari V. M., Root E. R., Germuth F. G., Jr, Robson A. M. Glomerular charge and urinary protein excretion: effects of systemic and intrarenal polycation infusion in the rat. Kidney Int. 1982 Aug;22(2):127–135. doi: 10.1038/ki.1982.144. [DOI] [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Winn R., Nadir B., Gleisner J., Stothert J., Hildebrandt J. Deficiencies in pore-membrane models of microvascular fluid and solute transudation. J Appl Physiol Respir Environ Exerc Physiol. 1981 Dec;51(6):1574–1580. doi: 10.1152/jappl.1981.51.6.1574. [DOI] [PubMed] [Google Scholar]


