Abstract
Background
Lack of evidence-based data on the spectrum of cardiovascular disease (CVD) in pregnancy or in the postpartum period, as well as on maternal and fetal outcome, provides challenges for treating physicians, particularly in areas of low resources. The objectives of this study were to investigate the spectrum of disease, mode of presentation and maternal and fetal outcome of patients referred to a dedicated Cardiac Disease and Maternity Clinic (CDM).
Methods
The prospective cohort study was conducted at a single tertiary care centre in South Africa. Two hundred and twenty-five women presenting with CVD in pregnancy, or within 6 months postpartum, were studied over a period of 2 years. Clinical assessment, echocardiography and laboratory tests were performed at baseline and follow-up visits. Prepartum, peripartum and postpartum complications were grouped into cardiac, neonatal and obstetric events.
Results
Ethnicity was black African (45%), mixed ethnicity (32%), white (15%), Indian/others (8%) and 12% were HIV positive. Of the 225 consecutive women (mean age 28.8±6.4), 196 (86.7%) presented prepartum and 73 in modified WHO class I. The 152 women presenting in a higher risk group (modified WHO class II–IV) were offered close follow-up at the CDM clinic and were diagnosed with congenital heart disease (32%, 15 operated previously), valvular heart disease (26%, 15 operated previously), cardiomyopathy (27%) and other (15%). Women presenting with symptoms of CVD or heart failure postpartum (n=30) presented in a higher New York Heart Association, had higher heart rates (p<0.001) and NTproBNP levels (p<0.0005). Of the 152 patients, 9 (6%) died within the 6-month follow-up period. Eight of the nine patients died >42 days postpartum. Perinatal death occurred in 1/152 (0.7%)—translating to a perinatal mortality rate of 7/1000 live births.
Conclusions
Disease patterns were markedly different to that seen in the developed world. However, joint obstetric–cardiac care in the low-resource cohort was associated with excellent survival outcome rates of pregnant mothers (even with complex diseases) and their offspring and was similar to that seen in the western world. Mortality typically occurred in the postpartum period, beyond the standard date of recording maternal death.
Keywords: QUALITY OF CARE AND OUTCOMES
Introduction
There is generally a paucity of data describing cardiovascular disease (CVD) in women from Africa or other developing regions. In particular, few data describe CVD related to pregnancy with its unique disease patterns and presentations.1 2 This has to be seen in the context of a general shortage of physicians in Africa; for example, South Africa has only 165 registered cardiologists for a population of 50 million. In April 2010, a dedicated weekly ‘Cardiac Disease and Maternity Clinic (CDM)’ was established at the Division of Cardiology, Department of Medicine, Groote Schuur Hospital, University of Cape Town, to provide multidisciplinary systematic care for women with suspected or previously known CVD, presenting in pregnancy or postpartum. In July 2010, a prospective single-centre study was initiated, which aimed to investigate the spectrum of CVD presenting prepartum or postpartum, as well as the maternal and fetal outcome using a comprehensive system of care by a team of obstetricians, anaesthesiologists and cardiologists.
Methods
Study design
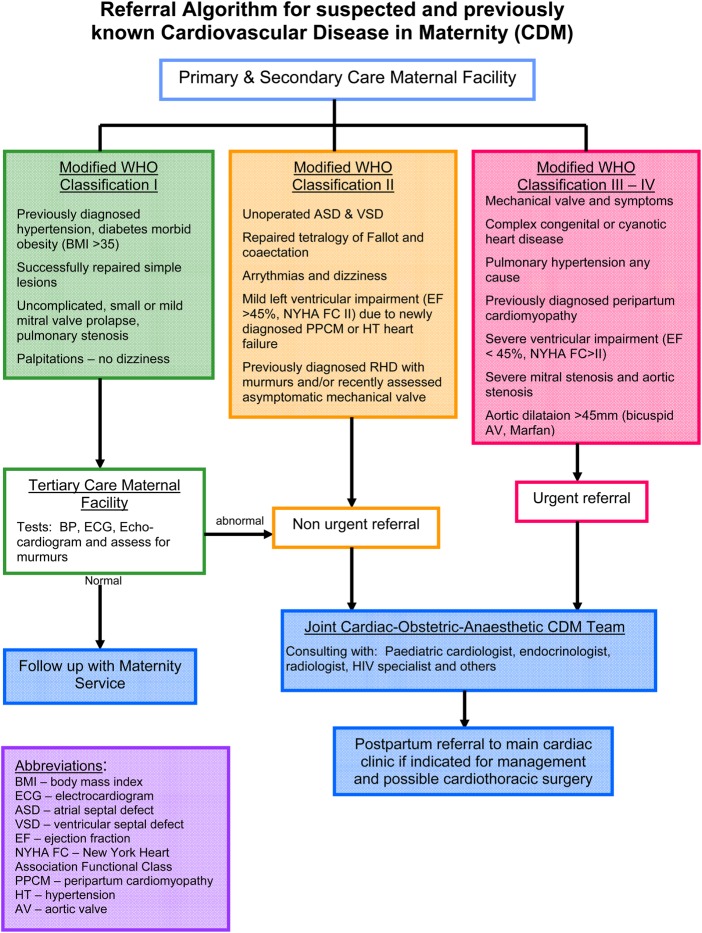
Between 1 July 2010 and 30 June 2012, 225 consecutive patients with suspected or previously diagnosed CVD, including blood pressure and heart rate, were assessed at first visit to the joint cardiac–obstetric clinic, having been sent there via a referral algorithm from primary care and secondary care facilities in Cape Town and from within the tertiary hospital (figure 1). Risk stratification after hours or via the emergency room were included and assessed in the wards or at the next available clinic date. The study was approved by the Ethics Committee of the University of Cape Town (HEC ref: 173/2010).
Figure 1.
Referral algorithm to joint Cardiac Maternity Clinic, Groote Schuur Hospital. BMI, body mass index; BP, blood pressure; ESC, European Society of Cardiology; ASD, atrial septal defect; VSD, ventricular septal defect; NYHA FC, New York Heart Association Functional Class; PPCM, peripartum cardiomyopathy; RHD, rheumatic heart disease; HT, hormone therapy; AV, aortic valve.
All patients referred to this clinic were seen jointly by a senior cardiology and obstetric consultant and physicians from other disciplines, for example, radiology, endocrinology and anaesthetics, were consulted. Patients were managed jointly throughout their pregnancy and those presenting postpartum were seen once at this clinic and managed further at the general cardiac clinic or a dedicated cardiomyopathy clinic, Groote Schuur Hospital (KS). Patients were booked according to standard management, which could include a waiting period of up to 3 months.
Data
Baseline data recorded at the first visit included sociodemographic factors, family history of CVD, history of pre-eclampsia or chronic hypertension, HIV status, onset of symptoms and signs, parity, prior cardiac events, prior surgery or cardiac interventions and use of medication. In addition, New York Heart Association Functional Class (NYHAFC), ECG and transthoracic echocardiography (KS) were recorded, including assessments of left and right ventricular function, Doppler quantification of inflow and outflow obstruction, quantification of valvular regurgitation and systolic pulmonary artery pressure were measured according to standard practice guidelines.3 The EF was calculated according to standard criteria, usually using M-mode and, in some cases, applying the modified Simpson method.3
Patients were stratified into four risk groups using a modified WHO risk classification for pregnant women with cardiac disease. Depending on diagnosis and severity of disease, risk classification ranged from class I (low risk) to class IV (contraindication for pregnancy), as recently used in the European Society of Cardiology (ESC) Guidelines on the management of CVD during pregnancy.4 Diseases not accounted for by this classification were scored by two authors, a cardiologist (KS) and an obstetrician (JA). The modified WHO classification has a prediction value in the management of pregnant women but is also based on the underlying severity of heart disease; uniformity in the classification has been secured by applying this classification to both antenatal and postpartum patients.
Poor outcome was as previously defined,5 using a combined endpoint of death, EF <45% or remaining in NYHA FC III/IV as the cohort was relatively small.
Patients with a history of CVD (but no symptoms or signs) and a normal clinical examination, together with absent or minimal echocardiography changes—such as operated congenital heart disease (CHD) or a prior history of rheumatic fever without clinical sequelae—were only seen once and then referred to secondary-level obstetric care. These patients were classified as modified WHO stage I. As many of those patients were referred from peripheral hospitals, records were not available to document maternal and fetal outcomes.
Patients with symptoms, signs or a WHO stage II–IV classification had clinical visits during the second trimester (<28 weeks), third trimester (28–37 weeks), peripartum period (onset of labour until hospital discharge) and at 6 weeks and 6 months postpartum. The frequency of visits was adapted to the severity of the disease and transport logistics of the patient. For many women, frequent hospital visits are not affordable. Mode of delivery, use of tocolytic therapy and perinatal outcome were obtained from patients, referring physicians and by examining the obstetric records. Newborns of mothers with CHD underwent paediatric echocardiography.
Peripartum complications were grouped into cardiac, neonatal and obstetric events. Cardiac events were defined by (re)admission for heart failure, symptomatic tachycardia requiring therapy, arrhythmia, stroke and cardiac death. Neonatal events were defined as any of the following: premature birth (<37 weeks gestation), small-for-gestational age birthweight (<10th centile), respiratory distress syndrome, intraventricular haemorrhage, fetal death (>20 weeks’ gestation) or neonatal death (within 28 days after birth). Perinatal mortality rates were calculated based on the number of fetal deaths and early neonatal deaths per 1000 live births. Obstetric events that were documented included non-cardiac death, pregnancy-induced hypertension, pre-eclampsia and postpartum haemorrhage (PPH). PPH was defined as blood loss >500 mL (vaginal delivery) or >1000 mL (caesarean section), which required transfusion or was accompanied by a drop in haemoglobin >2.0 g/L.
Blood tests
Routine laboratory workup on all patients included a haemoglobin and HIV test. Other blood tests, as determined by the physician, were performed in certain cases. Plasma was collected at baseline and, in consenting patients, also postdelivery. Aliquots were stored at −80°C for future analysis. Plasma NT-proBNP was measured by electrochemiluminescence immunoassay (BNP Fragment EIA, BIOMEDICA GRUPPE gmbHa Co, Vienna, Austria).6
Data collection
Data were entered into a specifically developed web-based platform for this project, which included uploading of ECGs in PDF or jpeg format and echo-images as still pictures or in AVI mode.
Statistical analysis
Cardiac, neonatal and obstetric events were analysed separately. Database management and statistical analyses were performed with SAS software, V.9.2 statistical program (SAS, Institute, Cary, North Carolina, USA). Continuous data were expressed as mean±SD or median (range). Comparison of means and proportions between subgroups at baseline was performed by independent t test and χ2 statistics (or Fisher's exact test where necessary), respectively, and where data were not normally distributed, a Wilcoxon rank-sum test was used.
Univariate and stepwise multiple logistic regression analyses were performed to establish independent predictors of poor outcome. Significance was assumed at a two-sided value of p<0.05.
Results
The characteristics of the 225 patient cohort is shown in table 1.
Table 1.
Baseline maternal characteristics of 225 cohorts presenting in modified WHO I–IV
| All patients (n=225) | WHO I (n=73) | WHO II–IV (n=152) | p Value | |
|---|---|---|---|---|
| Age at enrolment (years) | 28.6±6.4 | 28.8±7.0 | 28.5±6.1 | 0.97 |
| Ethnicity, n (%) | ||||
| African or black | 101 (45) | 23 (32) | 79 (52) | <0.0001 |
| Mixed | 72 (32) | 15 (20) | 56 (37) | |
| White | 34 (15) | 19 (26) | 15 (10) | |
| Other (Arab, Indian, other) | 18 (8) | 16 (22) | 2 (1) | |
| General medical history, n (%) | ||||
| Chronic hypertension | 12 (5) | 2 (3) | 10 (7) | 0.35 |
| Hypercholesterolaemia | 3 (1) | 0 (0) | 3 (2) | 0.55 |
| HIV | 38 (12) | 2 (3) | 36 (24) | <0.0001 |
| Syphilis | 1 (0.4) | 0 (0) | 1 (1) | 1.00 |
| Tuberculosis | 4 (2) | 0 (0) | 4 (3) | 0.31 |
| Clinical history and presentation, n (%) | ||||
| Previously known CVD | 93 (41) | 40 (55) | 53 (35) | 0.006 |
| Previously operated CVD | 57 (25) | 27 (37) | 30 (20) | 0.007 |
| NYHA FC I-II | 193 (86) | 73 (100) | 120 (79) | <0.0001 |
| NYHA FC III-IV | 32 (14) | 0 (0) | 32 (21) | |
| SBP in mmHg | 119±15 | 118±13 | 119±16 | 0.97 |
| DBP in mm Hg | 74±11 | 75±10 | 74±11 | 0.53 |
| Heart rate in bpm | 86±18 | 78±12 | 90±19 | <0.0001 |
| Weight in kg | 72.1±16.8 | 72.5±12.5 | 72.1+18.6 | 0.30 |
| Obstetric history, n (%) | ||||
| Gestational age at presentation | n=188 | n=66 | n=122 | |
| <12 weeks | 7 (4) | 3 (5) | 4 (3) | |
| 12–24 weeks | 84 (45) | 32 (48) | 52 (43) | 0.65 |
| >24 weeks | 97 (52) | 31 (47) | 66 (54) | |
| Gravida (median, range) | 2 (1–7) | 2 (1–6) | 2 (1–7) | 0.0002 |
| Para (median, range) | 1 (0–5) | 1 (0–4) | 1 (0–5) | 0.003 |
| Nulliparous, n (%) | 65 (29) | 29 (40) | 36 (24) | 0.02 |
| Twin pregnancies | 3 (1) | 0 (0) | 3 (2) | 0.30 |
A number of women were either not pregnant or presented postpartum. Gestational age at presentation percentages calculated towards 187, 66 and 121, respectively.
CVD, cardiovascular disease; DBP, diastolic blood pressure; NYHA FC, New York Heart Association Functional Class; SBP, systolic blood pressure.
Black patients presented significantly more frequently with advanced disease (WHO II–IV) compared with other ethnic groups (p<0.0001). There were significant differences in heart rate, gravidity and parity in patients presenting with a diagnosis in the modified WHO class I versus those in class II–IV. Generally, comorbidity, including HIV infection, was associated with more severe cardiac disease, measured in terms of WHO classification (p<0.0001). Patients with a high-risk category defined by modified WHO class presented with more symptomatic disease, defined by the NYHAFC and a higher heart rate (p<0.0001).
Diagnosis
The diagnosis of women classified as WHO class I (n=73) fell into one of four categories: history of rheumatic heart disease (RHD) or minor RHD lesion (n=16; 22%), minor or operated CHD with no significant residual structural abnormality (n=26; 35%), chronic hypertension with no end-organ damage (n=4; 7%) or they had been referred for other reasons such as history of palpitations or the need for preconception counselling (n=27; 36%). Diagnoses of the 152 patients presenting in WHO class II–IV and needing close follow-up were CHD (32%—ventricular septum defect n=6; atrial septum defect n=8; coarctation n=3 and others; only 15 operated previously), RHD (26%, 15 operated previously) and cardiomyopathy (27%—11 had idiopathic dilated cardiomyopathy; 21 peripartum cardiomyopathy (PPCM) and 9 hypertension-related cardiomyopathy). Fifteen per cent had other diagnosis such as Takayasu's disease (n=4), Marfan's disease (n=4), ventricular arrhythmias (n=5), constrictive pericarditis (n=3) and others. None of the women presented with ischaemic heart disease.
Demographics, medical and obstetric history of patients presenting in WHO II–IV needing follow-up
Table 2 shows ethnic group/ethnicity defined by language, educational level, disposable income, general medical history (including smoking), cardiac and obstetric history for patients presenting prepartum or postpartum. One hundred and twenty-two women (80%) presented prepartum with 66 (54%) patients presenting for the first time to our clinic with a gestational age at entry of >24 weeks. Black patients were represented in a higher percentage in the postpartum cohort (p=0.009). Patients in modified WHO class II–IV had significantly higher gravidity (p=0.0002). Women presenting in the postpartum period (n=30) presented with more advanced NYHA (p<0.001) and had a significantly higher heart rate (p<0.001) and NTproBNP (p<0.0001).
Table 2.
Baseline characteristics: demographics and medical history modified WHO II–IV
| All patients (n=152) | Presenting prepartum (n=122) | Presenting postpartum (n=30) | |
|---|---|---|---|
| Age at enrolment (years) | 28.5±6.1 | 28.2±6.2 | 29.6±5.8 |
| Ethnicity, n (%) | |||
| African or black | 79 (52) | 55 (45) | 24 (80)* |
| Mixed | 56 (37) | 50 (41) | 6 (20)* |
| White | 15 (10) | 15 (12) | 0 (0) |
| Other (Arab, Indian, other) | 2 (1) | 2 (2) | 0 (0) |
| Language, n (%) | |||
| Afrikaans | 43 (28) | 40 (33) | 3 (10) |
| English | 33 (22) | 29 (24) | 4 (13) |
| isiXhosa | 58 (38) | 41 (34) | 17 (57) |
| isiZulu | 12 (8) | 9 (7) | 3 (10) |
| Other | 6 (4) | 3 (2) | 3 (10) |
| Education level, n (%) | |||
| No school | 1 (1) | 0 (0) | 1 (3) |
| Year 1–7 | 46 (30) | 36 (30) | 10 (33) |
| Year 8–11 | 92 (61) | 73 (60) | 19 (63) |
| Year 12/>Year 12 | 13 (8) | 13 (10) | 0 |
| Income per month, n (%) (ZAR) | |||
| <300 | 56 (37) | 39 (33) | 17 (57) |
| 300–999 | 38 (25) | 31 (26) | 7 (23) |
| 1000–9999 | 54 (36) | 48 (40) | 6 (20) |
| ≥10 000 | 2 (1) | 2 (2) | 0 (0) |
| General medical history (%) | |||
| Chronic hypertension | 10 (7) | 10 (8) | 0 (0) |
| Hypercholesterolaemia | 3 (2) | 3 (2) | 0 (0) |
| HIV | 36 (24) | 26 (21) | 10 (33) |
| Syphilis | 1 (1) | 1 (1) | 0 (0) |
| Tuberculosis | 4 (3) | 3 (2) | 1 (3) |
| Family history of CVD | 23 (15) | 21 (17) | 2 (7) |
| Family history of PPCM/CMO | 11 (7) | 10 (8) | 1 (3) |
| Heart valve replacement/repair | 16 (11) | 16 (13) | 0 (0) |
| Obstetric history, n (%) | |||
| Gestational age at presentation, women presenting prepartum | |||
| 12–24 weeks | 52 (43) | 52 (43) | 0 |
| >24 weeks | 66 (54) | 66 (54) | 0 |
| Nulliparous n (%) | 36 (24) | 32 (26) | 4 (13) |
| Parous n (%) | 116 (76) | 90 (74) | 26 (87) |
| Social history, n (%) | |||
| Smoking | |||
| Current | 10 (6) | 8 (7) | 2 (7) |
| Former | 2 (1) | 2 (2) | 0 (0) |
| Alcohol use | |||
| Current | 3 (2) | 1 (1) | 2 (7) |
| Former | 2 (1) | 2 (2) | 0 (0) |
*p<0.009.
CVD, cardiovascular disease; CMO, cardiomyopathy; PPCM, peripartum cardiomyopathy.
Presenting symptoms, laboratory tests, ECG, echocardiographic findings and medication are shown in table 3. Patients developing symptoms postpartum presented with significantly larger left ventricular dimensions and with a markedly lower echocardiography EF (44.7±11.8 vs 54.5±12.4, p<0.003).
Table 3.
Symptoms, examination and medication at presentation for patients in modified WHO II–IV
| All patients (n=152) | Presenting prepartum (n=122) | Presenting postpartum (n=30) | p Value | |
|---|---|---|---|---|
| Symptoms and severity of disease, n (%) | ||||
| Palpitations | 64 (42) | 54 (44) | 10 (33) | 0.27 |
| Presyncope/syncope | 1 (0) | 1 (0) | 0 (0) | 0.58 |
| Chest pain | 5 (3) | 4 (3) | 1 (3) | 1.00 |
| NYHA FC | ||||
| I/II | 120 (79) | 108 (89) | 12 (40) | <0.001 |
| III | 32 (21) | 14 (11) | 18 (60) | <0.001 |
| Modified WHO group | ||||
| II-III | 98 (64) | 98 (80) | 0 (0) | <0.001 |
| IV | 54 (36) | 24 (20) | 30 (100) | <0.001 |
| Vital signs | ||||
| Heart rate in bpm | 90±19 | 87±17 | 103±19 | <0.0001 |
| SBP in mm Hg | 119±16 | 121±15 | 111±17 | 0.001 |
| DBP in mm Hg | 74±11 | 74±12 | 74±11 | 0.79 |
| Physical examination, n (%) | ||||
| Jugular venous pressure >5 | 42 (28) | 20 (16) | 22 (73) | <0.001 |
| Crepitations | 40 (26) | 21 (18) | 19 (63) | <0.001 |
| Oedema | 29 (19) | 15 (12) | 14 (47) | <0.001 |
| Systolic heart murmur | 86 (57) | 60 (49) | 26 (87) | <0.001 |
| Diastolic heart murmur | 7 (5) | 7 (6) | 0 (0) | 0.17 |
| Laboratory tests | ||||
| Haemoglobin (g/dL) | 11.5±1.9 | 11.5±1.7 | 11.4±2.4 | 0.11 |
| HIV positive | 36 (24) | 26 (21) | 10 (36) | 0.17 |
| NTProBNP* (median, range) | 629.3 (130–6400) | 459.6 (130–4885) | 1589.9 (130–6400) | <0.0001 |
| Log NTProBNP | 6.38±1.04 | 6.15+0.98 | 7.16+0.89 | <0.0001 |
| ECG, n (%) | ||||
| Sinus rhythm | 127 (83) | 103 (84) | 24 (86) | 0.79 |
| Sinus tachycardia | 13 (9) | 8 (7) | 5 (17) | 0.09 |
| Atrial fibrillation | 1 (1) | 1 (3) | 0 (0) | 0.34 |
| Echocardiogram | ||||
| LVEDD (mm) | 50.6±8.5 | 48.6±7.7 | 58.7±6.5 | <0.0001 |
| LVESD (mm) | 36.9±10.1 | 33.4±7.6 | 50.4±6.9 | <0.0001 |
| EF (%) (n=62) | 51.1±13.0 | 54.5±12.4 | 44.7±11.8 | 0.003 |
| Medications at baseline visit, n (%) | ||||
| ACE inhibitor | 5 | 0 | 5 | – |
| Aldosterone antagonist | 1 | 0 | 1 | – |
| Aspirin | 3 | 3 | 0 | – |
| Beta blocker | 10 | 9 | 1 | – |
| Bromocriptine | 5 | 0 | 5 | – |
| Calcium channel blocker | 7 | 6 | 1 | – |
| Digoxin | 1 | 1 | 0 | – |
| Hydralazine | 1 | 1 | 0 | – |
| Nitrate | 0 | 0 | 0 | – |
| Furosemide | 34 | 18 | 16 | – |
| Thiazide | 16 | 15 | 1 | – |
| Warfarin | 9 | 9 | 0 | – |
*NTproBNP measurements were only performed in 110 patients.
DBP, diastolic blood pressure; LVESD, left ventricle in end-systolic diameter; LVEDD, left ventricle in end-diastolic diameter; NYHA FC, New York Heart Association Functional Class; SBP, systolic blood pressure; ACE inhibitor, angiotensin-converting-enzyme inhibitor.
Of the women diagnosed with CVD prepartum, 27% received diuretics, 7.3% beta-blockers and 7.3% warfarin while pregnant (table 3).
Overall and cardiac outcome
Only one patient of the diagnosed prepartum women had no postpartum visit. Thus, 142 patients had follow-up data.
Maternal mortality rate was 9/152: 5.92%, 95% CI (2.15% to 9.65%) within the 6-month postpartum follow-up period. The diagnoses of women who died were familial and PPCM (n=7) and two cases of prosthetic valve complications (thrombosis and sepsis) (table 4). All patients who died belonged to the mod WHO class III or IV risk group. Eight of the nine patients died after 42 days of postpartum. Thirty women had poor outcome as predefined using a combined endpoint of death, EF and FC.
Table 4.
Maternal mortality in women with structural heart disease
| Diagnosis | Timing of presentation | Modified WHO class | NYHA class | Age (years) | EF (%) | When | Reason | Fetal outcome |
|---|---|---|---|---|---|---|---|---|
| PPCM with subsequent pregnancy | Prepartum | IV | II | 24 | 45 | 61 days postpartum | Sudden death | Fetal survival |
| PPCM | Postpartum | IV | II | 32 | 32 | 130 days postpartum | CCF | Fetal survival |
| Rheumatic HD with DVR | Prepartum | IV | I | 19 | 30 | 50 days postpartum | Valve thrombosis | Fetal survival |
| PPCM | Postpartum | IV | III | 24 | 24 | 122 days postpartum | CCF | Fetal survival |
| Familial CMO | Prepartum | IV | II | 43 | 30 | 44 day spostpartum | CCF | Fetal survival |
| PPCM | Prepartum | IV | II | 32 | 25 | 95 days postpartum | Sudden death | Fetal survival |
| Familial CMO | Prepartum | IV | III | 25 | 26 | 92 days postpartum | CCF | Fetal survival |
| PPCM post miscarriage | Postpartum | IV | IV | 24 | 31 | 150 postpartum | CCF | Miscarriage 20 weeks |
| Rheumatic HD with MVR | Prepartum | IV | I | 25 | 60 | 16 weeks prepartum | SBE | Fetal death |
CCF, congestive cardiac failure; CMO, cardiomyopathy; DVR, double valve replacement; HD, heart disease; MVR, mitral valve replacement; NYHA, New York Heart Association; PPCM, peripartum cardiomyopathy; SBE, subacute bacterial endocardititis.
Of the women that were diagnosed prepartum, 34% developed signs and symptoms of heart disease while pregnant, leading to admission in 20% of cases. At last follow-up, 11% of the patients were in NYHA functional class III or IV.
Of the 30 patients diagnosed postpartum, 3 died before their 6-month visit. Of the remaining patients, 44% felt minimally to moderately better, 12% unchanged, 44% minimally or moderately worse compared with first presentation.
Obstetric and fetal outcome
The mean gestational stage was 36.7±4.2 with a significantly longer gestational period in women presenting with antenatal cardiac disease versus presentation postpartum (p=0.04). There was a high overall rate of operative delivery with 46 of 152 women (30%) having had a caesarean section. There were no significant differences between the different modified WHO class disease categories (II–IV) in terms of obstetric outcome. In addition to their structural CVD, 11 patients developed gestational hypertension while pregnant. Preeclampsia was found in 1:30 nullipara and 1:60 multiparous women (n=3 in this cohort).
Perinatal death occurred in 1/152 (0.66%, 95% CI (0.00 to 1.94)) due to one fetal death, translating to a perinatal mortality of 7/1000 live births. In addition, there were two medically indicated terminations and two miscarriages.
Mean birth weight for the entire cohort was 2850.8±552.7 g, with 33/148 born weighing <2500 g and 46/148 born preterm (<37 weeks’ duration).
Of 152 babies, 19 had an Apgar score of <7 at 5 min. Women presenting with cardiac disease postpartum had a greater proportion of neonates who had lower Apgar scores (30% vs 8%; p=0.001). There were no significant differences between modified WHO groups in terms of neonatal outcome.
Predictors of poor maternal outcome
In the univariate logistic regression analysis, increased heart rate (p=0.009), increased log NTproBNP (p=0.001), lower income (p=0.005), more severe NYHA FC at baseline (class I+II vs III+IV: p=0.048) and decreased EF (p<0.0001) predicted poor outcome. In multivariate analysis, only the echocardiography EF prevailed as an independent predictor of poor outcome (p<0.0001: OR=0.93 (0.90 to 0.96)), after adjusting for age, HR, systolic blood pressure, log NTproBNP, income, level of education, HIV status, WHO class and NYHA FC at baseline.
Discussion
A number of registries on women with heart disease in pregnancy have been reported in the past.7–10 However, they are predominantly from the developed world with a focus on women presenting with operated CHD.9 10 They, therefore, do not cover the entire burden of CVD in pregnancy particular to an African population. This study evaluated the clinical outcome, based upon a referral algorithm deemed practical and appropriate for the local clinical service, in which patients often do not have prediagnosed illness because they have poor access to healthcare outside of pregnancy and are referred to a tertiary service only late in their disease due to a lack of awareness of cardiac symptoms by both patients and primary healthcare nurses. A weekly clinic with a senior cardiologist and obstetrician allowed speedy assessment of complex cases and appropriate workup, including evaluation by an anaesthetist prior to delivery and prenatal evaluation of the fetus.
The study has shown a disease pattern markedly different to that seen in the developed world with RHD, cardiomyopathies and CHD, some previously not diagnosed, being major problems, often complicated by HIV/AIDS as a comorbidity.
The data presented demonstrate a significantly higher maternal mortality rate with nine of 152 cases (5.9%) compared with 1% in a recent study performed by ESC.7 Interestingly, most of the deaths were attributable to different forms of cardiomyopathies, with only two being related to complications attributable to sepsis and thrombosis affecting prosthetic heart valves. These observations are relevant to the general pattern of maternal mortality described in South. The confidential inquiry into maternal death in South Africa reported that, of the 4867 death reported over 2 years, 14% were due to hypertensive disorders, with another 8.8% due to medical and surgical conditions.11 The definitions employed describe maternal death as death occurring during pregnancy or within 42 days of delivery as it is international standard.12 The definition of death within 42 days applied to our data would have completely underestimated the number of cardiac deaths related to pregnancy because eight out of nine deaths would not have been captured. Our data suggest that care needs to be stepped up covering the postpartum period with earlier referral to the general cardiac or cardiomyopathy clinic and adequate counselling about the risks of future pregnancy and contraceptive services be provided. Our findings are likely to be applicable to other regions where cardiomyopathies due to a number of conditions are common.13 14
A recent systematic review of the burden of antenatal heart disease in South Africa reported a prevalence of heart disease, ranging from 123 to 943 per 100 000 deliveries with RHD being the most common abnormality, followed by cardiomyopathies.15 The results of the Global Burden of Disease Study 2013 on global, regional and national levels and causes of maternal mortality during 1990–2013, published in 2014, reported on an ongoing high maternal mortality in South Africa, with 171.5/100 000 live births.16 Although substantial progress has been made, South Africa will not meet the fifth Millennium Developmental Goal. Heart disease, chronic hypertension and preeclampsia contributed to more than 20% of 3024 cases with severe maternal outcome in the WHO Multicountry Survey on maternal and newborn health.17
In addition to the high number of postnatal deaths, our data showed that 18% of women who survived and attended follow-up clinics had symptoms that were worse after the pregnancy, with 44% having moderate-to-severe disability. There were no discriminating criteria according to diagnosis that allowed any prediction of who would become more symptomatic, although the EF was significantly worse in the cardiomyopathy group.
The obstetric outcome for the cohort studied was good. There were no stillbirths and only one neonatal death. Although 30% of the patients delivered before 37 weeks, the mean gestational age at delivery fell just short of 37 weeks and the mean birth weight was 2.85 kg. There was a high rate of obstetric intervention evident as a 30% caesarean section rate, similar to the 27% in the Canadian CARPREG study8 and 41% in the European cohort.7 In addition, one in five pregnancies required hospitalisation for cardiac assessment and treatment during the course of the pregnancy.
The perinatal outcome in an environment of intensive surveillance and management was found to be generally good. This has to be seen in the context of a high overall perinatal mortality of 34/1000 live births in South Africa.11
With regards to the clinical service provision, a large proportion of the women studied had RHD due to the sequelae of group A streptococcal infection. These women all benefit from access to clinics such as the Cardiac Disease and Maternity Clinic (CDMC), where multidisciplinary care contributes to the likelihood of safe motherhood. In the future, echocardiography-based screening of patients may further improve identification and referral of high-risk cases, although the cost-effectiveness of this intervention is yet to be established.18 19 B-type natriuretic peptides have also been shown to be effective biomarkers in pregnant women, correlating with outcome,20 and need further exploration as a bed-side test in the developing world.
Limitations
This study has several limitations as it is an observational study confined to a particular area. The data are therefore at variance with the previously published literature. In addition, women with subclinical disease, milder forms of CVD or fatal events prior to admission were not captured. Only M-mode echocardiography has been performed to qualify left ventricular function in a number of cases. We have adhered to the published STROBE guidelines relating to reporting of this type of study.21
Conclusion
Our data show a disease pattern in South Africa, markedly different to that of the developed world. Joint obstetric–cardiac care is associated with excellent survival rates of mothers presenting while pregnant, even with complex diseases, and their offspring. The greatest risk of adverse outcome is attributable to late presentation and left ventricular failure with death commonly occurring outside the 42-day maternal mortality reporting period. The perinatal outcome in an environment of intensive surveillance and management was found to be generally good.
Key messages.
What is already known on this subject?
A number of registries on women with heart disease in pregnancy have been reported in the past years. However, they are predominantly from the developed world with a focus on women presenting with operated congenital heart disease.
What might this study add?
We report on 225 women with heart disease in pregnancy. We found a disease pattern markedly different to that in the higher-income countries. Rheumatic heart disease, cardiomyopathy and hypertensive heart failure are major problems often complicated by HIV/AIDS as comorbidity.
How might this impact on clinical practice?
Joint obstetric–cardiac care showed survival rate of mothers presenting while pregnant, even those with complex diseases similar to that seen in the western world, but also that of their offspring. However, high mortality occurred in the postpartum period. As traditionally maternal mortality is reported as death <42 days postdelivery, eight of nine deaths would have not been recorded as maternal death, which could imply underreporting of maternal deaths in the context of an overall very high maternal death rate in South Africa.
Acknowledgments
The authors would like to acknowledge the support of Mrs Sylvia Dennis, Hatter Institute for Cardiovascular Research in Africa, in preparing the manuscript. This research could not have been conducted without the funding support of The University of Cape Town, the Medical Research Council South Africa, the Medtronic Foundation, Maurice Hatter Foundation and Servier. We also would like to thank Dr Lori Blauwet and Dr Laetitia Aquah, Mayo Clinic, Rochester, USA, and other physicians and nurses at the Department of Cardiology and Obstetrics for their support.
Footnotes
Contributors: KS, CE, FT and JA have participated in the original conception and design of the study. EL was fundamentally involved in the data analysis and interpretation. LZ, AO, JR-H and TL have participated in the interpretation of the data, drafting and critical revision of the paper. KS takes responsibility for the overall content as guarantor.
Funding: University of Cape Town, South Africa.
Competing interests: None.
Ethics approval: Human Ethics Committee, University of Cape Town.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mocumbi AO, Sliwa K. Women's cardiovascular health in Africa. Heart 2012;98:450–5. [DOI] [PubMed] [Google Scholar]
- 2.Sliwa K, Mayosi BM. Recent advances in the epidemiology, pathogenesis and prognosis of acute heart failure and cardiomyopathy in Africa. Heart 2013;99:1317–22. [DOI] [PubMed] [Google Scholar]
- 3.Sahn DJ, Demaria A, Kisslo J, et al. Recommendations regarding quantitation in M-Mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978;58:1072–83. [DOI] [PubMed] [Google Scholar]
- 4.Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2011;32:3147–97. [DOI] [PubMed] [Google Scholar]
- 5.Blauwet LA, Libhaber E, Forster O, et al. Predictors of outcome in 176 South African patients with peripartum cardiomyopathy. Heart 2013;99:308–13. [DOI] [PubMed] [Google Scholar]
- 6.Prickett TC, Yandle TG, Nicholls MG, et al. Identification of amino-terminal pro-C-type natriuretic peptide in human plasma. Biochem Biophys Res Commun 2001;286:513–17. [DOI] [PubMed] [Google Scholar]
- 7.Roos-Hesselink JW, Ruys TP, Stein JI, et al. Outcome of pregnancy in patients with structural or ischaemic heart disease: results of a registry of the European Society of Cardiology. Eur Heart J 2013;34:657–65. [DOI] [PubMed] [Google Scholar]
- 8.Siu SC, Sermer M, Colman JM, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation 2001;104:515–21. [DOI] [PubMed] [Google Scholar]
- 9.Pieper PG, Balci A, Aarnoudse JG, et al. Uteroplacental blood flow, cardiac function, and pregnancy outcome in women with congenital heart disease. Circulation 2013;128:2478–87. [DOI] [PubMed] [Google Scholar]
- 10.Stangl V, Schad J, Gossing G, et al. Maternal heart disease and pregnancy outcome: a single-centre experience. Eur J Heart Fail 2008;10:855–60. [DOI] [PubMed] [Google Scholar]
- 11.NCCEEMD. National Committee for the Confidential Enquiries into Maternal Death (NCCEMD) in South Africa 2012. http://wwwdohgovza/docs/reports/2012/Report
- 12.World Health Organization. Maternal mortality in 2005: estimates developed by WHO, UNICEF, UNFPA, and the World Bank. ISBN 978 92 4 159621 3 2007.
- 13.Sliwa K, Hilfiker-Kleiner D, Petrie M, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on Peripartum Cardiomyopathy. Eur Heart J 2010;12:767–78. [DOI] [PubMed] [Google Scholar]
- 14.Sliwa K, Hilfiker-Kleiner D, Mebaaza A, et al. EURObservational Research Programme: a worldwide registry on peripartum cardiomyopathy (PPCM) in conjunction with the Heart Failure Association of the European Society of Cardiology Working Group on PPCM. Eur J Heart Fail 2014;16:583–91. [DOI] [PubMed] [Google Scholar]
- 15.Watkins DA, Sebitloane M, Engel ME, et al. The burden of antenatal heart disease in South Africa: a systematic review. BMC Cardiovasc Disord 2012;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014. doi:10.1016/S0140-6736(14)60696-6. [Epub ahead of print 2 May 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souza JP, Gulmezoglu AM, Vogel J, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross-sectional study. Lancet 2013;381:1747–55. [DOI] [PubMed] [Google Scholar]
- 18.Mayosi BM, Flisher AJ, Lalloo UG, et al. The burden of non-communicable diseases in South Africa. Lancet 2009;374:934–47. [DOI] [PubMed] [Google Scholar]
- 19.Sliwa K, Zilla P. Rheumatic heart disease: the tip of the iceberg. Circulation 2012;125:3060–2. [DOI] [PubMed] [Google Scholar]
- 20.Tanous D, Siu SC, Mason J, et al. B-type natriuretic peptide in pregnant women with heart disease. J Am Coll Cardiol 2010;56:1247–53. [DOI] [PubMed] [Google Scholar]
- 21.von Elm E, Altman D, Egger M, et al. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ 2007;85:867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]